Abstract
Preharvest sprouting (PHS) is a deleterious phenotype that occurs frequently in rice-growing regions where the temperature and precipitation are high. It negatively affects yield, quality, and downstream grain processing. Seed dormancy is a trait related to PHS. Longer seed dormancy is preferred for rice production as it can prevent PHS. Here, we map QTLs associated with rice seed dormancy and clone Seed Dormancy 3.1 (SDR3.1) underlying one major QTL. SDR3.1 encodes a mediator of OsbZIP46 deactivation and degradation (MODD). We show that SDR3.1 negatively regulates seed dormancy by inhibiting the transcriptional activity of ABIs. In addition, we reveal two critical amino acids of SDR3.1 that are critical for the differences in seed dormancy between the **an/indica and Geng/japonica cultivars. Further, SDR3.1 has been artificially selected during rice domestication. We propose a two-line model for the process of rice seed dormancy domestication from wild rice to modern cultivars. We believe the candidate gene and germplasm studied in this study would be beneficial for the genetic improvement of rice seed dormancy.
Similar content being viewed by others
Introduction
Crop domestication is necessary and fundamental for historical development. The food surplus brought about by domestication has promoted the development of human civilization through urbanization and the evolution of the nation state1. In turn, the diverse cultural preferences of different historical periods have determined the direction of crop domestication that can meet the particular needs of humans2,3,4,5. Rice is one of the oldest domesticated crops, and is cultivated approximately 11,000 years ago6. Over time, rice has become the main source of energy for more than half of the world’s population7. During the domestication of wild rice, many physiological traits and morphological features were considerably altered. These transformations include changes in awn development8,9,10, seed shattering11,12,13, grain quality6, plant architecture14,15,16,17, grain size18, heading date19, panicle size20, and seed dormancy21.
An important goal of rice domestication is to increase yield to meet demand and overcome unfavorable environmental conditions. Yield is usually affected by PHS, which generally occurs in high-temperature and high-humidity environments in the middle and lower reaches of the Yangtze River. PHS can cause enormous economic losses in crop production, including declines in seed yield and quality22. Longer seed dormancy can prevent PHS, thereby reducing losses and increasing yield. Unfortunately, during domestication, rice seed dormancy is largely lost23. Therefore, it is necessary to identify rice cultivars that have longer seed dormancy and clone seed dormancy genes to improve rice yield.
Seed dormancy is a complex trait, and many dormancy-related QTLs have been identified in rice;24,25,26,27,28 however, few of these QTLs have been cloned21,29,30,31. The cloned seed dormancy genes are associated with either abscisic acid (ABA) or gibberellin (GA), two key hormones that regulate seed dormancy21. Sdr4 encodes an unknown function protein, and its regulation of seed dormancy depends on VP1. Only Sdr4-n occurs in japonica cultivars, whereas both Sdr4-n and Sdr4-k occur in indica cultivars, which seems to indicate that Sdr4 is involved in the rice seed dormancy domestication21. qSD7-1 is cloned from the weedy rice cultivar SS18-2 and encodes a basic helix-loop-helix protein. It increases the ABA content in seeds by upregulating the expression of ABA synthesis genes29. In addition, qSD7-1 interacts with VP1 and OsC1 to increase the seeds sensitivity to ABA33. qSD1-2 is isolated from SS18-2. It regulates seed dormancy via the GA pathway30. Recently, SD6, which is cloned from Kasalath, together with ICE2 antagonistically balanced the expression of ABA metabolic genes to control rice seed dormancy by responding to temperature signals31. However, if seed dormancy is too persistent, it is difficult to sprout, which increases the cost of timely farming; conversely, if dormancy is too weak, PHS will occur. Thus, it is necessary to appropriately regulate seed dormancy by identifying additional seed dormancy genes.
ABA is an important hormone that regulates seed dormancy. Its signal transduction involves many genes, such as the transcription factors ABI3 and ABI5. These genes positively regulate the transcription factors involved in rice seed dormancy34,35,36. Other ABA signaling response factors, such as embryonic abundant protein (EM1)37, late embryogenesis abundant protein (LEA3)
qSDR3.1 is a major QTL that regulates seed dormancy To determine the genetic mechanism underlying rice seed dormancy, we used ZH11 as the recipient parent and MJLXL as the donor parent to construct a backcross inbred lines population (Fig. 1a). First, we examined the germination rate of 153 lines derived from the BC3F2 population, which were selfbred from a single BC3F1 plant (the genotype of BC3F1 is shown in Supplementary Data 1). The germination rate of this population exhibited a normal distribution, indicating that seed dormancy was controlled by QTLs (Supplementary Fig. 2a). Then, we identified three QTLs of seed dormancy, designated qSDR2.1, qSDR3.1, and qSDR3.2, which were located on the second and third chromosomes, respectively (Supplementary Fig. 2b). qSDR3.1 had the highest logarithm of odds value (11.75) and was mapped between markers 3-15 and 3-21 on chromosome 3 (Supplementary Fig. 2c; Supplementary Table 1). Genetic analysis of the BC3F2 individuals suggested that qSDR3.1 is an incomplete dominant gene (Supplementary Fig. 2d, e). a Roadmap of constructing near-isogenic lines and fine-map** populations. b SDR3.1 was located between markers Indel27 and Indel29 on chromosome 3. c Identification of candidate genes of SDR3.1. d Germination rate of ZH11, the NIL, and three complementary lines 35 DAH (n = 3 independent experiments). e Copy numbers of SDR3.1 in ZH11 and three complementary lines. f PHS phenotypes of ZH11, the NIL, and three complementary lines 35 DAH. Data are presented as the mean ± SD. Source data are provided as a Source Data file. The six homozygous recombinant lines (selected from 4146 BC4F2 individuals) in the QTL region were analyzed for fine map** (Fig. 1b). The 7-day germination rate phenotype of the recombinant lines ranged from 20.16% to 72.14%. The 