Abstract
Thermal behavior, molecular aggregation structure, and water repellency for poly{2-[(perfluorooctylethyl)carbamate]ethyl}acrylate (PFAUr-C8) were studied by differential scanning calorimetry (DSC), polarized optical microscopy (POM), wide-angle X-ray diffraction (WAXD), grazing-incidence wide-angle X-ray diffraction (GI-WAXD), contact angle measurements, and X-ray photoelectron spectroscopy (XPS). The impact of the carbamate linker was verified by comparison with poly[2-(perfluorooctyl)ethyl]acrylate (PFA-C8). PFAUr-C8 exhibited two phase transitions at 357 K and 416 K, whereas PFA-C8 showed a single-phase transition at 347 K. Both PFAUr-C8 and PFA-C8 formed a bilayered hexatic smectic-B phase at room temperature. Although the lamellar structure for the fluoroalkyl (Rf) groups was immediately lost in PFA-C8 after disordering of the lateral translational order, PFAUr-C8 maintained a stacked lamellar structure without lateral translational order. PFAUr-C8 exhibited excellent water repellency at room temperature similar to PFA-C8. The outstanding thermal stability of the lamellar structure in PFAUr-C8 is attributed to the cohesive interaction of the carbamate linkage.
Similar content being viewed by others
Introduction
Comb-shaped fluoropolymers bearing pendant perfluoroalkyl (Rf) side chains have attracted extensive attention in the field of surface science and engineering over the past several decades [1,2,3,4,5,6,7]. Poly[2-(perfluorooctyl)ethyl]acrylate (PFA-C8) produces a tilted hexatic smectic-B phase in which the orientational order of Rf groups breaks rotational symmetry within layers [8, 9]. The Rf side chains have the role of a mesogen to form a bilayer lamellar structure with lateral hexagonal translational order. In the PFA-C8 thin films, the Rf groups are segregated to the outermost surface because of the low surface energy of CF3 groups at the Rf chain ends following which a highly oriented anisotropic lamellar structure parallel to the surface is produced. As the surface is covered with an array of CF3 groups without considerable defects, the surface exhibits excellent liquid repellency. Therefore, the extremely low surface energy of the comb-shaped fluoropolymers is coupled with the ordered smectic arrangement of the Rf groups. Meanwhile, it has already been demonstrated that the ordered structure relies on several structural factors, including the number of fluorinated carbons [10, 11], main chain rigidity [12, 13], and linkers between Rf side chains and hydrocarbon backbones [14,15,16]. Although the comb-shaped polymers with long-chain Rf groups for which the number of fluorinated carbons ≥ 7 produce a well-ordered structure and exhibit better liquid repellency than the analogs with short-chain Rf chains, the potential toxicity and bioaccumulation of these long-chain Rf groups have been of concern [17, 18]. Therefore, it is imperative to develop environmentally friendly and high-performance alternatives.
Linkers that connect Rf side chains to hydrocarbon backbones have profound impacts on the aggregation structures and surface properties of comb-shaped fluoropolymers. The linkers can be roughly divided into two categories: flexible methylene linkers and polar linkers such as the N-methylsulfonamide linker [–N(CH3)SO2–]. The flexible methylene linkers generally yield flexibility and mobility for the Rf side chains, consequentially impairing the ordered structure and surface properties of the comb-shaped fluoropolymers [15]. To the best of our knowledge, only a few types of polar linkers have been reported, with their impact on aggregation structures and surface properties still not well-disclosed. Corpart et al. [16] reported that polyacrylates with a N-methylsulfonamide linker in the perfluorohexylethyl side chains showed a smectic-ordered structure and liquid repellency, comparable to PFA-C8. They claimed that the dipole–dipole interactions between N-methylsulfonamide groups induced the arrangement of the Rf groups [16]. The effect of the ɑ-substituents (methyl, fluoride, and chloride substituents) on the wetting behavior and molecular motion at the surface of poly(fluoroalkyl acrylate) thin films has also been investigated [12, 13]. Although poly(meth)acrylates bearing perfluorobutyl side chains were amorphous even following the incorporation of bulky α-substituents, the α-substituents significantly increased the glass transition temperature (Tg). Therefore, the main chain mobility was restricted, leading to pronounced water-repellent performance for the polymers.
In this study, we address the effect of a carbamate linker (–NHCOO–) on the ordered structure and water repellency of comb-shape fluoropolymers to expand the understanding of polar cohesive linkers. Poly{2-[(perfluorooctylethyl)carbamate]ethyl}acrylate (PFAUr-C8, Scheme 1) was prepared by a simple alcoholysis reaction of isocyanate, with its molecular aggregation structure and water repellency investigated by differential scanning calorimetry (DSC), polarized optical microscopy (POM), wide-angle X-ray diffraction (WAXD), grazing-incidence WAXD (GI-WAXD), contact angle measurements, and X-ray photoelectron spectroscopy (XPS). The role of the carbamate linker in the aggregation structure and surface properties was verified through comparison with PFA-C8.
Experimental sections
Materials
2-Isocyanatoethyl acrylate ( > 97%, