Abstract
De novo mutations and copy number deletions in NRXN1 (2p16.3) pose a significant risk for schizophrenia (SCZ). It is unclear how NRXN1 deletions impact cortical development in a cell type-specific manner and disease background modulates these phenotypes. Here, we leveraged human pluripotent stem cell-derived forebrain organoid models carrying NRXN1 heterozygous deletions in isogenic and SCZ patient genetic backgrounds and conducted single-cell transcriptomic analysis over the course of brain organoid development from 3 weeks to 3.5 months. Intriguingly, while both deletions similarly impacted molecular pathways associated with ubiquitin-proteasome system, alternative splicing, and synaptic signaling in maturing glutamatergic and GABAergic neurons, SCZ-NRXN1 deletions specifically perturbed developmental trajectories of early neural progenitors and accumulated disease-specific transcriptomic signatures. Using calcium imaging, we found that both deletions led to long-lasting changes in spontaneous and synchronous neuronal networks, implicating synaptic dysfunction. Our study reveals developmental-timing- and cell-type-dependent actions of NRXN1 deletions in unique genetic contexts.
Similar content being viewed by others
Introduction
De novo mutations and copy number variations (CNVs) in 2p16.3 have been repeatedly observed in patients with autism spectrum disorders (ASDs), SCZ, and intellectual disability1,2,3. Albeit rare, these CNV losses usually manifest in heterozygous deletions, and present a significant increase in risk for multiple neuropsychiatric disorders4. Neurexin-1 (NRXN1), the single gene present in this locus, encodes a type I membrane cell adhesion molecule that functions as a synaptic organizer at central synapses5. As a presynaptic molecule, NRXN1 associates with multiple soluble and transmembrane molecules, thereby endowing specific synapses with unique synaptic signaling and transmission properties6,7,8,9. NRXN1 also undergoes extensive alternative splicing, further enriching the diversity of these interactions10,11,12,13. Due to this pan-synaptic role throughout the brain, it is not surprising to find strong prevalence of NRXN1 genetic lesions in multiple neurodevelopmental and psychiatric disorders. Most often, these lesions are large deletions (up to ~1 Mb) affecting the long isoform NRXN1α specifically, as well as NRXN1α /β lesions affecting both the long and short isoforms. However, it remains unknown why or how the same NRXN1 deletion results in phenotypically distinct disorders in individuals. It is often hypothesized that the interaction between common variants (disease genetic background) and NRXN1 CNVs drives these differences. Yet, experimentally demonstrating this hypothesis has been challenging.
Separate from its canonical function at synapses which occurs post-neurogenesis, recent evidence suggests possible roles of NRXN1 in early cortical development. In fact, NRXN1 mRNAs are abundantly detected in human embryonic neocortex, as early as gestational week (GW) 14, reaching peak at birth before slowly decreasing with age14. Knockdown of NRXN1 in human neural progenitor cells (NPCs) results in decreased levels of glial progenitor marker GFAP, thereby potentially skewing the ratio of neurons to astrocytes15. A bi-allelic NRXN1α deletion in human iPSC-derived neural cells has been shown to impair maturation of neurons and shift NPC differentiation potential towards glial rather than neuronal fate16. More recently, in vivo CRISPR KO of nrxn1 in Xenopus tropicalis embryos led to increased telencephalon size attributed to the increased proliferation of NPCs17. Separate validation using human cortical NPCs and iPSC-derived organoids showed increased proliferation of NPCs and an increase in neurogenesis in NRXN1 mutants17. Though these studies provide some clues as to which roles NRXN1 may play during early corticogenesis, the outcomes from these distinct models are inconsistent due to the differences in genetic lesions and the dosage of NRXN1 being manipulated at different developmental time points. Therefore, it is worth investigating whether disease-associated NRXN1 mutations in human cells lead to aberrant cortical development and maturation trajectories of neuronal populations in the cortex, thereby ultimately impacting cortical circuitry and synaptic function.
Human pluripotent stem cell (hPSC) derived brain organoids have been proven useful to model early developmental processes associated with neuropsychiatric disorders18,19,20,21,22,1 and 2), indicative of active neurogenesis and the start of astrogenesis. At this time point and beyond, presynaptic markers (SYNAPSIN and SYNAPTOPHYSIN) and postsynaptic marker (HOMER) were also detected along MAP2+ dendrites (Supplementary Figs. S1 and 2), suggesting that the develo** neurons are actively forming synapses in this organoid model. Thus, we chose these three developmental time points for 10X single-cell RNA sequencing (scRNAseq), thereby capturing the different pools of cell identities undergoing fate specification and maturation.
a Schematic of brain organoid generation protocol and the corresponding representative brightfield images over development. Scale bars – 250 μm for d-1, 0, 3; 100 μm for d35+. b Schematic showing genotypes used for scRNAseq and other experiments in the study. c Uniform Manifold Approximation and Projection (UMAP) showing distributions of cell classes of the integrated single-cell data. Abbreviations: NECs neural precursor cells, oRG outer radial glial cells, RG-like radial glial-like cells, IPC intermediate precursor cells, CN cortical excitatory neurons, and IN cortical GABAergic inhibitory neurons. d Heatmap of gene signatures across cell classes and cell groups. Student’s t tests were used to compute differentially expressed genes (DEGs) of each cell class in the integrated scRNA-seq data. Top 50 DEGs for each cell type are shown in a diagonal manner while normalized expression levels are scaled for each row. e UMAP showing distribution of pseudotime values of integrated single-cell data inferred by Monocle3. f NRXN1 mRNA expression (Seurat scaled expression values) across pseudotime in control donor-derived brain organoids. A higher pseudotime value indicates greater maturity as indicated by the number of various neuronal cell classes.
Having established a reliable protocol, we then subjected a cohort of previously characterized hPSC lines30,31 (Table 1) to brain organoid differentiation and collection for scRNAseq (Fig. 1b, Supplementary Data 1). We generated brain organoids from the NRXN1 cKO hESC line, which produced a single replicate of control (Flp) and deletion (Cre) at day 23, two replicates of control and deletion samples at day 50, and two replicates of control and deletion samples at day d100/112. In addition, two sets of SCZ patient and control donor iPSC pairs (2 SCZ-NRXN1del lines and 2 control lines) were sequenced at days 22 and 50 and four sets of SCZ patient and control donor iPSCs (4 SCZ-NRXN1del lines and 4 control lines) were sequenced at day 100/101 time point. For simplicity, we designate these time points as ~3 wk (days 21–23), ~2 mo (day 50), and ~3.5 mo (days 100–112), and hereafter refer to NRXN1 cKO samples as ‘engineered’ and SCZ-NRXN1del lines and controls as ‘donor’. After rigorous quality control data processing steps (Supplementary Figs. S3 and 4), we generated a total of 156,966 high-quality single-cell transcriptomes from 26 brain organoid samples for downstream analysis (Supplementary Fig. S5; n = 10 engineered and n = 16 donor).