7-day germination rate of R2, R4, and R6 were similar to that of MJLXL, and the 7-day germination rate of R1, R3, and R5 were similar to that of ZH11. Finally, the location of qSDR3.1 was narrowed to a 26.9-kb region between markers Indel27 and Indel29 (Fig. 1b). This region contained 4 candidate genes, namely, LOC_Os03g11520, LOC_Os03g11530, LOC_Os03g11540 and LOC_Os03g11550 (Supplementary Table 2). LOC_Os03g11520 encode a proteins with unknown function. There was no difference between the parents in LOC_Os03g11530 and LOC_Os03g11540. And there were three bases differences between the two parents in LOC_Os03g11550, which created two amino acid differences (Fig. 1c; Supplementary Fig. 3a-b). In addition, the cDNA of LOC_Os03g11550 of R3 was consistent with that of ZH11, whereas the cDNA of LOC_Os03g11550 of R2 and R4 were consistent with that of MJLXL, indicating that the LOC_Os03g11550 genotypes cosegregated with the seed dormancy phenotypes (Supplementary Fig. 3c-e). According to the Rice Genome Annotation Database (http://rice.uga.edu/, RGAP 7), LOC_Os03g11550 comprises three exons and two introns. It encodes MODD39. MODD negatively regulates ABA signaling and drought resistance in rice39. Therefore, LOC_Os03g11550 was selected as the candidate gene of SDR3.1. To further identify SDR3.1, six chromosome segment substitution lines from the BC3F3 population were selected: R15, R32, and R152, which had a high germination rate; R16, R23, and R100, which had a low germination rate. The genotypes of SDR3.1 of R15, R32, and R152 were the same as that of ZH11, whereas the genotypes of SDR3.1 of R16, R23, and R100 were the same as that of MJLXL (Fig. 1c). We complemented SDR3.1 from ZH11 into NIL. The copy numbers of COM-1, COM-2, and COM-3 were 13, 7 and 7, respectively (Fig. 1e). The 7-day germination rate of the T2 generation seeds of the three complementary lines (COM-1: 90.06%; COM-2: 50.95%; COM-3: 52.50%) were significantly higher than that of NIL (19.01%) (Fig. 1d, f). These results demonstrated that LOC_Os03g11550 is the target gene of SDR3.1. To clarify the function of SDR3.1, we disrupted it in ZH11 via CRISPR/Cas9, and two homozygous mutants, KO-1 and KO-2, were screened through sequencing. These mutants contained a 1-bp frame-shift insertion or deletion in the first exon (Supplementary Fig. 4a, Supplementary Fig. 5). The expression and protein levels of SDR3.1 in both mutant lines were significantly lower than those of ZH11 (Supplementary Fig. 4b-c). Similarly, the 7-day germination rate of the two mutant lines (KO-1 and KO-2: 9.59% and 17.09%, respectively) were significantly lower than that of ZH11 (82.56%) (Supplementary Fig. 4d-e). Thus, we concluded that SDR3.1 negatively regulates seed dormancy. In rice, PHS frequently leads to severe decreases in grain yield and quality. Enhanced seed dormancy is useful for preventing PHS40. To determine whether SDR3.1 regulates PHS, we measured the PHS of ZH11, MJLXL, NIL, and the two mutant lines. On the 6th day, the PHS of NIL and MJLXL significantly decreased by 82.83% and 83.51% compare with that of ZH11, respectively. On the 5th day, the PHS of KO-1 and KO-2 significantly decreased by 48.21% and 47.79% compare with that of ZH11, respectively (Supplementary Fig. 6). Combined with the enhanced seed dormancy in NIL and the two mutant lines (Fig. 1d; Supplementary Fig. 4d, e), indicating that SDR3.1 regulates PHS. qRT-PCR showed that SDR3.1 is widely expressed in 10-day leaf, glume, anther, 5-day root, 20-day root, and 5-day leaf, with the highest transcript level detected in the pistil (Supplementary Fig. 7a). Then, the dynamic transcript levels of SDR3.1 were investigated during seed development. The transcript began to accumulate 5 days after fertilization and peaked at 15 days (Supplementary Fig. 7a). To study the biological function of SDR3.1, we fused the coding region to the N-terminus of GFP. The empty GFP vector was used as a control and mCherry (LOC_Os11g01330) was used as a marker. The results showed that the 35 S::SDR3.1-GFP protein was localized in the nucleus (Supplementary Fig. 7b). To determine whether SDR3.1 is a transcription factor, the recombinant vector pGBKT7-SDR3.1 was transferred into the yeast (Saccharomyces cerevisiae) strain Y2HGold. Cells expressing SDR3.1 protein could not grow on the systematic tri-deficient medium (Fig. 2a), indicating that SDR3.1 was not a transcription factor. a Verification of SDR3.1 transcriptional activation; pGBK-Negative, negative control; pGBK-Positive, positive control. b The yeast two-hybrid assay showed that SDR3.1 interacted with ABI5. pGADT7-T and pGBKT7-53 were used as positive controls; empty vectors were used as negative controls. c Bimolecular fluorescence complementation analysis. Fluorescence was observed only in the nuclei of protoplast, which resulted from complementation of the N-terminal region of YFP fused with SDR3.1 (SDR3.1-YFPN) with the C-terminal region of YFP fused with ABI5 (ABI5-YFPC). Bar = 10 μm. The experiments were replicated 3 times with similar results. d In vitro pull-down assays of SDR3.1 with ABI5. Affinity-purified His-ABI5 was incubated with GST-SDR3.1. Pull-down proteins were subjected to immunoblotting with anti-His antibodies. The experiments were replicated 3 times with similar results. e Schematic of the effectors and reporter used in the transient transactivation assays. f SDR3.1 repressed ABI5 to activate EM1 in response to 5 μM ABA. g SDR3.1 repressed ABI5 to activate LEA3 in response to 5 μM ABA. In f, g n = 3 independent experiments. REN, Renilla LUC. Data are presented as the mean values ± SD, and P values are indicated by two-tailed Student’s t test. Source data are provided as a Source Data file. Because MODD is homologous to the ABI5-binding protein AFP in Arabidopsis thaliana39, we speculated that SDR3.1 could interact with ABI5 in rice. To test this hypothesis, we performed a yeast two-hybrid (Y2H) assay. The results showed that SDR3.1 interacted with ABI5 (Fig. 2b), which was further confirmed by the bimolecular fluorescence complementation (BiFC) assay (Fig. 2c). Moreover, a protein‒protein pull-down assay showed that GST-SDR3.1 bound His-ABI5 (Fig. 2d), indicating that SDR3.1 interacted with ABI5 in vitro. In summary, SDR3.1 could interact with ABI5, implying that SDR3.1 mediates the ABA response to regulate seed dormancy. To identify the protein sites necessary