Abstract
Background
Continuous-wave transscleral cyclophotocoagulation (CW-TSCP) is usually reserved for advanced/refractory glaucoma. Micropulse transscleral laser therapy (MPTLT) utilises short energy pulses separated by ‘off’-periods. MPTLT is postulated to have fewer complications, but its relative efficacy is not known. The National Institute for Health and Care Excellence (NICE) has deemed the evidence supporting MPTLT use of inadequate quality, limiting its use to research. This study aims to evaluate MPTLT efficacy and safety compared to CW-TSCP.
Methods
This 24-month follow-up retrospective audit included 85 CW-TSCP and 173 MPTLT eyes at a London tertiary referral centre. Primary outcome was success rate at the last follow-up; defined as at least 20% intraocular pressure (IOP) reduction with the same/fewer medications, and IOP between 6 and 18 mmHg. Secondary outcomes were acetazolamide use and success rates per glaucoma type. Safety outcomes were reported as complication rates.
Results
By 24-months, mean IOP reduced from 34.6[±1.4]mmHg to 19.0[ ± 3.0]mmHg post-CW-TSCP (p < 0.0001); and from 26.1[±0.8]mmHg to 19.1[±2.2]mmHg post-MPTLT (p < 0.0001). Average IOP decreased by 45.1% post-CW-TSCP, and 26.8% post-MPTLT. Both interventions reduced medication requirements (p ≤ 0.05). More CW-TSCP patients discontinued acetazolamide (p = 0.047). Overall success rate was 26.6% for CW-TSCP and 30.6% for MPTLT (p = 0.83). Only primary closed-angle glaucoma saw a significantly higher success rate following CW-TSCP (p = 0.014). CW-TSCP complication rate was significantly higher than MPTLT (p = 0.0048).
Conclusion
Both treatments significantly reduced IOP and medication load. CW-TSCP had a greater absolute/proportionate IOP-lowering effect, but it carried a significantly greater risk of sight-threatening complications. Further prospective studies are required to evaluate MPTLT compared to CW-TSCP.
Similar content being viewed by others
Introduction
Glaucoma is one of the leading causes of irreversible blindness worldwide [1]. Elevated intraocular pressure (IOP) may be associated with chronic progressive optic nerve neuropathy characterised by damage to the optic nerve head (ONH), loss of retinal ganglion cells and visual field loss [1]. Raised IOP is the main known modifiable risk factor for glaucoma; all current glaucoma therapies aim to slow or halt disease progression, either by increasing aqueous humour (AH) outflow or by decreasing its production [2].
The ciliary body (CB) consists of an anterior portion, the pars plicata, and posterior portion, the pars plana [3]. The pars plicata epithelium is the principal source of AH [4, 5]. Destruction of the CB secretory epithelium, via transscleral cyclophotocoagulation (TSCP), has a well-established role in the treatment of uncontrolled glaucoma [6]. CW-TSCP transmits laser energy through the overlying conjunctiva and sclera, to target ciliary epithelium and stroma where it has a coagulative effect; the contact probe causes compression of the tissues, aiding transmission by minimising absorption as the energy passes through the ocular surface [7].
The original form of CW-TSCP delivers laser energy in a constant manner while the laser is ‘on’ (i.e., being applied), leading to the term continuous-wave transscleral cyclophotocoagulation (CW-TSCP) [8]. When directed at the pars plicata, the 810 nm laser energy causes epithelium ablation, resulting in homogenous blanching and shrinking of the ciliary processes, reducing AH production [9]. CW-TSCP (often referred to in the UK as ‘cyclodiode’) was initially reserved for refractory glaucoma or eyes with poor visual potential due to reports of reduced visual acuity following treatment and its perceived risk of sight-threatening complications [10]. Later publications propose, and largely support, its use earlier in the treatment process [11, 12].
There is some evidence that CW-TSCP, and other earlier forms of CW-TSCP such as neodymium:yttrium aluminium garnet (NdYAG)-TSCP, may also act as an outflow procedure [13, 14]. This might be explained by damage to the CB that renders it more permeable to aqueous outflow.