Single cell transcriptomic atlas from develo** organoids informs relevant cell types with prominent NRXN1 expression
After data processing, normalization, and clustering, a total of 29 cell clusters were further annotated by marker expression (Supplementary Figs. S6b and S7), which consisted of both cycling and non-cycling neural progenitors (NECs), radial glia-like cells (RG-like cells), outer radial glial cells (oRGs), astroglia, intermediate cells, and intermediate progenitor cells (IPCs) that give rise to distinct subpopulations of glutamatergic excitatory neurons (CNs) and GABAergic inhibitory neurons (INs; Fig. 1c, d; Supplementary Figs. S6 and S7). We further validated our cell annotations by comparing gene expression signatures of our scRNAseq cell clusters to published brain organoid datasets as reference (Supplementary Fig. S6a)20,33,NRXN1 engineered deletions affect mature time point and alter gene expression programs in ubiquitin proteasome system, alternative splicing, and synaptic signaling To study the impact of developmental-timing- and cell-type-specific effects of isogenic NRXN1 deletions in brain organoid development, we analyzed the engineered samples throughout the three time points by 1) constructing single-cell developmental trajectories across pseudotime and 2) analyzing cell-type-specific differentially expressed gene (DEG) networks. By calculating the densities of cells across pseudotime values, we drew density plots for cells with (Cre) or without engineered NRXN1 deletions (Flp) across three time points, representing the dynamic cell abundance changes and cellular transitions throughout their developmental trajectory (Fig. 2a, b). Interestingly, we found that engineered deletions followed similar developmental trajectories as controls at 3 wk and 2 mo until reaching 3.5 mo, which showed a subtle but noticeable difference at two peaks between the control and deletion (Fig. 2b). Engineered deletion organoids displayed deviations in developmental trajectories impacting RG-like cells, oRGs, and astroglia (first peak) and IPCs and CNs/INs (second peak), suggesting that the timing of cellular differentiation and maturation in brain organoids may be affected. Importantly, at 3 to 4 month time period, brain organoids reach the peak of neuronal diversity and amplification and the beginnings of astrogenesis19,33,39, indicating that engineered NRXN1 deletions may impact gene expression programs that regulate active neurogenesis, gliogenesis, and synapse development. a UMAPs showing distributions of cell classes (left), time points (top right), and genotypes (bottom right) of NRXN1 cKO engineered brain organoids. A total of 10 samples were processed for deletion (Cre) control (Flp) – n = 1 for 3 weeks (3 wk) Cre and Flp; n = 2 for 2 months (2 mo Cre and Flp; n = 2 for 3.5 months (3.5 mo) Cre and Flp. b Ridge plots showing the density of cell abundance across the dimension of pseudotime for 3 wk, 2 mo, and 3.5 mo engineered brain organoids. c, d Dot plots showing significant differential gene expression in deletion vs. control across time points and cell types. The size and color of each dot show the number and significance of overlap** DEGs for each comparison of two cell classes. The significance was measured by \(-{\log }_{10}({FDR\; adjusted\; p\; values})\) of hypergeometric tests (see Methods). e Representative enriched gene sets were shown for GSEA results of DEGs of multiple cell classes using ToppGene. Different categories of Gene Ontology are shown in different colors (blue: molecular function, orange: biological process, and red: pathway). Enrichment scores were defined as \(-{{lo}g}_{10}({FDR\; adjusted\; p\; values})\) to represent the associations between DEG sets and Gene Ontology gene sets. To explore DEG networks in engineered deletions vs. controls, we performed DEG analysis (FDR-adj. p < 0.05) in each cell type associated with each time point. Total DEG number increased from 2 mo to 3.5 mo with the greatest perturbation at 3.5 mo (Fig. 2c–e, Supplementary Data 2). In 3.5 mo engineered organoids, DEG patterns were most pronounced in astroglia, RG-like cells, oRGs, IPC3, CNs, and INs (Fig. 2c–e, Supplementary Data 2), which correlated with the trajectory analysis (Fig. 2b). To better understand the contribution of specific gene modules associated with cell-type-specific DEGs across each developmental time point, we explored whether there exists any overlap between DEGs in different cell types using a hypergeometric test. Interestingly, DEG overlap** patterns were present within CNs and INs at 2 mo and 3.5 mo, showing similar DEGs impacted within neuronal subtypes (Fig. 2d, Supplementary Data 3). Using 3.5 mo cell-type-specific DEGs, we performed gene set enrichment analysis (GSEA, ToppGene40) to examine whether specific molecular functions, biological processes and/or biochemical pathways were significantly enriched. Collectively, DEG sets from astroglia, oRGs, IPC3, CNs, and INs were specifically enriched for RNA splicing/processing and Ubiquitin (Ub)-mediated processes (Fig. 2e, Supplementary Fig. S9, Supplementary Data 4). Notably, DEGs representing both glutamatergic excitatory neurons (CN3/4) and GABAergic neurons (IN2/7) were enriched for synaptic genes as expected, as well as components of the Ub-mediated proteolysis and RNA splicing (Fig. 2e, Supplementary Fig. S9, Supplementary Data 4). Intrigued by the specificity of the DEG enrichment in the components of mRNA splicing and ubiquitin-proteasome system (UPS), we further queried for these gene signatures across time points and cell types. Splicing- and UPS-specific DEGs are detected early as 2 mo, and by 3 mo, the impact of these DEGs became more enhanced (Supplementary Fig. S10 and 11), suggesting that perturbation effects become amplified as cells mature. Altogether, GSEA suggests that regulators of RNA splicing and UPS are consistently perturbed from oRGs to differentiated neuronal subtypes in the engineered deletions. Using similar analysis methods, we probed how SCZ-NRXN1 deletions affect single-cell developmental trajectories and DEG networks. To our surprise, we found a significant and consistent deviation of pseudotime developmental trajectories from 3 wk to 3.5 mo in the donor deletions vs. controls (Fig. 3a, b). Most notably, donor deletions induced abnormal developmental patterns of NECs in addition to other develo** cell types, an effect that was not observed in the engineered deletion organoids. Mirroring this finding, we observed significant DEG occurrences in NECs from 3 wk time point, which was maintained up to 3.5 mo (Fig. 3c, d, Supplementary Data 3 and 5). At 3.5 mo, there was a great degree of DEG overlaps across cell types, indicating possible overlap of common biological effects. In summary, the DEG significance, overlap** DEG patterns, and abnormal developmental trajectories were all enhanced in the donor deletions compared to the engineered deletions. a UMAPs showing distributions of cell classes (left), time points (top right), and genotypes (bottom right) of SCZ-NRXN1del donor derived brain organoids. A total of 16 samples were processed – 2 SCZ-NRXN1del donors and 2 Ctrl donors for 3 weeks (3 wk); 2 SCZ-NRXN1del donors and 2 Ctrl donors for 2 months (2 mo); 4 SCZ-NRXN1del donors and 4 Ctrl donors for 3.5 months (3.5 mo). b Ridge plots showing the density of cell abundance across the dimension of pseudotime for 3 wk, 2 mo, and 3.5 mo engineered brain organoids. c, d Dot plots showing significant differential gene expression in deletion vs. control across time points and cell types. The size and color of each dot show the number and significance of overlap** DEGs for each comparison of two cell classes. The significance was measured by \(-{\log }_{10}({FDR\; adjusted\; p\; values})\) of hypergeometric tests (see Methods). e Representative enriched gene sets were shown for GSEA results of DEGs of multiple cell classes using ToppGene. Different categories of Gene Ontology are shown in different colors (blue: molecular function, orange: biological process, and red: pathway). Enrichment scores were defined as \(-{\log }_{10}({FDR\; adjusted\; p\; values})\) to represent the associations between DEG sets and Gene Ontology gene sets. We then investigated the functional significance of DEGs in donor deletions and whether they represent similar or different gene set enrichment patterns compared to engineered deletions. Therefore, we performed GSEA on overlap** DEGs found in similar cell types organized into separate cell groups – all NECs, RG-like cells/intermediate cells/IPC3, astroglia, oRGs, CN2/3, and IN4/5. Intriguingly, while each cell group showed specific DEG enrichment of biological processes relevant to its cell type functions, i.e. cell cycle-related genes in dividing cells and cell signaling genes in neurons, we again identified two distinct biological processes that were repeatedly observed across cell groups – RNA splicing and UPS (Fig. 