for interaction with ABI5, we divided SDR3.1 into three segments according to the NCBI conservative domains database (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi), namely, the N-terminus containing the EAR domain (BD-SDR3.1-N), the M-terminus containing the NINJA_B domain (BD-SDR3.1-M), and the C-terminus containing the Jas domain (BD-SDR3.1-C; Supplementary Fig. 8a). Deletion of the N- and M-terminus residues did not affect the interaction of SDR3.1 with ABI5; however, deletion of the C-terminus fragment of SDR3.1 abolished its interaction with ABI5 (Supplementary Fig. 8b). Therefore, the C-terminus of SDR3.1 was necessary for the SDR3.1-ABI5 interaction. Similarly, we divided ABI5 into two segments; an N-terminus domain (AD-ABI5-N) and a C-terminus bZIP domain (AD-ABI5-C; Supplementary Fig. 8c). Deletion of the C-terminus residue of ABI5 did not affect its interaction with SDR3.1, whereas deletion of the N-terminus fragment abolished the interaction of ABI5 with SDR3.1 (Supplementary Fig. 8d). Therefore, the N-terminus of ABI5 was essential for the SDR3.1-ABI5 interaction. To clarify the function of ABI5, we disrupted it (LOC_Os01g64000) in ZH11 via CRISPR/Cas9, and two homozygous mutants, abi5-1 and abi5-2, were obtained through sequencing. These mutants contained a 1-bp frame-shift insertion or deletion in the first exon (Supplementary Fig. 9a), and the mutants had lower ABI5 expression levels than that of ZH11 (Supplementary Fig. 9b). Moreover, we overexpressed ABI5 in ZH11 (Supplementary Fig. 9c). The results indicated that the mutant lines (97.92% and 98.33%) had higher germination rate than that of ZH11 (88.75%). In contrast, the germination rate of the overexpressed lines (36.67% and 46.67%) were significantly lower than that of ZH11 (88.75%) (Supplementary Fig. 9d-e). These results indicated that ABI5 promoted seed dormancy. Because SDR3.1 inhibits seed dormancy and ABI5 promotes seed dormancy, we investigated whether the SDR3.1 protein inhibits the transcriptional activation of ABI5. Therefore, identifying ABI5 target genes was necessary. In Arabidopsis, the EMBRYONIC ABUNDANT PROTEIN1 (EM1) is a target of ABI541, but EM1 has not been reported as a target gene of ABI5 in rice. Therefore, we used a yeast one-hybrid (Y1H) assay to confirm that ABI5 could bind to the promoter of EM1 in rice (Supplementary Fig. 10a), and we narrowed the binding region within 200 bp (Supplementary Fig. 10b). In addition, we used an electrophoretic mobility shift assay (EMSA) to confirm the interaction between ABI5 and the promoter of EM1 (Supplementary Fig. 10c). To identify additional downstream target genes of ABI5, we used Cleavage Under Targets and Tagmentation (cut&Tag) technology combined with Y1H and EMSA to verify that ABI5 could bind to the promoter of LEA3 (Supplementary Fig. 10d-f). Furthermore, we disrupted EM1 (LOC_Os05g28210) and LEA3 (LOC_Os05g46480) in ZH11 via CRISPR/Cas9, and four homozygous mutants, namely, em1-1, em1-2, lea3-1 and lea3-2 were obtained through sequencing. These mutants contained a 32-bp or 28-bp frame-shift deletion in the first exon of EM1 and an 89-bp frame-shift deletion or 1-bp frame-shift insertion in the second exon of LEA3 (Supplementary Fig. 11a-b). The em1/lea3 plants had lower gene expression levels than that of ZH11 (Supplementary Fig. 11c). Moreover, we overexpressed EM1 and LEA3 in ZH11, respectively (Supplementary Fig. 11c). The mutant lines had higher germination rate than that of ZH11. In contrast, the germination rate of the overexpressed lines were significantly lower than that of ZH11 (Supplementary Fig. 11d-e). These results indicated that EM1 and LEA3 are important downstream genes involved in the seed dormancy regulation pathway. Since EM1-ABI5 and LEA3-ABI5 interact in rice, we fused the LUC gene to the EM1 and LEA3 promoters (Fig. 2e). In trans, we expressed SDR3.1, ABI5, and GFP with the CaMV 35 S promoter (Fig. 2e). The EM1 and LEA3 reporters were significantly activated by the ABI5 effector, when compared with the GFP effector (Fig. 2f-g). However, the coexpression of SDR3.1 with ABI5 disrupted the ABI5-activated LUC expression (Fig. 2f-g). These results indicated that SDR3.1 repressed the transcriptional activity of ABI5. To verify the relationship between SDR3.1 and ABI5, we constructed a double mutant in ZH11 (Supplementary Fig. 12a-b). The germination rate of the double mutants had no significantly difference compare with that of ZH11 (Supplementary Fig. 12c-d). These results further verified that SDR3.1 repressed the transcriptional activity of ABI5. Because ABI5 is the central factor corresponding to ABA signaling, we examined the expression levels of ABA response genes in ZH11, MJLXL and transgenic plants. In addition to OsEM1 and OsLEA3, we also detected the expression levels of three genes that are related to ABA regulation (OsbZIP72, OsRab16A and OsTRAB1). The results showed that the expressions were suppressed significantly by SDR3.1 (Fig. 3a-e). However, the expression levels of OsbZIP72, OsRab16A and OsTRAB1 were not activated by ABI5 (Fig. 3c-e). Therefore, SDR3.1 might regulate the response to ABA signals through more than just ABI5. Based on these findings, we tested whether SDR3.1 inhibits another seed dormancy-related transcription factor, OsABI3. The results showed that SDR3.1 inhibited the transcriptional activity of OsABI3 (Supplementary Fig. 13a-b). a–e The relative expression levels of ABA-responsive genes between ZH11 and MJLXL, among the NIL and three COM lines, among ZH11 and two SDR3.1 knockout lines, among ZH11, two ABI5 knockout lines and two OE lines. f ABA contents between ZH11 and MJLXL, among the NIL and three COM lines; among ZH11 and two SDR3.1 knockout lines; and among ZH11, two ABI5 knockout lines and two OE lines. g Germination performance of two SDR3.1 knockout lines and ZH11 seeds treated with 0, 5, or 10 μM ABA at 4 days after imbibition (DAI). Bar = 1 cm. h Germination rate of two SDR3.1 knockout lines and ZH11 seeds treated with 0, 5, or 10 μM ABA at 4 DAI. i Germination rate of two SDR3.1 knockout lines and ZH11 seeds treated with 0, 5, or 10 μM ABA at 6 DAI. In a–f, h, i n = 3 independent experiments. Data are presented as the mean values ± SD, and P values are indicated by two-tailed Student’s t test. Source data are provided as a Source Data file. In addition, we examined the ABA and GA contents in seeds, and