A more recent modality of transscleral laser energy delivery, micropulse transscleral laser therapy (MPTLT), administers laser energy directed at the pars plana in a series of repetitive, short pulses separated by rest (‘off’) periods [15]. It is theorised that the ‘off’ periods allow adjacent tissues to dissipate heat energy, preventing them reaching coagulative threshold [15]; this has therefore led to the perception that micropulse is a ‘non-thermal’ laser treatment. MPTLT, proposed to be as effective as CW-TSCP with fewer complications, was widely adopted in the UK from 2016 in earlier disease and in better sighted eyes than had been routinely treated by CW-TSCP [16].
It has been proposed that MPTLT may also act in part on aqueous outflow, based initially on an experimental study of autopsy eyes that used discrete spots of applied laser energy as opposed to the swept form of treatment employed in MPTLT [22].
All patients received a sub-conjunctival injection with betamethasone 0.5 ml of 4 mg/ml at the conclusion of surgery, followed by dexamethasone drops 0.1% 4x/day for at least 4 weeks, longer at the discretion of the operating surgeon [22].
Data
Baseline parameters measured included: age at operation, patient-identified gender, reported ethnicity, co-morbidities, glaucoma type, and details of previous ocular surgeries.
The following variables were reviewed pre-operatively, at 1 day, 1 week, and 1, 3, 6, 12, 18, and 24 months after treatment: IOP measured by Goldmann applanation tonometry, number/type of glaucoma medications, complications, repeat procedures, and the need for subsequent alternative glaucoma surgery. We aimed to collect data from appointment records as close as possible to the desired follow-up time where available.
Surgical success
The primary outcome was treatment success, classified as success at the last available follow-up. Follow-up was categorised into success or failure. Success was defined as at least 20% IOP reduction from the pre-operative measurement resulting in an absolute IOP between 6 and 18 mmHg, with the same or fewer glaucoma medications [16, 24, 25]. Failure was defined as an inability to reach success at two consecutive visits, an increased number of glaucoma medications, and a need for an additional or alternative glaucoma surgery.
Definition of success required both a proportional IOP reduction and quantitative value within a set range, to prevent undue favourable results for eyes with pre-operative IOP within a normal range.
Secondary outcomes of efficacy were the proportion of patients who discontinued oral acetazolamide (Diamox®) use, post-laser number of repeat/further treatments, and success rate per glaucoma type. Patients requiring further surgery were included up until the last available follow-up before the next intervention.
Safety outcomes were the incidence of individual complications and the overall complication rate following both procedures. Individual complications included hypotony, persistent hypotony, macular oedema, uveitis, hyphaema, phthisis bulbi, corneal epithelial defect, corneal oedema/Descemet’s folds. Hypotony was defined as an IOP less than or equal to 5 mmHg. Persistent hypotony was defined as hypotony over two consecutive follow-up visits lasting more than 90 days, or hypotony leading to choroidal detachment/effusion [26]. The overall complication rate was defined as the proportion of patients that experienced at least one complication during the follow-up period.
Statistical analysis
All statistical analyses were performed using GraphPad Prism analysis software. A p value of ≤0.05 was considered statically significant. All graphs were produced using GraphPad Prism, all tables were produced using Microsoft Excel.
When comparing two independent proportions, a z-test was used. Chi-square testing was used for categorical values. A Shapiro-Wilk test was used to test for normality of continuous data. For non-parametric data, a Wilcoxon pairs signed-rank test (paired samples) or a Mann–Whitney U test (independent samples) was used. Student’s paired t test was used for parametric data.
The probability of success was analysed using a Kaplan-Meier graph with subsequent Mantel-Cox testing.
Results
Table 1 summarises patient demographic information, glaucoma aetiologies and prior interventions. A total of 85 eyes underwent CW-TSCP and 173 eyes underwent MPTLT. The MPTLT treatment arm had significantly more patients with primary open angle glaucoma (POAG), whereas, the CW-TSCP treatment arm had significantly more patients with neovascular and aqueous misdirection glaucoma. The two groups did not differ significantly in terms of age or self-declared gender.