3e, Supplementary Fig. S12, Supplementary Data 6). As mentioned above, these two pathways were also found disrupted in engineered deletions. Specifically in neuronal subtypes (CN2/3 and IN4/5), a strong enrichment of synaptic signaling genes was present (Fig. 3e, Supplementary Fig. S12, Supplementary Data 6). We next tested for the presence of RNA splicing- and UPS-specific DEGs in all cell types across timepoints and found that donor deletions showed a significant enrichment of these DEGs throughout development with most significant patterns reached at 3.5 mo (Supplementary Fig. S10 and S11). Collectively, these results highlight that, in the develo** brain organoids, gene networks involving UPS and RNA splicing are commonly disrupted across isogenic and SCZ-NRXN1 deletion backgrounds but manifest in different temporal and cell-type-specific manners. To gain better insight into how RNA splicing could be misregulated in our brain organoid models, we examined the expression patterns of established neuronal splicing regulators including PTBPs, MBNLs, RBFOXs, NOVAs, SSRMs, and KHDRBSs in both the donor and engineered deletion contexts at 3.5 mo (Supplementary Data 7). Interestingly, we found that the vast majority were downregulated, suggesting that these splicing regulators may not function properly to promote global splicing patterns of mature neuronal programs41,42,43,44,45. Based on the predicted functions of these RNA binding proteins in regulating alternative splicing during neural development and their associated downregulated gene signatures, we reasoned that NRXN1 deletions could render an overall immature splicing program and that global alternative splicing could potentially be misregulated. Moreover, since many of these neuronal splicing regulators control alternative splicing of neurexins46, we questioned whether alternative splicing of NRXN1 gene itself was changed as previously reported in postmortem tissue samples from SCZ patients47. To test whether NRXN1 deletion induces changes in alternative splicing programs in neurons, we explored a previously published bulk RNA sequencing dataset generated from mature glutamatergic excitatory induced neurons (iNs) from the same set of donor derived iPSC lines and an engineered NRXN1 cKO iPSC line31. We first confirmed that neuronal splicing regulators are differentially expressed with an overall downregulation pattern in iNs with NRXN1 deletion (Fig. 4a). Using RSEM48, we detected a total of 184 differentially expressed isoforms (DEIs) enriched in engineered iNs with NRXN1 deletions (Cre/Flp) and 213 DEIs in SCZ donor iNs with NRXN1 deletions (NRXN1dels/Ctrls) across three pairs of healthy control and SCZ-NRXN1 deletion carriers (Fig. 4b, Supplementary Data 8). These DEIs mapped to 165 and 153 unique gene IDs for engineered iNs and donor iNs, respectively. Among the differentially spliced genes, ~10% consisted of synaptic genes identified by SYNGO enrichment analysis49, implicating a major functional impact on synaptic signaling. Interestingly, while 5 genes (AHI1, CHD4, DHX36, NRXN1, STAMBP) overlapped between engineered and donor iNs, the majority of genes were unique to each iN type (Fig. 4b), showing differential abundance of DEIs in the engineered vs. donor genetic background. Interestingly, synaptically localized CHD4 and NRXN1 showed similar differential expression patterns of isoforms in both deletion contexts (Fig. 4c, d). a Differential gene expression (adjusted p values < 0.05) of neuronal splicing regulators in SCZ-NRXN1del1 compared to Ctrl.1 iN cells (n = 3 replicates). Log2 fold changes are plotted on the y-axis with example DEGs on the x-axis. Blue bars represent downregulation and red bars represent upregulation. b Venn diagram representing differential isoform abundance derived from RSEM analysis across iN cells from engineered NRXN1 deletions (n = 3 replicates from NRXN1 cKO iPSC) and donor-derived SCZ-NRXN1 deletions (3 healthy controls and 3 SCZ-NRXN1 deletion carriers; n = 3 replicates per line, 18 replicates total). Number of differentially expressed isoforms (184 for engineered iNs and 213 donor derived iNs) mapped to 160 and 148 unique gene IDs respectively. Among the unique genes, example SYNGO-mapped genes are highlighted. c, d Percentage of isoform ratio change (differential transcript usage, see methods) for NRXN1 and CHD4 in NRXN1 deletions from engineered (eng.; yellow) and donor (green) backgrounds. Error bars represent +/−S.E.M. e Detection and visualization of local differential splicing using Majiq and ggSashimi. Change in percent spliced in index (dPSI) is calculated for differential exon usage in the NRXN1 gene (exons 19–21) in the SCZ-NRXN1 deletion vs. NRXN1 cKO engineered deletion iNs. Number of detected reads is plotted on the y-axis with location of genomic coordinates on the x-axis. In addition to differential isoform expression, we analyzed differential local splicing in iNs using Majiq50. In this analysis, one can integrate both de novo splicing change detection as well as known splicing events to quantify the change in PSI (percent spliced in index), which reports how often specific sequences are spliced into transcripts. Out of 33 specific local splicing variations (LSVs) present in the NRXN1 gene in iNs, we found 4 LSVs which were similarly changed in both donor and engineered iNs, 15 LSVs that were different, and 14 LSVs that were not significantly changed in any genotype (Supplementary Data 9). More specifically, we found a biologically significant LSV in exon 20, where inclusion of this exon was increased in the deletions compared to controls in both isogenic and SCZ-NRXN1 deletion backgrounds (Fig. 4e). Differential splicing of this exon, which contains splice site #4, could have functional implications in ligand binding and synaptic properties5,7. Altogether, splicing analysis of mature glutamatergic iNs show that NRXN1 deletions result in changes in spliced variant landscape of synaptic genes, including NRXN1 itself, which could impact synaptic function by differential isoform representation. To test whether the observed developmental abnormalities and gene expression programs translate to functional and sustained differences in neuronal connectivity, we performed live Ca2+ imaging in 4 mo brain organoids from SCZ-NRXN1 deletion vs. control (NRXN1del2 vs. Ctrl2). Two weeks prior to imaging, brains organoids were infected with AAVs expressing a genetically encoded Ca2+ indicator, somaGCaMP6fs construct51. Under basal conditions (without any stimulation), we measured the frequency and amplitude of spontaneous Ca2+ transients, which are indicative of spontaneous neuronal network activities. In addition, we quantified the frequency of synchronous firing, which indicates how often neurons fire together, thereby producing synchronized bursts of activities. Compared to controls, NRXN1del2 brain organoids showed a significant decrease in the frequency of spontaneous Ca2+ transients without a change in the amplitude of the responses, as measured by dF/F0 intensity (Fig. 5a–c). In addition, there was an overall decrease in the synchronous firing rate in these brain organoids, demonstrating a significant decrease in the neuronal network bursts (Fig. 5a–c). a Representative fluorescent images of intact donor derived organoids (Ctrl.2 and NRXN1del2) expressing soma-GCaMP6f2 at ~120 days prior to live Ca2+ imaging. b Colored raw intensity traces are shown in the boxed graphs with averaged intensities plotted in bolded black. c Averaged data for synchronous firing rates (number of detected synchronous spikes/minute) representative of network activity, as well as amplitudes (dF/F0) and frequencies (total number of detected peaks/minute) of spontaneous spike activity, are shown in scatter plots. Each data point represents averaged data from a single field of view (FOV) consisting of 4-5 ROIs per FOV. At least 4–6 FOVs were taken from each organoid and 5 organoids per genotype were used for experiments. N = 29–30 for Ctrl; N = 23–25 for NRXN1del2. d Experimental flow of live Ca2+ imaging with glycine stimulation. e Synchronous firing rate is plotted in genotypes with or without stimulation. Each data point represents averaged data from a single field of view (FOV) consisting of 4–5 ROIs per FOV. At least 4–6 FOVs were taken from each organoid and 5 organoids per genotype were used for experiments. N = 24–25 for Ctrl; N = 16–19 for NRXN1del2. Error bars represent +/− S.E.M. Statistical significance is represented by asterisks: *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. Student’s t test (two-sided) was performed for data shown for c and one-way ANOVA with multiple comparisons (Tukey’s test) was used for data shown on e. Source data and statistics are provided as a Source Data file. Next, we explored the possibility that N-methyl-D-aspartate receptor (NMDAR) function might be abrogated in these organoids, thus contributing to the overall neuronal network