the results showed that SDR3.1 regulated seed dormancy not by regulating ABA and GA contents (Fig. 3f; Supplementary Fig. 13c-f). Then, we examined the sensitivity of ZH11 and the two mutant lines to ABA after breaking seed dormancy. As expected, under normal conditions, there was no significant differences in germination rate between ZH11 and the two mutant lines (Fig. 3g-i). However, after 5 μM or 10 μM ABA treatment, the germination rate of the two mutant lines were significantly lower than that of ZH11 (Fig. 3g-i). Therefore, the two mutant lines were more sensitive to ABA. The above results indicated that SDR3.1 negatively regulated ABA signaling by repressing ABIs. The SDR3.1 encoded protein contains 401 amino acids. Homologous proteins were detected by BLAST searches of the UniProt database (https://www.uniprot.org/). Species with more than 50% similarity to SDR3.1 protein sequences were selected for multiple sequence alignment, and a phylogenetic tree was constructed using the neighborhood connection method. Homologs of SDR3.1 were identified only in monocotyledons, including Setaria viridis, Setaria italica, Panicum miliaceum, Dichanthelium oligosanthes, Sorghum bicolor, Zea mays, and Eragrostis curvula. The highest identities observed of SDR3.1 protein sequences were from the wild rice species Oryza glumipatula (99.8%), Oryza barthii (99.5%), Oryza meridionalis (96.8%) and Oryza punctata (93.5%) (Supplementary Fig. 14). These results implied that SDR3.1 performs an irreplaceable function in monocotyledons. Given that seed dormancy is an ancient trait of wild rice13 and that most modern rice cultivars exhibit shorter seed dormancy. We analyzed the polymorphisms of SDR3.1 in the 3 K RG dataset (www.rmbreeding.cn/index.php). Using five single-nucleotide polymorphisms (SNPs) in the coding sequence, we detected five major haplotypes (Hap) of SDR3.1 in 2584 accessions in the 3 K RG dataset (only haplotypes with more than 100 accessions were selected) (Fig. 4b). These were divided mainly into **an/indica (58.6%), Geng/japonica (29.8%), and Aus (6.9%) cultivars. Further analysis revealed that **an/indica was mainly Hap1 (52.5%) and Hap2 (44.4%), Geng/japonica was mainly Hap3 (51.5%) and Hap4 (48.5%), and Aus was mainly Hap5 (69.1%), Hap2 (18.0%) and Hap1 (12.4%) (Supplementary Fig. 15). Further, we analyzed 2356 cultivars that the exact geographic information was available. Each haplotype was distributed in the region where rice was grown (Fig. 4a), and the Shannon evenness (EH) value of SDR3.1 was 0.19242, indicating that the genetic diversity of SDR3.1 was relatively low among cultivars. a Geographic distribution of the five haplotypes among 2356 accessions of 3 K RGs. N: North latitude; S: South latitude; W: West longitude; E: East longitude. b Haplotypes of SDR3.1 in 2584 accessions of 3 K RG according to five SNPs in the coding sequences region. c The 7-day germination rate of the five haplotypes (291 rice accessions) 35 DAH. The short horizontal lines represent the number of cultivars. d Rice subspecies distribution of each haplotypes. e The 7-day germination rate of the three combinations (291 rice accessions) 35 DAH. The short horizontal lines represent the number of cultivars. f Rice subspecies distribution of each amino acid sequences. g The differences of germination rate between the **an/indica and Geng/japonica in the combination of Ser102 + Asp156. The short horizontal lines represent the number of cultivars. In c, e Data are presented as the mean ± SD, and P values are indicated by two-tailed Student’s t test. Source data are provided as a Source Data file. We collected 291 rice accessions to determine the association between genotypes and seed dormancy phenotypes. The average germination rate of Hap3 (67.68%) and Hap4 (59.73%) were the highest, followed by those of Hap1 (31.87%) and Hap2 (24.28%), and the average germination rate of Hap5 (4.95%) was the lowest (Fig. 4c; Supplementary Figs. 16-20). Hap1 was mainly **an/indica and Aus, accounting for 63.77% and 24.64%, respectively. Hap2 was mainly **an/indica and Aus, accounting for 45.78% and 44.58%, respectively. Hap3 and Hap4 were dominated by Geng/japonica, accounting for 77.56% and 97.22%, respectively, while 98.28% of the Hap5 was Aus (Fig. 4d). These results suggested that the function of SDR3.1 differs between the **an/indica and Geng/japonica cultivars. Due to the similar germination rate between Hap1 and Hap2, as well as between Hap3 and Hap4, we speculated that different SNPs may have caused synonymous mutations. We subsequently analyzed the differences in amino acids at the five different SNP sites. We found only two of five SNPs (SNP-3399 and -305 in Fig. 4b) changed the amino acids; the other three SNPs (SNP-3591, -348 and -75 in Fig. 4b) did not change the amino acids. Therefore, the amino acid sequences of SDR3.1 of Hap1 and Hap2 were the same (Ser102+Asp156), the amino acid sequences of SDR3.1 of Hap3 and Hap4 were also the same (Asn102+Glu156), and Hap5 was Ser102+Glu156. Among the combinations tested, Asn102+Glu156 was associated with the highest germination rate (64.13%), followed by Ser102+Asp156 (27.72%), and Ser102+Glu156 was associated with the lowest germination rate (4.95%) (Fig. 4e). The combination of Asn102+Glu156 was mostly appear in the Geng/japonica cultivar (85.19%), the combination of Ser102+Asp156 was mostly appear in the **an/indica cultivar (53.95%), and the combination of Ser102+Glu156 was mostly appear in the Aus cultivar (98.28%) (Fig. 4f). These results implied that Aus had longest seed dormancy, followed by **an/indica, while Geng/japonica had the shortest seed dormancy. The genetic effects of different alleles are strongly affected by the population structure as Asian cultivated rice exhibit dramatic differences in **an/indica and Geng/japonica rice cultivars. Therefore, we compared seed dormancy between the **an/indica and Geng/japonica cultivars in both Hap1 and Hap2 with Ser102+Asp156, and the results showed that the germination rate of Geng/japonica was significantly higher than that of **an/indica (Fig. 4g), indicating that the genetic effect of dormancy was influenced by population structure. To investigate whether the effect of SDR3.1 on seed dormancy was related to its expression levels, we measured its expression levels in some rice cultivars from each haplotype. There was no significant correlation between