Type II diabetes mellitus (T2DM) was present in 22.4% and 20.8% of CW-TSCP patients and MPTLT patients, respectively. Similarly, 51.8% of CW-TSCP patients and 46.2% of MPTLT patients had systemic hypertension. 25.9% of CW-TSCP patients and 18.5% of MPTLT patients also had dyslipidaemia. The proportion of patients with these co-morbidities was not significantly different between both groups (p > 0.05, Z-test).
Effect on IOP
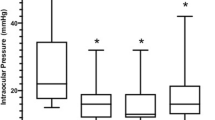
Following both CW-TSCP and MPTLT, IOP reduced significantly compared to baseline at all follow-up intervals up to 24 months (Fig. 1a).
A The effect of CW-TSCP (white circle) and MPTLT (black circle) on IOP. B The effect of CW-TSCP (white bar) and MPTLT (black bar) on the average number of glaucoma medications required. Vertical error bars represent SEM. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001—Wilcoxon matched pairs signed-rank test comparing pre-operative vs follow-up IOP after CW-TSCP (grey asterisks)/MPTLT (black asterisks). Paired t-test used for day-1, 3-months, 6-months MPTLT (parametric). Mann-Whitney U test comparing pre-operative and follow-up average number of medications after CW-TSCP (grey asterisks)/MPTLT (black asterisks). CW-TSCP continuous wave transscleral cyclophotocoagulation, MPTLT micropulse transscleral laser therapy, IOP intraocular pressure, n number of eyes at follow-up.
After CW-TSCP, mean IOP reduced from 34.6[ ± 1.4] mmHg pre-op to 19.0[ ± 3.0] mmHg at 24 months (45.1% reduction); after MPTLT, mean IOP reduced from 26.1[ ± 0.8] mmHg pre-op to [19.1 ± 2.2] mmHg at 24 months (26.8% reduction)—see Fig. 1a.
Effect on glaucoma medications
Figure 1b illustrates the average number of glaucoma medications throughout follow-up.
Both interventions significantly spared glaucoma treatments at 24 months (p ≤ 0.05); after CW-TSCP, medication use decreased from a mean of 2.1[ ± 1.1] to 1.5[ ± 0.2] agents, and after MPTLT, from 2.4[ ± 1.2] to 1.7[ ± 0.2] agents—see Fig. 1b.
In total, 57 eyes and 31 eyes undergoing MPTLT and CW-TSCP respectively, required acetazolamide pre-treatment. Significantly more of these patients were able to discontinue acetazolamide use after CW-TSCP (77.4%) compared to MPTLT (56.1%, p = 0.047).
Overall treatment success
Kaplan-Meier survival analysis showed comparable probability of overall success for both treatments by 24 months; for CW-TSCP 26.6[ ± 6.2]% and for MPTLT 30.6[ ± 4.3]%—see Fig. 2.
Overall success: success at the last available follow-up. Success at follow-up: ≥ 20% IOP reduction AND IOP 6–18 mmHg, with the same or fewer glaucoma medications. Failure: an inability to reach either success criteria at 2 successive visits/an increased number of glaucoma medications/a need for an additional laser treatment/a need for alternative glaucoma surgery. 85 eyes underwent CW-TSCP, 173 eyes underwent MPTLT. Vertical bars represent SEM. Mantel-Cox test was used to test for significance between two curves (p = 0.94). CW-TSCP continuous wave transscleral cyclophotocoagulation, MPTLT micropulse transscleral laser therapy, IOP intraocular pressure.
Significantly more CW-TSCP eyes received repeat laser treatment (21/85 eyes, 24.7%) compared to MPTLT (21/173 eyes, 12.1%), p = 0.01.
However, a significantly higher proportion of MPTLT (54/173, 31.2%) eyes required a further alternative glaucoma intervention post-treatment compared to CW-TSCP (9/85, 10.6%, p = 0.0003).
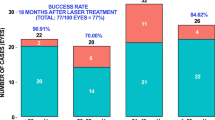
Success rate per glaucoma type
Table 2 summarises the overall success rate for each glaucoma type. Patients with primary closed angle (PCA) had a significantly higher success rate following CW-TSCP compared to MPTLT.