dysfunction. Based on DEG analysis of 3.5 mo brain organoids, we identified differential expression in NMDAR subunit 2B (GRIN2B) and fatty acid binding protein 7 (FABP7), both of which have been linked to SCZ pathogenesis52,53,54,55,56. It is well documented that NMDAR hypofunction underlies SCZ pathology57,58, and recent exome sequencing and GWAS studies identified the NMDAR subunit GRIN2A as a significant SCZ risk allele59. Genetic variants in FABP7 have been identified in SCZ and ASD patients, and its function has been linked to NMDAR signaling regulation52,53,54,60. Furthermore, while searching for neuronal-specific DEGs that were consistently perturbed between engineered and donor deletions, we found GRIN2B as a commonly perturbed gene in GABAergic neuronal subtypes across genetic backgrounds (engineered IN2 vs. donor IN4/5; Supplementary Data 2 and 5). These findings suggest that misregulated NMDAR signaling in GABAergic neurons could potentially impact synaptic connectivity and signaling in these brain organoid models. To test this idea, we developed an assay mimicking chemical long term potentiation (cLTP) whereby the functionality of NMDARs can be tested with the addition of glycine, a co-agonist for NMDARs61 (Supplementary Fig. S13a). In control brain organoids, glycine stimulation potentiates neuronal activity as shown by sustained increases in synchronous firing rate measured at 30 min and 60 min post-stimulation (Supplementary Fig. S13b, d, e). This potentiated activity is mediated by NMDARs, as the addition of selective NMDAR antagonist APV blocks this effect (Supplementary Fig. S13c–e). Indeed, when stimulated with glycine, brain organoids with SCZ-NRXN1 deletion failed to potentiate glycine-induced activity compared to control brain organoids (Fig. 5e), suggesting disruption in NMDAR activity. At more mature time points, (~4.5–5 months), we found that both synchronous neuronal activity as well as spontaneous neuronal spikes was still decreased in the SCZ-NRXN1 deletions (NRXN1del3) compared to controls (Supplementary Fig. S14a–d), suggesting synaptic network dysfunction continues through maturation in these models. Interestingly, NRXN1 engineered deletions produced a slightly different phenotype in which the frequency of spontaneous Ca2+ transients was increased without any changes in the amplitude of the responses as well as the synchronicity of spontaneous firing events (Supplementary Fig. S14e–h). Recorded Ca2+ transients represent true neuronal signals as they were sensitive to synaptic blockers (Supplementary Fig. S15). These data suggest that although spontaneous neuronal activities are uniformly altered in the brain organoids carrying NRXN1 deletions, depending on the genetic background, different phenotypic outcomes manifest, reflective of the differences in transcriptomic landscape and developmental origins of such changes in these brain organoids. To test whether up- and down-regulated DEGs identified from donor-derived and engineered brain organoids were associated with specific neuropsychiatric disease gene signatures, we computed a ‘disease enrichment’ score (-log10 (FDR-adjusted p values)) based on a previously established curated list of dysregulated gene sets obtained from SCZ, bipolar disorder (BD), major depression disorder (MDD), and ASD-associated samples62. Excitingly, in 3.5 mo brain organoids, DEGs from both donor and engineered deletions were significantly enriched for ASD and SCZ gene signatures (Fig. 6a). Interestingly, there was no significant enrichment of the DEGs in MDD- and BD-related gene sets across the cell types, suggesting that the DEG pool from our brain organoids most closely resembles dysregulated transcriptional signatures related to SCZ and ASD, similar to what has been reported regarding shared genetic signals between SCZ and ASD63. a Heatmap showing results from a subset of gene enrichment analyses of DEGs performed on cells of both brain organoid types (engineered and donor derived brain organoids) across all time points using neurological disorder dysregulated gene sets in several categories (ASD autism spectrum disorders, BP bipolar disorder, MDD mood disorder, SCZ schizophrenia) (columns). Data shown here are from 3.5 mo time point only. Body Mass Index (BMI) was used as control (−). Gene Set Enrichment Analysis (GSEA) was employed using the package GSEAPY, and significance scores were defined as \(-{\log }_{10}({FDR\; adjusted\; p\; values})\) to represent the associations between DEG sets and neurological disorders. Scores were trimmed to 0~10 (see Methods). Significance levels were represented by numbers of asterisks (*: adjusted p values < 0.05; **: adjusted p values < 0.01; ***: adjusted p values < 0.001). b Significance of differential expression of prioritized genes obtained from PGC wave 3 and SCHMEA consortium51,63. The size of each dot represents the level of DE significance of each gene in each cell class of 3.5 mo donor brain organoids (left; n = 4 for Ctrls and n = 4 for SCZ-NRXN1dels) and 3.5 mo engineered brain organoids (right; n = 2 for Flp and n = 2 for Cre). Two-sided Wilcoxon rank-sum tests were employed for the DE tests, with FDR-adjusted p-values utilized. Next, we independently examined the enrichment score of rare and common variants of SCZ in the 3.5 mo organoid DEG sets by comparing them to a list of risk genes recently reported by SCZ-GWAS PGC wave3 and SCHEMA consortium59,63. Remarkably, several of the SCZ risk genes were represented across cell types in the SCZ-NRXN1 deletions (Fig. 6b), which highlights SCZ-specific transcriptional signatures present in the patient genetic backgrounds. Engineered NRXN1 deletion-associated DEGs showed minimal overlap with SCZ-associated risk variants, clearly demonstrating strong association of SCZ-specific loci in the patient disease background compared to the isogenic background (Fig. 6b). A separate survey of DEG overlaps with the GWAS-loci across neuropsychiatric disorders64,65,66,67 confirmed this finding, where we observed the presence of many more disease-associated DEGs in the donor vs. engineered deletions (Supplementary Data 10).SCZ-NRXN1 deletions induce early developmental perturbation effects which are maintained throughout maturation with consistent DEG patterns as engineered deletions
Alternative splicing dysregulation in glutamatergic excitatory neurons with NRXN1 deletions
Impaired neuronal network function in brain organoids carrying NRXN1 deletions
Differential enrichment of disease-associated signatures in SCZ-NRXN1 deletions vs. engineered NRXN1 deletions
Discussion
Here we provide a systematic analysis of the developmental-timing- and cell-type-dependent perturbations induced by NRXN1 deletions in the develo** human brain organoids using single cell transcriptomics. We initially had two specific goals in mind – 1) to understand the developmental effects of NRXN1 heterozygous deletions in an isogenic background and uncover which time points and cell types are important for NRXN1 function, and 2) to utilize SCZ-NRXN1del patient iPSC-derived organoids as a model to study the molecular and cellular biology of SCZ. By profiling the single cell transcriptomes of NRXN1 cKO brain organoids, we found that cellular phenotypes associated with NRXN1 haploinsufficiency manifest during a developmental window of brain organoids at the peak of neurogenesis and early astrogenesis. Moreover, developmental trajectories and gene expression profiles of maturing glutamatergic and GABAergic neurons are impacted by NRXN1 deletions.
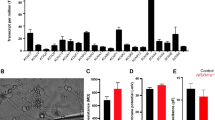
By comparing engineered and donor-derived organoids side by side, we found both commonalities and differences, reflective of the contribution of genetic background effects. We unbiasedly found shared molecular programs that are perturbed across organoid types including alternative splicing, UPS, and synaptic signaling. Synaptic signaling misregulation was expected since NRXN1 deletions in 2D glutamatergic iNs decrease probability of neurotransmitter release and synaptic strength31. In our study, NMDAR subunit GRIN2B was differentially expressed in GABAergic neurons across genetic backgrounds (in engineered IN2 and donor IN4/5). This finding is significant as it confirms previous results showing that mouse Nrxn1 signals through NMDARs7,8 and SCZ-NRXN1del glutamatergic iNs carry upregulated levels of the endogenous NMDAR antagonist KYAT331. Moreover, genetic variants in the NMDAR subunits, GRIN2B and GRIN2A, are both observed in SCZ populations59,68,69. Lastly, NMDAR hypofunction in SCZ has been a longstanding hypothesis supported by multiple post-mortem studies and brain imaging studies from SCZ patients as well as mouse models of NMDAR blockade through ketamine and phencyclidine57,58. In agreement with transcriptomic data, we showed that SCZ-NRXN1del organoids fail to potentiate glycine-induced augmentation in neuronal activity (i.e. cLTP), which validates NMDAR dysfunction.