the germination rate and the expression levels of each haplotype (Supplementary Fig. 21a-e). There were also no significant differences in expression levels among the five haplotypes, except between haplotype 4 and haplotype 5 or between haplotype 1 and haplotype 4 (Supplementary Fig. 21f). These results indicated that the regulation of seed dormancy by SDR3.1 depended on its protein activity more than its expression levels. To clarify the evolution of SDR3.1 in rice, we analyzed the amino acid sequences of the 4 wild rice accessions (Supplementary Fig. 14). The amino acid sequences at positions 102 and 156 were Ser and Glu, respectively, which was consistent with the sequence of Hap5 (Fig. 5a). We subsequently sequenced 28 wild rice accessions and found that most of the amino acid sequences at positions 102 and 156 were Ser and Glu (Fig. 5b). The absolute Tajima values for ** was conducted in a B3C2 population with 153 plants derived from the cross between ZH11 and MJLXL. Approximately 4146 plants of the BC4F2 population were subjected to recombinant screening with markers P3 and P4. qSDR3.1 was ultimately narrowed to a 26.9 kb interval between markers Indel27 and Indel29. For the complementation experiments, the entire DNA sequence of SDR3.1 from ZH11 was inserted into the pCAMBIA1300 vector and subsequently transformed into NIL. The CRISPR/Cas9 vector BGK03 Biogle (Hangzhou, China) was used for genome editing. The primers used in this assay are listed in Supplementary Data 2. Total RNA was extracted with TRIzol (TransGen). RNA reverse transcription was performed using ReverTra Ace (TOYOBO Biotech). qRT‒PCR was performed using Applied Biosystems QuantStudio 3 Real-Time PCR System (Thermo Fisher Scientific, USA). The primers used in this assay are listed in Supplementary Data 2. The copy number of the COMs were detected via qRT‒PCR. The pUC plasmid containing the SDR3.1 gene fragment was used as a standard to generate a standard curve. The primers used in this assay are listed in Supplementary Data 2. A triple quadrupole mass spectrometer coupled with an electrospray ionization and high-performance liquid chromatography (HPLC‒ESI‒MS/MS) system (Shimadzu LC system, pump model: LC-10ADvp; oven model: CTO-10Avp; system controller model: SCL-10Avp; ABI 4000; Applied Biosystems)71 was used to quantify phytohormones at Nan**g Ruiyuan Biotechnology Co., Ltd. (Nan**g, China). The coding sequences of SDR3.1 without a stop codon were amplified from the cDNA of ZH11, subsequently inserted into pYBA1132. The recombinant 35 S::SDR3.1-GFP vector and the control construct were subsequently transformed into rice protoplasts through polyethylene glycol (PEG4000)-mediated transformation72. In brief, rice stem tissues from 11-day-old plants were collected and used as source materials for protoplast extraction; the materials were isolated with an enzymatic digestion solution (0.6 M mannitol, 1.6% cellulose R-10, 0.75% Macerozyme R-10, 0.1% BSA, 10 mM 2-(Nmorpholino)ethanesulfonic acid, 1 mM CaCl2, and 0.4% b-mercaptoethanol). The protoplasts were washed with W5 solution (154 mM NaCl, 125 mM CaCl2·2H2O, 5 mM KCl, 5 mM glucose, 2 mM MES). Plasmid DNA was mixed with the rice protoplasts, and an equal volume of 40% PEG solution (40% [w/v] PEG 4000, 0.1 M CaCl2·2H2O, 0.2 M mannitol) was added and mixed gently. After 20 hours of cultivation under dark conditions at 28 °C, fluorescence was detected with a Zeiss LSM 710 NLO confocal microscope. The coding sequences of SDR3.1 were inserted into pGBKT7 (Clontech, Dalian, China) as a ‘bait’ vector, and the coding sequences of ABI5 were inserted into pGADT7 (Clontech) as a ‘prey’ vector. The two plasmid vectors were cotransformed into the yeast strain Y2HGold. Transformants were spotted on SD-Leu-Trp media. After 28 °C cultivation for 3 days, five colonies were randomly selected, spotted on SD-Leu-Trp-His-Ade media and incubated for 3 days. The primers used for cloning are listed in Supplementary Data 2. The coding sequences of SDR3.1 were inserted into the pGEX-4T-1 vector to create a fusion protein with GST. The coding sequences of ABI5 were inserted into pET28a to generate a His fusion protein. Both the empty pGEX-4T-1 vector and the corresponding vector were introduced into the E. coli strain Rosetta. The GST fusion protein was induced with 0.1 mmol/L isopropyl β-D-1-thiogalactopyranoside (IPTG) at 24 °C for 12 h. The His fusion protein was induced with 1.0 mmol/L IPTG at 16 °C for 12 h. For pull-down assays, 2 μg of His::ABI5 prey protein was incubated with 2 μg of immobilized GST or GST::SDR3.1 bait proteins. The mixtures were incubated on a shaker for 5 h (all operations were performed at 4 °C), after which the proteins were separated via 8% SDS–PAGE. Then the pulled down proteins were immunoblotted with anti-GST or anti-His antibodies. The mouse monoclonal anti-GST, mouse monoclonal anti-His and HRP-goat anti-mouse antibodies used for this study were obtained from Beyotime at a dilution of 1:10,000 (Beyotime: AF0174, AF2870 and A0192). The primers used in this assay are listed in Supplementary Data 2. The coding sequences of SDR3.1 were connected to the vector pSPYCE, and the coding sequences of ABI5 was connected to the vector pSPYNE. The plasmids were subsequently introduced into rice protoplasts72. Fluorescence was detected with a Zeiss LSM 710 NLO confocal microscope. The primers used in this assay are listed in Supplementary Data 2. Total plant protein was extracted from 7-day-old embryos. Extraction buffer (25 mM Tris-HCl, pH=7.4; 150 mM NaCl; 1 mM EDTA; 1% Nonidet P-40; 5% Glycetol; 0.144 g/L protease inhibitor; Roche: 5892791001) (750 µL) was added. The material was dispersed, mixed well, and then centrifuged at 13,000 × g for 20 min; the supernatant was collected. Denatured proteins were separated via SDS‒PAGE and transferred to PVDF membranes for immunoblotting with anti-SDR3.1. The rabbit monoclonal anti-SDR3.1 antibody used for this study was obtained from AtaGenix at a dilution of 1:10,000. The β-actin protein was used as a control. The mouse monoclonal anti-actin, HRP-goat anti-mouse and HRP-goat anti-rabbit antibodies used for this study were obtained from Beyotime and used at a dilution of 1:10,000 (Beyotime: AF0003, A0192 and A0208). The coding sequences of ABI5 were inserted into pB42AD (Clontech), and the promoter of EM1 was inserted into pLacz (Clontech). The vectors were subsequently cotransformed into the yeast strain EGY48. Transformants were spotted on SD-Ura-Trp media. After 28 °C cultivation for 3 days, colonies were randomly selected and spotted on SD-Ura-Trp media supplemented with 2% (w/v) galactose, 1% (w/v) raffinose, 1 × salt buffer (7 g/L Na2HPO4·PH2O, 3 g/L Na2HPO4, pH 7.0), and 80 mg/L 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside acid (Clontech). The plates were incubated for 1 day. The primers used in this assay are listed in Supplementary Data 2. An electromobility shift assay was performed with a Chemiluminescent EMSA Kit (Beyotime, Shanghai). 