Safety outcomes
Table 3 illustrates the proportion of eyes experiencing each type of complication. There were no cases of phthisis bulbi and hyphaema after MPTLT. The most common complication for both treatments was hypotony, with incidence of 18.8% and 8.1% for CW-TSCP and MPTLT, respectively. Following CW-TSCP, one of the patients with persistent hypotony experienced choroidal detachment. One CW-TSCP eye required evisceration. CW-TSCP was associated with a significantly higher overall complication rate than MPTLT (33.3% v 17.6%, p = 0.0048).
36.7% and 15.8% of POAG eyes had post-operative complications following CW-TSCP and MPTLT, respectively.
All PCA glaucoma eyes had complications after CW-TSCP, while only 25% had complications after MPTLT.
Discussion
This study demonstrates that both CW-TSCP and MPTLT procedures significantly reduce IOP, with a greater absolute and proportional IOP reduction seen after CW-TSCP.
The day-1 rapid reduction seen after both procedures suggests that the immediate but transient effect may be secondary to active inflammation, which increases uveoscleral outflow and decreases AH production [27].
Although pre-operative IOP in the CW-TSCP cohort was significantly higher than MPTLT, average IOP by the end of follow-up was lower for CW-TSCP than for MPTLT. These results may indicate that CW-TSCP has a more efficacious IOP-lowering effect, but a further confounding factor may be that clinicians tend to titrate medical treatments to a target after surgical or laser treatment. Further studies with similar pre-operative IOP in both treatment arms would be helpful to further investigate this [16, 24].
The initial IOP was higher in the CW-TSCP group, which could indicate a selection bias if the treatment was reserved for more advanced cases.
At the end of follow-up, both treatments had a comparable probability of success (26.6[ ± 6.2]% for CW-TSCP, 30.6[ ± 4.13]% for MPTLT). Tan et al. defined success as at least 30% IOP reduction or an IOP between 6 and 21 mmHg and reported a 73% success rate for MPTLT [7]. Williams et al. reported MPTLT success rate of 66% and required at least 20% IOP reduction or IOP between 6 and 21 mmHg [28]. It is evident that heterogeneity in success criteria used between studies may contribute to the disparity in reported treatment success rates.
Most studies require either a minimum percentage IOP reduction or an absolute IOP (‘or’ criteria), while the present study requires both (‘and’ criteria) [7, 16, 28, 29]. Although ‘and’ criteria may place high expectations of success, this criterion may help reduce undue favourable results [30]. Patients starting and remaining within normal IOP ranges would be regarded as success by default, when using ‘or’ criteria [31].
Souissi et al. report a similar MPTLT success rate to our study, utilising similar success criteria [32]. However, the inclusion of only treatment naïve patients does not reflect common clinical practice [21, 33]. Additionally, while repeat treatments are common in clinical practice, the lack of reporting success rate per treatment course in many studies further limits comparison [34, 35]. Therefore, both unified success criteria and eligibility criteria are vital to allow valid comparisons across studies.
Comorbidities prevalent study cohorts, such as T2DM, may have affected treatment outcomes, given that they may be associated with a risk of elevated IOP [36,37,38,39]. A further study refinement would be to more closely match for such characteristics.
Another confounder could be ethnicity. Studies have reported higher inflammation rates amongst black and Asian patients [28, 40]. Williams et al. report 3.6 greater odds of prolonged inflammation in black patients, possibly associated with darker pigmentation leading to increased energy absorption [28]. In our study, 10.6% of CW-TSCP and 13.3% of MPTLT patients identified as ethnically black. Nevertheless, undue significance should not be placed on the effect of ethnicity given the underrepresentation of some ethnic groups in our cohort.
The precise IOP-lowering mechanism of action of MPTLT has not been fully elucidated. Suggested mechanisms for the effects of MPTLT include decreased AH production, increased uveoscleral outflow and increased trabecular outflow [41]. Some cadaveric studies have not shown significant CB histological changes, suggesting that the effects of MPTLT may be at least partly independent of its effects on AH production [42]. Barac et al. reported that successfully treated patients showed increased choroid thickness suggesting a role for increased uveoscleral outflow—however, their sample size (n = 22) was too small to determine statistical significance [43].