Alternative splicing is highly regulated in the brain70,71 and is influenced by neuronally enriched splicing factors like NOVAs, PTBPs, RBFOXs, and MBLNs. The general notion is that RBFOXs and MBNLs promote mature splicing patterns whereas MBNLs antagonize this process, altogether tightly regulating alternative splice usage during cortical development and plasticity41,42,43,44,45,72. Interrogation of DEGs in our brain organoid dataset showed a clear misregulation of these neuronal-specific splicing regulators at the transcriptional level, suggestive of an immature splicing program in these models. Indeed, by analyzing splicing variation in 2D glutamatergic iNs differentiated from the same set of iPSC lines revealed changes in alternative splicing in the abundance of isoform representation and local splicing variation in both donor and engineered deletions. It has been shown that global splicing changes and alternative transcript usage are overrepresented in SCZ brains, more so than in ASDs and BD47. Differential splicing of various genes has been observed in the brain samples of SCZ patients compared to controls, including DRD2, NRG1, ERBB4, GRM3, and GRIN147,73,74,75,76. More recently, differential splicing effects of NRXN1 has been suggested in SCZ iPSC-derived neurons77 and in postmortem brains of SCZ and BD patients14,47, further highlighting the importance of splicing regulation in SCZ as a potential molecular mechanism.
In addition to splicing, DEGs responsible for UPS regulation have been identified in our study. These genes encode for proteins that are E2 conjugating enzymes (UBE2M, UBE2V2), deubiquitinase (UCHL1), Ub processing- (UBB) or autophagy-related factors (GABARPL2) as well as proteins, which are themselves or direct binding partners to E3 Ub ligases (NDFIP1, SKP1, RNF, STUB1). Though it is not clear from the list of DEGs whether or not these molecules are actively participating in the protein quality control or in the regulation of protein components involved in signal transduction, it has been hypothesized that alterations in proteostasis and Ub-mediated regulation of synaptic signaling contribute to SCZ pathogenesis78,79,80,81,82. Additionally, protein truncating variants of Ub ligases (CUL1 and HERC1) were recently found to be associated with SCZ at exome-wide scale63, further highlighting the importance of this molecular pathway in SCZ. Possibly more UPS genes are to be discovered for both rare and common variants in the future. Though it remains to be determined whether these changes are indeed causal or merely reporting a consequence of the disease, altered UPS does exist and this, in turn, could affect protein homeostasis in the brains of SCZ patients. Importantly, proteasome function at synapses is tightly regulated by NMDAR activity, as NMDAR activation regulates 26S proteosome assembly and catalytic activity83,84 and stability of proteasomes in the post-synaptic density85. Moreover, E3 Ub-ligases and deubiquitinases act in concert to regulate the ubiquitination, internalization and localization of NMDARs, AMPARs, and mGluRs, and therefore, actively participate in Hebbian and homeostatic plasticity86,87,88. Further investigations on the interplay between NMDAR signaling and UPS regulation during synaptic development would enhance our understanding of how these distinct biological pathways converge in the context of SCZ pathogenesis.
Interestingly, there were major differences between donor vs. engineered organoids that we observed. First, unlike the donor derived organoids, engineered organoids did not exhibit changes in the developmental trajectory or gene expression in the NEC subtypes. Moreover, the magnitude of gene expression changes in various cell types in the engineered organoids was minimal compared to donor derived organoids. These findings indicate that brain organoids derived from patient genetic background induce a greater degree of transcriptional perturbations and uncover NECs as a vulnerable cell type during cortical development. Neuronal subtypes, astroglial cells, and oRGs are commonly affected to varying degrees in both patient and engineered genetic backgrounds carrying NRXN1 deletions. The specificity of NEC phenotypes in the SCZ-NRXN1del genetic background is further supported by previous studies reporting alterations in the morphology, differentiation potential, and gene expression profiles from SCZ iPSC derived NECs and brain organoid models, all of which are in support of the neurodevelopmental hypothesis of SCZ89,90,91. Second, donor derived organoids showed a greater accumulation of disease-specific signatures related to SCZ. This finding makes sense, since donor derived organoids carry SCZ-relevant genetic background. Due to this effect, differences in the magnitude of gene expression changes and the directionality of those changes were observed in these organoid types. This is also apparent in the differences in the specific neuronal firing patterns observed in the isogenic engineered vs. donor derived organoids.
There are two main limitations to this study. First, despite obtaining high-quality dataset of ~157,000 single cells, the overall study is underpowered due to the small sample size of patient/control cohort with limited genetic backgrounds being represented. Larger sample size and multiple technical replicates would allow for a more granular analysis of mutational effects across brain organoid development. Second, while we focused on early developmental time points leading up to ~3.5 mo, which allows investigation of the molecular programs underlying peak of neuronal diversity and amplification, older time points could reveal postnatal gene signatures that are being missed here. For example, astrocytes are prominent cell types that are shared among engineered and donor derived organoids and produce changes in gene expression and developmental trajectory. This finding could be further explored using older organoid samples, as astrocyte development is initiating at ~day 100 and requires long term cultures to study their biology92. In addition, the developmental switch from GRIN2B- to GRIN2A-containing NMDARs occurs at ~300 day old brain organoids25, which could potentially allow one to study postnatal human brain biology.
In the future, it will be important to expand upon this work by comparing this dataset with single cell transcriptomes obtained from NRXN1 deletion carriers with other neuropsychiatric disorders like ASDs as well as those from healthy, unaffected individuals who also carry NRXN1 deletions. This type of experimental design would allow dissection of the contribution of disease-specific effects at a greater scale – common vs. distinct molecular features across neuropsychiatric disorders which uniformly affect brain development and synaptic function. Furthermore, detection and analysis of alternative splicing of full-length transcripts at the single cell level across brain organoid development in these genetic contexts will better inform splicing misregulation. Finally, at the mechanistic level, why or how NRXN1 deletions induce changes in alternative splicing will need to be determined. It is curious to know whether alternative splicing changes precede or is a response to synaptic dysfunction.