2 μg of His::ABI5 protein and 1 μL of biotin-labeled probe (1 ng) were mixed at 25 °C for 20 min. The unlabeled probe (1 ng) was added as a competitor. The sequences of these probes are listed in Supplementary Data 2. The promoter region of EM1 was inserted into the pGreenII 0800-LUC vector as a reporter plasmid. ABI5, SDR3.1, and GFP were independently inserted into the pGreenII 62-SK vector as effector plasmids. The combined plasmids were introduced into rice protoplasts. The transformed cells were incubated for 24 hours at 28 °C with or without 5 μM ABA, and the relative LUC activity was measured with a dual-luciferase reporter assay instrument (Promega). The primers used for cloning are listed in Supplementary Data 2. We used the UniProt database (https://www.uniprot.org/) to obtain proteins homologous to SDR3.1. Species with protein homology greater than 50% were selected for the construction of a phylogenetic tree and comparison with DNAMAN software (Lynnon Biosoft, USA). Haplotype analysis was performed on the 3 K website to determine the haplotype classification of SDR3.1, and the coding sequences were chosen as the gene region for analysis. Filtering was performed based on MAF (minor allele frequency) being greater than or equal to 0.01. Cygwin and vcftools (v0.1.16) were used to calculate nucleotide diversity (π) and Tajima’s D for the 2-Mb region flanking SDR3.1 (each 50-kb across the genome as a window and with an overlap** 5-kb step size). A world map was downloaded from Origin 2022 (https://www.originlab.com). We harvested the cells and then centrifuged them for 3 min at 600 × g and 25 °C. Subsequently, the cell nucleus was extracted via CelLytic TM PN Isolation/Extraction Kit (Weibo: 3424255; refer to the manual for the specific steps). Then, the samples were incubated with primary antibodies (mouse anti-GFP-Tag mAb, ABclonal, AE012; dilution 1:100) overnight at 4 °C on a rotating platform. After removing the primary antibody, the cells were incubated at 25 °C for 30 minutes in secondary antibody (HRP-conjugated mouse anti-GFP-Tag mAb, ABclonal, AE030; dilution 1:100) diluted in DigWash buffer. After incubating in pA-Tn5 adapter complex diluent for 1 hour, the nucleus was resuspended in labeled buffer and incubated at 37 °C for 1 hour. The labeling reaction was stopped, and the products were amplified and sequenced with an Illumina NovaSeq 6000, after which the raw data were obtained. The raw data were evaluated using FastQC software (v0.11.5). Then, Trimmomatic software (v0.39) was used for data filtering, and the adapter sequence at the end was truncated. Next, bwa software (v0.7.17-r1188) was used to perform unique comparisons, screening and duplication processing on the obtained reads. Finally, MACS2 software (v2.2.7.1) was used to find the protein binding sites (peaks). IBM SPSS Statistics software (v25) was used for Student’s t test. Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
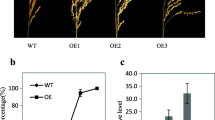
SDR3.1 negatively regulates seed dormancy
Expression and subcellular localization of SDR3.1
SDR3.1 physically interacts with ABI5 and represses its transcriptional activation
SDR3.1 negatively regulates ABA signaling
Evolutionary analysis of SDR3.1
Natural variations of SDR3.1 associated with differences in seed dormancy
Conversion of longer dormancy in wild rice to shorter dormancy in **an/indica and Geng/japonica requires SDR3.1 aa102 and aa156
Expression analysis
Copy number analysis
ABA and GA concentration measurements
Subcellular localization
Y2H assay
Pull-down assay
Bimolecular fluorescence complementation assay
Protein extraction and immunoblotting
Y1H assay
Electromobility shift assay
Transient transactivation assay
Evolutionary analysis and population genetic analysis of SDR3.1
Cut&Tag sequencing and analysis
Statistical analysis
Reporting summary
Data availability
The sequence data from this study can be found at Rice Genome Annotation Project website (http://rice.uga.edu/) under the following accession numbers: SDR3.1/MODD (LOC_Os03g11550 [http://rice.uga.edu/cgi-bin/ORF_infopage.cgi?orf=LOC_Os03g11550]), OsABI5 (LOC_Os01g64000 [http://rice.uga.edu/cgi-bin/ORF_infopage.cgi?orf=LOC_Os01g64000]), OsABI3 (LOC_Os01g68370 [http://rice.uga.edu/cgi-bin/ORF_infopage.cgi?orf=LOC_Os01g68370]), OsEM1 (LOC_Os05g28210 [http://rice.uga.edu/cgi-bin/ORF_infopage.cgi?orf=LOC_Os05g28210]), OsLEA3 (LOC_Os05g46480 [http://rice.uga.edu/cgi-bin/ORF_infopage.cgi?orf=LOC_Os05g46480]), OsTRAB1 (LOC_Os08g36790 [http://rice.uga.edu/cgi-bin/ORF_infopage.cgi?orf=LOC_Os08g36790]), OsRab16A (LOC_Os11g26790 [http://rice.uga.edu/cgi-bin/ORF_infopage.cgi?orf=LOC_Os11g26790]), and OsbZIP72 (LOC_Os09g28310 [http://rice.uga.edu/cgi-bin/ORF_infopage.cgi?orf=LOC_Os09g28310]). Source data are provided with this paper.
References
Diamond, J. Evolution, consequences and future of plant and animal domestication. Nature 418, 700–707 (2002).
Doebley, J. F., Gaut, B. S. & Smith, B. D. The molecular genetics of crop domestication. Cell 127, 1309–1321 (2006).
Purugganan, M. D. & Fuller, D. Q. The nature of selection during plant domestication. Nature 457, 843–848 (2009).
Gross, B. L. & Olsen, K. M. Genetic perspectives on crop domestication. Trends Plant Sci. 15, 529–537 (2010).
Olsen, K. M. & Wendel, J. F. A bountiful harvest: genomic insights into crop domestication phenotypes. Annu. Rev. Plant Biol. 64, 47–70 (2013).
Olsen, K. M. et al. Selection under domestication: evidence for a sweep in the rice waxy genomic region. Genetics 173, 975–983 (2006).
Ashikari, M. et al. Cytokinin oxidase regulates rice grain production. Science 309, 741–745 (2005).
Luo, J. et al. An-1 encodes a basic helix-loop-helix protein that regulates awn development, grain size, and grain number in rice. Plant Cell 25, 3360–3376 (2013).