As mentioned previously, an experimental study by Johnstone et al. on monkey eyes reported shortening of the CB longitudinal muscles, which has been interpreted as showing that enlarged trabecular spaces may facilitate outflow [51, 52].
In total, 36.7% and 15.8% of POAG eyes had post-operative complications following CW-TSCP and MPTLT, respectively. Previous studies, however, with shorter follow-up found no complications in POAG eyes after MPTLT [53].
Interestingly, all PCA eyes had complications after CW-TSCP compared to 25% seen post-MPTLT. Undue significance should not be placed on this finding given the small sample size of PCA eyes.
Limitations of our retrospective study include the disparity in the size of both arms reducing the statistical power of our study.
From 2013 to 16 only CW-TSCP was available; although both treatments were available from 2016 onwards, an increasing proportion of cases being treated with MPTLT after that time.
The COVID pandemic also adversely affected patient follow-up, limiting our ability to draw conclusions on mean IOP reduction. Virtual clinics limited the availability of IOP measurements [54]. Furthermore, during the pandemic MPTLT and CW-TSCP were commonly used as a temporizing procedure instead of more invasive surgeries [55]. This led to a change in treatment patterns with larger numbers of MPTLT procedures performed in situations when this treatment may not previously have been the first-line treatment choice. This may have affected its success rates and might have contributed to the observation that significantly more MPTLT patients required an alternative intervention.
Conclusions
In conclusion, our study suggests that CW-TSCP is more efficacious in lowering IOP, with a greater absolute and proportionate IOP-lowering effect than MPTLT. However, this needs to be balanced against its increased risk of sight-threatening effects. Both treatments resulted in a significant and sustained IOP reduction, with similar overall success rates by our criteria. Both treatments facilitated a significant reduction in topical medication load, although a greater proportion of patients were able to discontinue acetazolamide use after CW-TSCP than after MPTLT.
We recognise the need for multi-centre prospective studies to more accurately evaluate the efficacy of MPTLT compared to CW-TSCP. We propose the adoption of standardised success criteria, such as those utilised in this study, to facilitate future comparisons.
Summary
What was known before
-
Continuous-wave transscleral cyclophotocoagulation (CW-TSCP) is usually reserved for advanced/refractory glaucoma
-
Micropulse transscleral laser therapy (MPTLT) utilises short energy pulses separated by ‘off’-periods. It is postulated that MPTLT is associated with fewer complications.
-
NICE has deemed the evidence supporting MPTLT use of inadequate quality, limiting its use to research.
What this study adds
-
Both treatments significantly reduced IOP and medication load.
-
CW-TSCP had a greater absolute/proportionate IOP-lowering effect.
-
MPTLT had a significantly lower complication rate, while CW-TSCP carried significant risk of sight-threatening complications.
-
This study adds to the evidence base for MPTLT, to try and resolve what NICE deems as ‘inadequate’ evidence.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage.
References
Quigley H, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7.
Killer HE, Pircher A. What is the optimal glaucoma treatment: reducing aqueous humour production or facilitating its outflow? Eye (Basingstoke) Springe Nat. 2020;34:1719–21.
Tamm E The Role of the Ciliary Body in Aqueous Humor Dynamics Structural Aspects. In: Dartt Darlene A, editor. Encyclopedia of the Eye. p. 179–86. (2010).
Evans M, Yanoff M, Duker J Anatomy of the Uvea. In: Yanoff M, editor. Ophthalmology. 5th ed. p. 689–91. (2019).
Gelatt KN, Wilkie DA Chapter 9 - Surgical procedures of the anterior chamber and anterior uvea. In: Gelatt KN, Gelatt JP, editors. Veterinary Ophthalmic Surgery [Internet]. Edinburgh: W.B. Saunders; 2011. p. 237–62. Available from: https://www.sciencedirect.com/science/article/pii/B9780702034299000092.
Kosoko O, Gaasterland DE, Pollack IP, Enger CL. Long, term outcome of initial ciliary ablation with contact diode laser t ransscleral cyclophotocoagulation for severe glaucoma. Ophthalmology. 1996;103:1294–302.