Methods
hPSC culture and forebrain organoid generation
hESC carrying heterozygous NRXN1 deletions, as well as iPSCs from 4 SCZ donors with NRXN1 deletion (N3320a, N9540a, N1884a, N0575a) and 4 controls (C3141a, C9632, C8905a, C2320b) were generated in earlier studies30,31. More information about these lines are provided in Table 1. hESC and iPSCs were cultured on feeder-free conditions as previously described using mTeSR Plus medium (Stem Cell Technologies) on Matrigel (Corning)Gene modules from ToppCell We used ToppCell toolkit to generate gene modules of cell types and clusters in our single cell data (Supplementary Fig. S6a)97. We applied ToppCell to user-provided cell annotations and derived well-organized gene modules for all cell classes. Each gene module contains the top 200 DEGs from ToppCell, representing the most prominent transcriptomic profile of this cell class. ToppCell-derived gene modules were seamlessly enriched using ToppGene44 and ToppCluster98. Gene set enrichment analysis (GSEA) was conducted using ToppGene for gene sets from either ToppCell output or differential expression analysis. Gene ontologies were used to annotate molecular functions, biological processes and cellular components. In addition, we used the prerank function in GSEAPY package for the customized GSEA analysis. For UPS and alternative splicing enrichment gene plots (Supplementary Figs. S10 and S11), we consulted GSEA output from ToppGene as well as published literature reporting these genes. We used the manually curated neurological-disorder-associated gene sets65 as the reference for Fig. 6a, such as genes of autism spectrum disorder and schizophrenia. We calculated FDR adjusted p values of enrichment for differentially expressed genes to infer their associations with neurological diseases. For disease enrichment analysis for GWAS loci (Supplementary Data 10), we compiled a list of disease loci from the following publications: PMID: 28540026 (ASD)64, PMID: 31740837 (SCZ)65, PMID: 30718901 (MDD)66, and PMID: 31043756 (BP)67. We used Monocle342 to infer the pseudotime and trajectories of cell differentiations in the brain organoid single-cell data. We took advantage of the Seurat integration procedure and transferred Seurat objects into Monocle3 cell_data_set objects. Then we learned trajectories on the UMAP using the learn_graph function to get the pseudotime ordering of cells using the order_cells function. Cells with the highest expression levels of cell cycle genes in cycling NECs were selected as the start point of trajectories. In the end, every cell was assigned a pseudotime value, representing the estimated differentiation stages along the trajectory. Ridge plots were drawn based on the density of cells across pseudotime values. We collected human fetal cortex single-cell data from a large-scale single-cell dataset40. The normalized expression levels (log2(CPM + 1)) of NRXN1 were calculated for each cell and average normalized expression was derived for cells in each cell types across various developmental stages. We used bulk RNA-seq data from previous published induced neurons31. RNA-seq reads were aligned to the GRCh38.p13 reference genome using STAR version 2.7.9 using gene annotations from Gencode v41. Basic mode and “introMotif” were used for “twopassMode” and “outSAMstrandField” settings, respectively. Genes and isoforms were quanified with RSEM version 1.3.0. To measure the differential expression levels of genes and isoforms, we first loaded the output of RSEM with the package tximport, and then conducted differential gene expression and transcript usage analysis with the package DESeq248. Differential transcript usage analysis was conducted for genotypes by controlling the effects of cell lines. FDR adjusted p values were used to control the type I error. Majiq50 was used to quantify the alternative splicing. We first ran the Majiq Builder to de novo detect local splicing variations (LSVs) and build splicegraph. We then conducted Majiq Quantify to measure the Percent Splice In (PSI) and delta-PSI between genotypes. After quantification, we generated statistics of differential splicing variations and user interface for visualization using Volia. Additionally, we used ggsashimi93 to visualize the splicing events in sashimi plots with the bam file output from STAR. Several datasets were used for the cell type prediction in this study, including: Kanton et al. (2019)41: This is a single cell dataset of human cerebral organoids derived from iPSC- and embryonic stem cell (ESC)- derived cells (43,498 cells) at different time points (day 0 ~ day 120) during the differentiation. Paulsen et al. (2022)37: This is a single cell dataset of human cerebral cortex organoids with haploinsufficiency in three autism spectrum disorder (ASD) risk genes in multiple cell lines from different donors of more than 745,000 cells. Tanaka et al. (2020)38: This is synthetic analysis of single cell data from multiple brain organoid and fetal brain datasets. Data of 8 different protocols were collected and 190,022 cells were selected for the reannotation, where they classified 24 distinct clusters and 13 cell types. Velasco et al. (2019)24: This is a study to validate the reproducibility of brain organoids with single cell sequencing. They collected 166,242 cells from 21 individual organoids and identified indistinguishable compendiums of cell types and similar developmental trajectories. Zhong et al. (2018)39: This is a single cell dataset with more than 2,300 cells in develo** human prefrontal cortex from gestational weeks 8 to 26. Bhaduri et al. (2021)40: This is a large-scale single cell data of develo** human brain from gestation week (GW) 14 to GW 25. Multiple brain regions and neocortical areas were sampled for the data. The protocol utilized in this study has been replicated at a minimum of four culture batches for each cell line mentioned in this study (Fig. 1a; Table 1). Additional replicates were grown from these cell lines for other experimental needs as detailed in Supplementary Data 1. Immunostaining for all markers were performed across four culture batches and consistent expression of the markers were observed for each experiment (Supplementary Figs. S1 and S2). Calcium imaging experiment was replicated across seven cell lines. Specifically, GCAMP recordings were performed six times (Fig. 5a) and x-Rhod-1 dye recordings were performed six times (Supplementary Fig. S14). For generation of data in Supplementary Data 2, 5, and 7, two-sided Wilcoxon rank-sum tests were employed for the DE tests, with FDR-adjusted p-values utilized. For generation of data in Supplementary Data 4 and 6, Gene Set Enrichment Analysis (GSEA) was employed. Unadjusted p values (“p-value”), FDR-adjusted p values (“q-value FDR B&Y”), Bonferroni-adjusted p values (“q-value Bonferroni”), and p values adjusted by Benjamini & Yekutieli (2001) (“q-value FDR B&Y”) were provided. Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.Gene enrichment analysis
Trajectory inference and pseudotime analysis
NRXN1 expression analysis
Alternative splicing and isoform analysis
Reference datasets
Statistics and reproducibility
Reporting summary
Data availability
The single cell RNA-seq data generated in this study have been Omnibus database under accession code #GSE228315. Source data for all Ca2+ imaging analysis are provided with this paper. Source data are provided with this paper.
Code availability
Code used for single cell transcriptomic analysis can be found here (https://github.com/KANG-BIOINFO/NRXN1_effects). Calcium imaging analysis was performed in MATLAB using the previously published code (https://github.com/beccasbastian/CalciumIMG_Analysis).
References
Lowther, C. et al. Molecular characterization of NRXN1 deletions from 19,263 clinical microarray cases identifies exons important for neurodevelopmental disease expression. Genet Med. 19, 53–61 (2017).
Castronovo, P. et al. Phenotypic spectrum of NRXN1 mono- and bi-allelic deficiency: a systematic review. Clin. Genet 97, 125–137 (2020).
Marshall, C. R. et al. Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects. Nat. Genet. 49, 27–35 (2017).
Fuccillo, M. V. & Pak, C. Copy number variants in neurexin genes: phenotypes and mechanisms. Curr. Opin. Genet. Dev. 68, 64–70 (2021).
Sudhof, T. C. Synaptic neurexin complexes: a molecular code for the logic of neural circuits. Cell 171, 745–769 (2017).
Etherton, M. R., Blaiss, C. A., Powell, C. M. & Südhof, T. C. Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc. Natl Acad. Sci. USA 106, 17998–18003 (2009).
Dai, J., Aoto, J. & Südhof, T. C. Alternative splicing of presynaptic neurexins differentially controls postsynaptic NMDA and AMPA receptor responses. Neuron 102, 993–1008.e5 (2019).
Davatolhagh, M. F. & Fuccillo, M. V. Neurexin1α differentially regulates synaptic efficacy within striatal circuits. Cell Rep. 34, 108773 (2021).
Chen, L. Y., Jiang, M., Zhang, B., Gokce, O. & Südhof, T. C. Conditional deletion of all neurexins defines diversity of essential synaptic organizer functions for neurexins. Neuron 94, 611–625.e4 (2017).
Ullrich, B., Ushkaryov, Y. A. & Südhof, T. C. Cartography of neurexins: more than 1000 isoforms generated by alternative splicing and expressed in distinct subsets of neurons. Neuron 14, 497–507 (1995).
Tabuchi, K. & Südhof, T. C. Structure and evolution of neurexin genes: insight into the mechanism of alternative splicing. Genomics 79, 849–859 (2002).
Treutlein, B., Gokce, O., Quake, S. R. & Südhof, T. C. Cartography of neurexin alternative splicing mapped by single-molecule long-read mRNA sequencing. Proc. Natl Acad. Sci. USA 111, E1291–E1299 (2014).
Schreiner, D. et al. Targeted combinatorial alternative splicing generates brain region-specific repertoires of neurexins. Neuron 84, 386–398 (2014).