Gu, B. et al. An-2 Encodes a Cytokinin Synthesis Enzyme that Regulates Awn Length and Grain Production in Rice. Mol. Plant 8, 1635–1650 (2015).
Hua, L. et al. LABA1, a Domestication Gene Associated with Long, Barbed Awns in Wild Rice. Plant Cell 27, 1875–1888 (2015).
Li, C., Zhou, A. & Sang, T. Rice domestication by reducing shattering. Science 311, 1936–1939 (2006).
Zhang, L. B. et al. Selection on grain shattering genes and rates of rice domestication. New Phytol. 184, 708–720 (2009).
Ishikawa, R. et al. A stepwise route to domesticate rice by controlling seed shattering and panicle shape. Proc. Natl. Acad. Sci. USA 119, e2121692119 (2022).
**, J. et al. Genetic control of rice plant architecture under domestication. Nat. Genet. 40, 1365–1369 (2008).
Tan, L. et al. Control of a key transition from prostrate to erect growth in rice domestication. Nat. Genet. 40, 1360–1364 (2008).
Wang, Y. & Li, J. Rice, rising. Nat. Genet. 40, 1273–1275 (2008).
Asano, K. et al. Artificial selection for a green revolution gene during japonica rice domestication. Proc. Natl. Acad. Sci. USA 108, 11034–11039 (2011).
Shomura, A. et al. Deletion in a gene associated with grain size increased yields during rice domestication. Nat. Genet. 40, 1023–1028 (2008).
Xue, W. et al. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat. Genet. 40, 761–767 (2008).
Ishii, T. et al. OsLG1 regulates a closed panicle trait in domesticated rice. Nat. Genet. 45, 462–465 (2013). 465e461-462.
Sugimoto, K. et al. Molecular cloning of Sdr4, a regulator involved in seed dormancy and domestication of rice. Proc. Natl. Acad. Sci. USA 107, 5792–5797 (2010).
Wan, J. M. et al. Genetic dissection of the seed dormancy trait in cultivated rice (Oryza sativa L.). Plant Sci. 170, 786–792 (2006).
Zhao, B. et al. Sdr4 dominates pre-harvest sprouting and facilitates adaptation to local climatic condition in Asian cultivated rice. J. Integr. Plant Biol. 64, 1246–1263 (2022).
Takeuchi, Y., Lin, S. Y., Sasaki, T. & Yano, M. Fine linkage map** enables dissection of closely linked quantitative trait loci for seed dormancy and heading in rice. Theor. Appl. Genet. 107, 1174–1180 (2003).
Gu, X. Y., Liu, T., Feng, J., Suttle, J. C. & Gibbons, J. The qSD12 underlying gene promotes abscisic acid accumulation in early develo** seeds to induce primary dormancy in rice. Plant Mol. Biol. 73, 97–104 (2010).
Hori, K. et al. Detection of quantitative trait loci controlling pre-harvest sprouting resistance by using backcrossed populations of japonica rice cultivars. Theor. Appl. Genet. 120, 1547–1557 (2010).
Lu, B. et al. Map** two major effect grain dormancy QTL in rice. Mol. Breed. 28, 453–462 (2011).
Zhang, C. et al. Genetic Dissection of Seed Dormancy in Rice (Oryza sativa L.) by Using Two Map** Populations Derived from Common Parents. Rice (N Y) 13, 52 (2020).
Gu, X. Y. et al. Association between seed dormancy and pericarp color is controlled by a pleiotropic gene that regulates abscisic acid and flavonoid synthesis in weedy red rice. Genetics 189, 1515–1524 (2011).
Ye, H. et al. Map-Based Cloning of Seed Dormancy1-2 Identified a Gibberellin Synthesis Gene Regulating the Development of Endosperm-Imposed Dormancy in Rice. Plant Physiol. 169, 2152–2165 (2015).
Xu, F. et al. Antagonistic control of seed dormancy in rice by two bHLH transcription factors. Nat. Genet. 54, 1972–1982 (2022).
Shu, K., Liu, X. D., **e, Q. & He, Z. H. Two Faces of One Seed: Hormonal Regulation of Dormancy and Germination. Mol. Plant 9, 34–45 (2016).
Wang, J. et al. Transcription Factors Rc and OsVP1 Coordinately Regulate Preharvest Sprouting Tolerance in Red Pericarp Rice. J. Agric. Food Chem. 68, 14748–14757 (2020).
Zou, M., Guan, Y., Ren, H., Zhang, F. & Chen, F. A bZIP transcription factor, OsABI5, is involved in rice fertility and stress tolerance. Plant Mol. Biol. 66, 675–683 (2008).
Du, L. et al. Endosperm sugar accumulation caused by mutation of PHS8/ISA1 leads to pre-harvest sprouting in rice. Plant J. 95, 545–556 (2018).
Chen, W., Wang, W., Lyu, Y., Wu, Y. & Luo, J. OsVP1 activates Sdr4 expression to control rice seed dormancy via the ABA signaling pathway. Crop J. 9, 68–78 (2020).
Yu, J., Lai, Y., Wu, X., Wu, G. & Guo, C. Overexpression of OsEm1 encoding a group I LEA protein confers enhanced drought tolerance in rice. Biochem. Biophys. Res. Commun. 478, 703–709 (2016).
**ao, B., Huang, Y., Tang, N. & **ong, L. Over-expression of a LEA gene in rice improves drought resistance under the field conditions. Theor. Appl. Genet. 115, 35–46 (2007).
Tang, N. et al. MODD Mediates Deactivation and Degradation of OsbZIP46 to Negatively Regulate ABA Signaling and Drought Resistance in Rice. Plant Cell 28, 2161–2177 (2016).
Miao, C. et al. The grain yield modulator miR156 regulates seed dormancy through the gibberellin pathway in rice. Nat. Commun. 10, 3822 (2019).
Yang, M., Han, X., Yang, J., Jiang, Y. & Hu, Y. The Arabidopsis circadian clock protein PRR5 interacts with and stimulates ABI5 to modulate abscisic acid signaling during seed germination. Plant Cell 33, 3022–3041 (2021).
Zhang, F. et al. The landscape of gene-CDS-haplotype diversity in rice: Properties, population organization, footprints of domestication and breeding, and implications for genetic improvement. Mol. Plant 14, 787–804 (2021).
Zhu, B. F. et al. Genetic control of a transition from black to straw-white seed hull in rice domestication. Plant Physiol. 155, 1301–1311 (2011).
Yoshida, H. et al. Genome-wide association study identifies a gene responsible for temperature-dependent rice germination. Nat. Commun. 13, 5665 (2022).