Tan AM, Chockalingam M, Aquino MC, Lim ZIL, See JLS, Chew PT. Micropulse transscleral diode laser cyclophotocoagulation in the treatment of refractory glaucoma. Clin Exp Ophthalmol. 2010;38:266–72.
Yelenskiy A, Gillette TB, Arosemena A, Stern AG, Garris WJ, Young CT, et al. Patient outcomes following micropulse transscleral cyclophotocoagulation: Intermediate-term results. J Glaucoma. 2018;27:920–5.
Allbon D, Meyer JJ. Cyclodiode laser glaucoma therapy. StatPearls. (2021). Available from: https://www.ncbi.nlm.nih.gov/books/NBK574570/.
Bloom PA, Tsai JC, Sharma K, Miller MH, Rice NSC, Hitchings RA, et al. “Cyclodiode” Trans… scleral Diode laser cyclophotocoagulation in the treatment of advanced refractory glaucoma. Ophthalmology. 1997;104:1508–19.
Rotchford AP, Jayasawal R, Madhusudhan S, Ho S, King AJ, Vernon SA. Transscleral diode laser cycloablation in patients with good vision. Br J Ophthalmol. 2010;94:1180–3.
Ansari E, Gandhewar J. Long-term efficacy and visual acuity following transscleral diode laser photocoagulation in cases of refractory and non-refractory glaucoma. Eye. 2007;21:936–40.
Schubert HD, Agarwala A, Arbizo V. Changes in aqueous outflow after in vitro neodymium: yttrium aluminum garnet laser cyclophotocoagulation. Investig Ophthalmol Vis Sci. 1990;31:1834–8.
Bloom PA, Dharmaraj S. Endoscopic and transscleral cyclophotocoagulation. Br J Ophthalmol. 2006;90:665–6.
Malik KJ, Sampat KM, Mansouri A, Steiner JN, Glaser BM. Low-intensity/high-density subthreshold micropulse diode laser for chronic central serous chorioretinopathy [Internet]. Available from: http://journals.lww.com/retinajournal.
Aquino MCD, Barton K, Tan AMWT, Sng C, Li X, Loon SC, et al. Micropulse versus continuous wave transscleral diode cyclophotocoagulation in refractory glaucoma: A randomized exploratory study. Clin Exp Ophthalmol. 2015;43:40–6.
Johnstone MA, Song S, Padilla S, Wen K, **n C, Wen JC, et al. Microscope real-time video (MRTV), high- resolution OCT (HR-OCT) & histopathology (HP) to assess how transcleral micropulse laser (TML) affects the sclera, ciliary body (CB), muscle (CM), secretory epithelium (CBSE), suprachoroidal space (SCS) & aqueous outflow system. Invest Ophthalmol Vis Sci. 2019;60:2825.
NICE. Repetitive short-pulse transscleral cyclophotocoagulation for glaucoma Interventional procedures guidance Your responsibility Your responsibility [Internet]. 2021. Available from: www.nice.org.uk/guidance/ipg692.
National Institute for Health and Care Excellence IP1779 Repetitive short-pulse transscleral cyclophotocoagulation for glaucoma [Internet]. 2021. Available from: https://www.nice.org.uk/guidance/ng81/chapter/Recomm.
Grippo TM, de Crom RM, Giovingo M, Töteberg-Harms M, Francis BA, Jerkins B, et al. Evidence-based consensus guidelines series for micropulse transscleral laser therapy: dosimetry and patient selection. Clin Ophthalmol. 2022;ume 16:1837–46.
NICE. Glaucoma: diagnosis and management NICE guideline [Internet]. 2017. Available from: www.nice.org.uk/guidance/ng81.
Vig N, Ameen S, Bloom P, Crawley L, Normando E, Porteous A, et al. Micropulse transscleral cyclophotocoagulation: initial results using a reduced energy protocol in refractory glaucoma. Graefe’s Arch Clin Exp Ophthalmol. 2020;258:1073–9.
Iridex. Iridex Cyclo G6 ® Laser System Operator Manual. (2021).
Abdelrahman AM, El Sayed YM. Micropulse versus continuous wave transscleral cyclophotocoagulation in refractory pediatric glaucoma. J Glaucoma. 2018;27:900–5.