Jenkins, A. K. et al. Neurexin 1 (NRXN1) splice isoform expression during human neocortical development and aging. Mol. Psychiatry 21, 701–706 (2016).
Zeng, L. et al. Functional impacts of NRXN1 knockdown on neurodevelopment in stem cell models. PloS One 8, e59685 (2013).
Lam, M. et al. Single cell analysis of autism patient with bi-allelic NRXN1-alpha deletion reveals skewed fate choice in neural progenitors and impaired neuronal functionality. Exp. Cell Res. 383, 111469 (2019).
Willsey, H. R. et al. Parallel in vivo analysis of large-effect autism genes implicates cortical neurogenesis and estrogen in risk and resilience. Neuron 109, 788–804.e8 (2021).
Lancaster, M. A. et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013).
Paşca, A. M. et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 12, 671–678 (2015).
Velasco, S. et al. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature 570, 523–527 (2019).
Qian, X. et al. Brain-region-specific organoids using mini-bioreactors for modeling zikv exposure. Cell 165, 1238–1254 (2016).
Bershteyn, M. et al. Human iPSC-derived cerebral organoids model cellular features of lissencephaly and reveal prolonged mitosis of outer radial glia. Cell Stem Cell 20, 435–449.e4 (2017).
**ang, Y. et al. Fusion of regionally specified hPSC-derived organoids models human brain development and interneuron migration. Cell Stem Cell 21, 383–398.e7 (2017).
Sebastian, R., Song, Y. & Pak, C. Probing the molecular and cellular pathological mechanisms of schizophrenia using human induced pluripotent stem cell models. Schizophr. Res S0920-9964, 00263–00268 (2022).
Gordon, A. et al. Long-term maturation of human cortical organoids matches key early postnatal transitions. Nat. Neurosci. 24, 331–342 (2021).
Kathuria, A. et al. Transcriptomic landscape and functional characterization of induced pluripotent stem cell-derived cerebral organoids in schizophrenia. JAMA Psychiatry 77, 745–754 (2020).
Khan, T. A. et al. Neuronal defects in a human cellular model of 22q11.2 deletion syndrome. Nat. Med. 26, 1888–1898 (2020).
Stachowiak, E. K. et al. Cerebral organoids reveal early cortical maldevelopment in schizophrenia-computational anatomy and genomics, role of FGFR1. Transl. Psychiatry 7, 6 (2017).
Notaras, M. et al. Schizophrenia is defined by cell-specific neuropathology and multiple neurodevelopmental mechanisms in patient-derived cerebral organoids. Mol. Psychiatry 27, 1416–1434 (2022).
Pak, C. et al. Human neuropsychiatric disease modeling using conditional deletion reveals synaptic transmission defects caused by heterozygous mutations in NRXN1. Cell Stem Cell 17, 316–328 (2015).
Pak, C. et al. Cross-platform validation of neurotransmitter release impairments in schizophrenia patient-derived NRXN1-mutant neurons. Proc. Natl Acad. Sci. USA 118, e2025598118 (2021).
Yoon, S.-J. et al. Reliability of human cortical organoid generation. Nat. Methods 16, 75–78 (2019).
Paulsen, B. et al. Autism genes converge on asynchronous development of shared neuron classes. Nature 602, 268–273 (2022).
Tanaka, Y., Cakir, B., **ang, Y., Sullivan, G. J. & Park, I.-H. Synthetic analyses of single-cell transcriptomes from multiple brain organoids and fetal brain. Cell Rep. 30, 1682–1689.e3 (2020).
Zhong, S. et al. A single-cell RNA-seq survey of the developmental landscape of the human prefrontal cortex. Nature 555, 524–528 (2018).
Bhaduri, A. et al. An atlas of cortical arealization identifies dynamic molecular signatures. Nature 598, 200–204 (2021).
Kanton, S. et al. Organoid single-cell genomic atlas uncovers human-specific features of brain development. Nature 574, 418–422 (2019).
Cao, J. et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature 566, 496–502 (2019).
Uzquiano, A. et al. Proper acquisition of cell class identity in organoids allows definition of fate specification programs of the human cerebral cortex. Cell. 185, 3770–3788.e27 (2022).
Chen, J., Bardes, E. E., Aronow, B. J. & Jegga, A. G. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 37, W305–W311 (2009).
Weyn-Vanhentenryck, S. M. et al. HITS-CLIP and integrative modeling define the Rbfox splicing-regulatory network linked to brain development and autism. Cell Rep. 6, 1139–1152 (2014).
Zhang, C. et al. Integrative modeling defines the Nova splicing-regulatory network and its combinatorial controls. Science 329, 439–443 (2010).
Charizanis, K. et al. Muscleblind-like 2-mediated alternative splicing in the develo** brain and dysregulation in myotonic dystrophy. Neuron 75, 437–450 (2012).
Li, Q. et al. The splicing regulator PTBP2 controls a program of embryonic splicing required for neuronal maturation. Elife 3, e01201 (2014).
Licatalosi, D. D. et al. Ptbp2 represses adult-specific splicing to regulate the generation of neuronal precursors in the embryonic brain. Genes Dev. 26, 1626–1642 (2012).
Lukacsovich, D. et al. Single-cell RNA-seq reveals developmental origins and ontogenetic stability of neurexin alternative splicing profiles. Cell Rep. 27, 3752–3759.e4 (2019).
Gandal, M. J. et al. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science 362, eaat8127 (2018).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Koopmans, F. et al. SynGO: an evidence-based, expert-curated knowledge base for the synapse. Neuron 103, 217–234.e4 (2019).
Vaquero-Garcia, J. et al. A new view of transcriptome complexity and regulation through the lens of local splicing variations. eLife 5, e11752 (2016).
Shemesh, O. A. et al. Precision calcium imaging of dense neural populations via a cell-body-targeted calcium indicator. Neuron 107, 470–486.e11 (2020).
Watanabe, A. et al. Fabp7 maps to a quantitative trait locus for a schizophrenia endophenotype. PLoS Biol. 5, e297 (2007).
Maekawa, M. et al. Polymorphism screening of brain-expressed FABP7, 5 and 3 genes and association studies in autism and schizophrenia in Japanese subjects. J. Hum. Genet. 55, 127–130 (2010).
Koga, M. et al. Plasma fatty acid-binding protein 7 concentration correlates with depression/anxiety, cognition, and positive symptom in patients with schizophrenia. J. Psychiatr. Res. 144, 304–311 (2021).
Hu, C., Chen, W., Myers, S. J., Yuan, H. & Traynelis, S. F. Human GRIN2B variants in neurodevelopmental disorders. J. Pharmacol. Sci. 132, 115–121 (2016).
Takasaki, Y. et al. Mutation screening of GRIN2B in schizophrenia and autism spectrum disorder in a Japanese population. Sci. Rep. 6, 33311 (2016).
Coyle, J. T. NMDA receptor and schizophrenia: a brief history. Schizophr. Bull. 38, 920–926 (2012).
Nakazawa, K. & Sapkota, K. The origin of NMDA receptor hypofunction in schizophrenia. Pharm. Ther. 205, 107426 (2020).
Trubetskoy, V. et al. Map** genomic loci implicates genes and synaptic biology in schizophrenia. Nature 604, 502–508 (2022).
Shimamoto, C. et al. Functional characterization of FABP3, 5 and 7 gene variants identified in schizophrenia and autism spectrum disorder and mouse behavioral studies. Hum. Mol. Genet. 23, 6495–6511 (2014).
Lu, W. et al. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron 29, 243–254 (2001).
Wang, L. et al. Analyses of the autism-associated neuroligin-3 R451C mutation in human neurons reveal a gain-of-function synaptic mechanism. Mol. Psychiatry https://doi.org/10.1038/s41380-022-01834-x (2022).
Singh, T. et al. Rare coding variants in ten genes confer substantial risk for schizophrenia. Nature 604, 509–516 (2022).
Autism Spectrum Disorders Working Group of The Psychiatric Genomics Consortium. Meta-analysis of GWAS of over 16,000 individuals with autism spectrum disorder highlights a novel locus at 10q24.32 and a significant overlap with schizophrenia. Mol. Autism 8, 21 (2017).
Lam, M. et al. Comparative genetic architectures of schizophrenia in East Asian and European populations. Nat. Genet. 51, 1670–1678 (2019).
Howard, D. M. et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 22, 343–352 (2019).
Stahl, E. A. et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat. Genet. 51, 793–803 (2019).
Tarabeux, J. et al. Rare mutations in N-methyl-D-aspartate glutamate receptors in autism spectrum disorders and schizophrenia. Transl. Psychiatry 1, e55 (2011).
Yu, Y. et al. Rare loss of function mutations in N-methyl-D-aspartate glutamate receptors and their contributions to schizophrenia susceptibility. Transl. Psychiatry 8, 1–9 (2018).
Yeo, G., Holste, D., Kreiman, G. & Burge, C. B. Variation in alternative splicing across human tissues. Genome Biol. 5, R74 (2004).
Raj, B. & Blencowe, B. J. Alternative splicing in the mammalian nervous system: recent insights into mechanisms and functional roles. Neuron 87, 14–27 (2015).
Vuong, C. K., Black, D. L. & Zheng, S. The neurogenetics of alternative splicing. Nat. Rev. Neurosci. 17, 265–281 (2016).
Kaalund, S. S. et al. Contrasting changes in DRD1 and DRD2 splice variant expression in schizophrenia and affective disorders, and associations with SNPs in postmortem brain. Mol. Psychiatry 19, 1258–1266 (2014).
Tan, W. et al. Molecular cloning of a brain-specific, developmentally regulated neuregulin 1 (NRG1) isoform and identification of a functional promoter variant associated with schizophrenia. J. Biol. Chem. 282, 24343–24351 (2007).
Law, A. J., Kleinman, J. E., Weinberger, D. R. & Weickert, C. S. Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Hum. Mol. Genet. 16, 129–141 (2007).
Sartorius, L. J. et al. Expression of a GRM3 splice variant is increased in the dorsolateral prefrontal cortex of individuals carrying a schizophrenia risk SNP. Neuropsychopharmacology 33, 2626–2634 (2008).
Flaherty, E. et al. Neuronal impact of patient-specific aberrant NRXN1α splicing. Nat. Genet. 51, 1679–1690 (2019).
Luza, S. et al. The ubiquitin proteasome system and schizophrenia. Lancet Psychiatry 7, 528–537 (2020).
Nishimura, A. et al. The carbohydrate deposits detected by histochemical methods in the molecular layer of the dentate gyrus in the hippocampal formation of patients with schizophrenia, Down’s syndrome and dementia, and aged person. Glycoconj. J. 17, 815–822 (2000).
Altar, C. A. et al. Deficient hippocampal neuron expression of proteasome, ubiquitin, and mitochondrial genes in multiple schizophrenia cohorts. Biol. Psychiatry 58, 85–96 (2005).
Rubio, M. D., Wood, K., Haroutunian, V. & Meador-Woodruff, J. H. Dysfunction of the ubiquitin proteasome and ubiquitin-like systems in schizophrenia. Neuropsychopharmacology 38, 1910–1920 (2013).
Bousman, C. A. et al. Elevated ubiquitinated proteins in brain and blood of individuals with schizophrenia. Sci. Rep. 9, 2307 (2019).
Tai, H.-C., Besche, H., Goldberg, A. L. & Schuman, E. M. Characterization of the brain 26S proteasome and its interacting proteins. Front. Mol. Neurosci. 3, 12 (2010).
Bingol, B. et al. Autophosphorylated CaMKIIα acts as a scaffold to recruit proteasomes to dendritic spines. Cell 140, 567–578 (2010).
Ferreira, J. S. et al. GluN2B-containing NMDA receptors regulate AMPA receptor traffic through anchoring of the synaptic proteasome. J. Neurosci. 35, 8462–8479 (2015).
Scudder, S. L. et al. Synaptic strength is bidirectionally controlled by opposing activity-dependent regulation of Nedd4-1 and USP8. J. Neurosci. 34, 16637–16649 (2014).
Huo, Y. et al. The deubiquitinating enzyme USP46 regulates AMPA receptor ubiquitination and trafficking. J. Neurochem. 134, 1067–1080 (2015).
Fu, A. K. Y. et al. APCCdh1 mediates EphA4-dependent downregulation of AMPA receptors in homeostatic plasticity. Nat. Neurosci. 14, 181–189 (2011).
Weinberger, D. R. Future of days past: neurodevelopment and schizophrenia. Schizophr. Bull. 43, 1164–1168 (2017).
Murray, R. M. & Lewis, S. W. Is schizophrenia a neurodevelopmental disorder? Br. Med J. 295, 681–682 (1987).
Raedler, T. J., Knable, M. B. & Weinberger, D. R. Schizophrenia as a developmental disorder of the cerebral cortex. Curr. Opin. Neurobiol. 8, 157–161 (1998).
Sloan, S. A. et al. Human astrocyte maturation captured in 3D cerebral cortical spheroids derived from pluripotent stem cells. Neuron 95, 779–790.e6 (2017).
Garrido-Martín, D., Palumbo, E., Guigó, R. & Breschi, A. ggsashimi: Sashimi plot revised for browser- and annotation-independent splicing visualization. PLOS Comput. Biol. 14, e1006360 (2018).
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 https://doi.org/10.1186/s13059-017-1382-0 (2018).
Young, M. D. et al. Single-cell transcriptomes from human kidneys reveal the cellular identity of renal tumors. Science 361, 594–599 (2018).
Blighe, K., Rana, S. & Lewis, M. EnhancedVolcano: Publication-ready volcano plots with enhanced colouring and labeling. R package version 1.18.0, https://github.com/kevinblighe/EnhancedVolcano (2023).
**, K. et al. An interactive single cell web portal identifies gene and cell networks in COVID-19 host responses. iScience 24, 103115 https://doi.org/10.1016/j.isci.2021.103115 (2021).
Kaimal, V. et al. ToppCluster: a multiple gene list feature analyzer for comparative enrichment clustering and network-based dissection of biological systems. Nucleic Acids Res. 38, W96–102 https://doi.org/10.1093/nar/gkq418 (2010).
Acknowledgements
We thank Kelly Rangel (CCHMC gene expression core) for assistance with 10X scRNAseq. We also thank Dr. Jim Chambers (IALS Light Microscopy core) for assistance in gathering all microscopy data in the Light Microscopy Facility and Nikon Center of Excellence. We appreciate members of the Pak lab for experimental assistance and helpful discussions. We also thank Dr. Zhi** Pang for sharing the psychiatric risk summary gene list for disease enrichment analysis. This work was supported by NIMH (R01 MH122519 to C.P., R21 MH130843 to Y.S. and C.P.), UMass IALS/BMB faculty start up fund (to C.P.), Tourette Association of America (Young investigator award to C.P.), and NIGMS T32 BTP training program (T32 GM135096 to N.P.).
Author information
Authors and Affiliations
Contributions
R.S., R.B., Y.J.S., and J.B. cultured brain organoids and performed experiments. K.J. carried out all scRNAseq data analysis. R.S. and N.P. conducted live Ca2+ imaging and analysis. A.P. and R.B. optimized single cell dissociation protocol. R.G. optimized image analysis. R.S., K.J., Y.B.S., B.A., and C.P. designed the experiments and R.S., K.J., and C.P. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sebastian, R., **, K., Pavon, N. et al. Schizophrenia-associated NRXN1 deletions induce developmental-timing- and cell-type-specific vulnerabilities in human brain organoids. Nat Commun 14, 3770 (2023). https://doi.org/10.1038/s41467-023-39420-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-39420-6
- Springer Nature Limited