Sun, J. et al. Regain flood adaptation in rice through a 14-3-3 protein OsGF14h. Nat. Commun. 13, 5664 (2022).
Wang, M. et al. Parallel selection on a dormancy gene during domestication of crops from multiple families. Nat. Genet. 50, 1435–1441 (2018).
Wang, Y. et al. Copy number variation at the GL7 locus contributes to grain size diversity in rice. Nat. Genet. 47, 944–948 (2015).
Finch-Savage, W. E. & Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 171, 501–523 (2006).
Chen, Y. et al. ABA biosynthesis gene OsNCED3 contributes to pre-harvest sprouting resistance and grain development in rice. Plant Cell Environ. https://doi.org/10.1111/pce.14480. (2022).
Collin, A., Daszkowska-Golec, A. & Szarejko, I. Updates on the Role of ABSCISIC ACID INSENSITIVE 5 (ABI5) and ABSCISIC ACID-RESPONSIVE ELEMENT BINDING FACTORs (ABFs) in ABA Signaling in Different Developmental Stages in Plants. Cells 10, https://doi.org/10.3390/cells10081996. (2021).
Piskurewicz, U. et al. The gibberellic acid signaling repressor RGL2 inhibits Arabidopsis seed germination by stimulating abscisic acid synthesis and ABI5 activity. Plant Cell 20, 2729–2745 (2008).
Guan, C. et al. Cytokinin antagonizes abscisic acid-mediated inhibition of cotyledon greening by promoting the degradation of abscisic acid insensitive5 protein in Arabidopsis. Plant Physiol. 164, 1515–1526 (2014).
Zhao, X., Dou, L., Gong, Z., Wang, X. & Mao, T. BES1 hinders ABSCISIC ACID INSENSITIVE5 and promotes seed germination in Arabidopsis. New Phytol. 221, 908–918 (2019).
Ju, L. et al. JAZ proteins modulate seed germination through interaction with ABI5 in bread wheat and Arabidopsis. New Phytol. 223, 246–260 (2019).
Pan, J. et al. Molecular Mechanism Underlying the Synergetic Effect of Jasmonate on Abscisic Acid Signaling during Seed Germination in Arabidopsis. Plant Cell 32, 3846–3865 (2020).
Hu, Y. & Yu, D. BRASSINOSTEROID INSENSITIVE2 interacts with ABSCISIC ACID INSENSITIVE5 to mediate the antagonism of brassinosteroids to abscisic acid during seed germination in Arabidopsis. Plant Cell 26, 4394–4408 (2014).
Bi, C. et al. Arabidopsis ABI5 plays a role in regulating ROS homeostasis by activating CATALASE 1 transcription in seed germination. Plant Mol. Biol. 94, 197–213 (2017).
Zhang, Q. et al. Overexpression of a wheat α-amylase type 2 impact on starch metabolism and abscisic acid sensitivity during grain germination. Plant J. 108, 378–393 (2021).
Zong, Y. et al. Precise base editing in rice, wheat and maize with a Cas9-cytidine deaminase fusion. Nat. Biotechnol. 35, 438–440 (2017).
Lv, Y. et al. Targeted mutagenesis of POLYAMINE OXIDASE 5 that negatively regulates mesocotyl elongation enables the generation of direct-seeding rice with improved grain yield. Mol. Plant 14, 344–351 (2021).
Huang, X. et al. A map of rice genome variation reveals the origin of cultivated rice. Nature 490, 497–501 (2012).
Londo, J. P., Chiang, Y. C., Hung, K. H., Chiang, T. Y. & Schaal, B. A. Phylogeography of Asian wild rice, Oryza rufipogon, reveals multiple independent domestications of cultivated rice, Oryza sativa. Proc. Natl. Acad. Sci. USA 103, 9578–9583 (2006).
Gross, B. L. & Zhao, Z. Archaeological and genetic insights into the origins of domesticated rice. Proc. Natl. Acad. Sci. USA 111, 6190–6197 (2014).
Khoury, C. K. et al. Increasing homogeneity in global food supplies and the implications for food security. Proc. Natl. Acad. Sci. USA 111, 4001–4006 (2014).
Østerberg, J. T. et al. Accelerating the Domestication of New Crops: Feasibility and Approaches. Trends Plant Sci. 22, 373–384 (2017).
Yu, H. et al. A route to de novo domestication of wild allotetraploid rice. Cell 184, 1156–1170.e1114 (2021).
Yu, H. & Li, J. Breeding future crops to feed the world through de novo domestication. Nat. Commun. 13, 1171 (2022).
Massawe, F., Mayes, S. & Cheng, A. Crop Diversity: An Unexploited Treasure Trove for Food Security. Trends Plant Sci. 21, 365–368 (2016).
Renard, D. & Tilman, D. National food production stabilized by crop diversity. Nature 571, 257–260 (2019).
He, Y. et al. A quantitative trait locus, qSE3, promotes seed germination and seedling establishment under salinity stress in rice. Plant J. 97, 1089–1104 (2019).
Pan, X., Welti, R. & Wang, X. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat. Protoc. 5, 986–992 (2010).
Hu, Z. et al. A Novel QTL qTGW3 Encodes the GSK3/SHAGGY-Like Kinase OsGSK5/OsSK41 that Interacts with OsARF4 to Negatively Regulate Grain Size and Weight in Rice. Mol. Plant 11, 736–749 (2018).
Acknowledgements
We thank Ling Jiang (Nan**g Agricultural University), Zhigang Zhao (Nan**g Agricultural University) and Jiuyou Tang (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences) for their valuable suggestions and revisions to the manuscript. This research was financially supported by the National Natural Science Foundation of China (No.32188102, 31871597, 32071991), the Zhejiang Science and Technology Major Program on Agricultural New Variety Breeding (2021C02063-2), the Key Research and Development Program of the China National Rice Research Institute (CNRRI-2020-02), Zhejiang Provincial Natural Science Foundation of China (LDQ23C130001), Zhejiang Provincial Science and Technology Project (2020R51007), the Key Research and Development Program of Zhejiang Province (2022C02011).
Author information
Authors and Affiliations
Contributions
H.P. and S.Z. designed the research; G.N., T.S., W.Y., C.W., A.R., R.Z., H.S., T.S., W.X., S.G., J.G., X.L., W.L., C.Y. and Z.F. performed the experiments; S.Z. and G.N. analyzed the results and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Sibin Yu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, N., Tang, S., Wang, Y. et al. A mediator of OsbZIP46 deactivation and degradation negatively regulates seed dormancy in rice. Nat Commun 15, 1134 (2024). https://doi.org/10.1038/s41467-024-45402-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-45402-z
- Springer Nature Limited