Marchand M, Singh H, Agoumi Y. Micropulse trans-scleral laser therapy outcomes for uncontrolled glaucoma: a prospective 18-month study. Can J Ophthalmol. 2021;56:371–8.
Rabiolo A, Leadbetter D, Anand N. Hypotony-associated complications after deep sclerectomy: incidence, risk factors, and long-term outcomes. J Glaucoma. 2021;30:E314–26.
Toris CB, Pederson JE. Aqueous humor dynamics in experimental iridocyclitis. Invest Ophthalmol Vis Sci. 1987;28:477–81.
Williams AL, Moster MR, Rahmatnejad K, Resende AF, Horan T, Reynolds M, et al. Clinical efficacy and safety profile of micropulse transscleral cyclophotocoagulation in refractory glaucoma. J Glaucoma. 2018;27:445–9.
Cheung JJC, Li KKW, Tang SWK. Retrospective review on the outcome and safety of transscleral diode laser cyclophotocoagulation in refractory glaucoma in Chinese patients. Int Ophthalmol. 2019;39:41–6.
Shaarawy T, Grehn F, Sherwood M, World Glaucoma Association. WGA guidelines on design and reporting of glaucoma surgical trials. Kugler Publications; 83 p. (2009).
Yap TE, Ahmed F, Bloom PA Re: Gedde et al.: Treatment outcomes in the Primary Tube Versus Trabeculectomy (PTVT) study after 3 years of follow-up (Ophthalmology. 127:333–345). Vol. 127, Ophthalmology. Elsevier Inc.; 2020. p. e80–1. (2020).
Souissi S, Baudouin C, Labbé A, Hamard P. Micropulse transscleral cyclophotocoagulation using a standard protocol in patients with refractory glaucoma naive of cyclodestruction. Eur J Ophthalmol. 2021;31:112–9.
Malik R, Ellingham RB, Suleman H, Morgan WH. Refractory glaucoma - Tube or diode? Clin Exp Ophthalmol. 2006;34:771–7.
Pucci V, Tappainer F, Borin S, Bellucci R. Long-term follow-up after transscleral diode laser photocoagulation in refractory glaucoma. Ophthalmol [Internet]. 2003;217:279–83. https://www.karger.com/DOI/10.1159/000070635.
Spencer AF, Vernon SA. Cyclodiode”: results of a standard protocol. Br J Ophthalmol. 1999;83:311–6.
Okeke CO, Quigley HA, Jampel HD, Ying Gshuang, Plyler RJ, Jiang Y, et al. Adherence with topical glaucoma medication monitored electronically. Travatan Dosing Aid Study Ophthalmol. 2009;116:191–9.
Klein BEK, Klein R, Knudtson MD. Intraocular pressure and systemic blood pressure: Longitudinal perspective: The Beaver Dam Eye Study. Br J Ophthalmol. 2005;89:284–7.
Bulpitt CJ, Hodes C, Everitt MG. Intraocular pressure and systemic blood pressure in the elderly. Br J Ophthalmol. 1975;59:717–20.
Biswas S, Raman R, Koluthungan V, Sharma T. Intraocular pressure and its determinants in subjects with type 2 diabetes mellitus in India. J Prevent Med Public Health. 2011;44:157–66.
Madjedi KM, Stuart KV, Chua SYL, Luben RN, Warwick A, Pasquale LR, et al. The association between serum lipids and intraocular pressure in two large UK cohorts. Ophthalmology [Internet]. 2022;129:986–996. https://linkinghub.elsevier.com/retrieve/pii/S0161642022003116.
Emanuel ME, Grover DS, Fellman RL, Godfrey DG, Smith O, Butler MR, et al. Micropulse cyclophotocoagulation: initial results in refractory glaucoma. J Glaucoma. 2017;26:726–9.
Abdelmassih Y, Tomey K, Khoueir Z Micropulse transscleral cyclophotocoagulation. Journal of Current Glaucoma Practice. Jaypee Brothers Medical Publishers (P) Ltd; p. Vol. 15, 1–7. (2021).
Moussa K, Feinstein M, Pekmezci M, Lee JH, Bloomer M, Oldenburg C, et al. Histologic changes following continuous wave and micropulse transscleral cyclophotocoagulation: A randomized comparative study. Transl Vis Sci Technol. 2020;9:1–9.
Barac R, Vuzitas M, Balta F. Choroidal thickness increase after micropulse transscleral cyclophotocoagulation. Rom J Ophthalmol. 2018;61:144–8.
Schlote T, Derse M, Rassmann K, Nicaeus T, Dietz K, Thiel HJ. Efficacy and Safety of Contact Transscleral Diode Laser Cyclophotocoagulation for Advanced Glaucoma [Internet]. 2001. Available from: http://journals.lww.com/glaucomajournal.
Ishida K. Update on results and complications of cyclophotocoagulation. Curr Opin Ophthalmol. 2013;24:102–10.
Wong KYT, Aquino CM, Macasaet AM, Suwandono ME, Chew PTK, Koh VTC. MP3 plus: a modified micropulse transscleral cyclophototherapy technique for the treatment of refractory glaucoma. J Glaucoma. 2020;29:264–70.
Tekeli O, Köse HC. Outcomes of micropulse transscleral cyclophotocoagulation in primary open-angle glaucoma, pseudoexfoliation glaucoma, and secondary glaucoma. Eur J Ophthalmol. 2021;31:1113–21.
Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA—J Am Med Assoc Am Med Assoc. 2014;311:1901–11.
Schmack I, Völcker HE, Grossniklaus HE Chapter 54 - Phthisis bulbi. In: Levin LA, Albert DM, editors. Ocular Disease [Internet]. Edinburgh: W.B. Saunders; 2010. p. 415–23. Available from: https://www.sciencedirect.com/science/article/pii/B9780702029837000541.
Feldman R, El-Harazi SM, LoRusso FJ, McCash C, Warner PA. Histopathologic findings following contact transscleral semiconductor diode laser cyclophotocoagulation in a human eye. J Glaucoma. 1997;6:139–40.
Dastiridou AI, Katsanos A, Denis P, Francis BA, Mikropoulos DG, Teus MA, Konstas AG. Cyclodestructive procedures in glaucoma: a review of current and emerging options. Adv Ther [Internet]. 2018;35:2103–2127. https://doi.org/10.6084/m9.figshare.7291136.
Tong W, Shen T, Aquino M, Chew P, Lim D. One-year outcomes of micropulse cyclophototherapy for primary open-angle glaucoma. J Glaucoma. 2021;30:911–20.
Lakhani BK, Attzs MS, Stead R, Tambe K. The impact of the COVID-19 pandemic on ophthalmology services across the United Kingdom: a brief report on a cross-sectional survey of clinical leads. Ther Adv Ophthalmol [Internet]. 2021;13:25158414211010548 https://doi.org/10.1177/25158414211010549.
Holland LJ, Mercieca KJ, Kirwan JF. Effect of COVID-19 pandemic on glaucoma surgical practices in the UK. Br J Ophthalmol. 2022;106:1406–1410. https://doi.org/10.1136/bjophthalmol-2021-319062.
Acknowledgements
We would like to thank and acknowledge locum consultants (Dimitrios Besinis, Alastair Porteous, Joanna Tryfinopoulou, Ahmed Al-Nahrawy, Nada Mohamed), the fellows and trainees at the Western Eye Hospital for their contribution in caring for study patients.
Author information
Authors and Affiliations
Contributions
PB conceived and directed the project. MK was responsible for collected data, performed statistical analysis, and wrote the first draft of the manuscript. PB and EN were responsible for designing the review, contributed to writing the final report, and interpreting results. FC, LC, FA, NV and SA critically revised the manuscript and provided feedback on the report.
Corresponding author
Ethics declarations
Competing interests
PB received consultancy fees from BVI and travelling expenses and honoraria from Iridex. EMN has received travelling expenses from Iridex. MK, FMC, LC, FA, SA, NV declare no potential conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kelada, M., Normando, E.M., Cordeiro, F.M. et al. Cyclodiode vs micropulse transscleral laser treatment. Eye 38, 1477–1484 (2024). https://doi.org/10.1038/s41433-024-02929-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-024-02929-1
- Springer Nature Limited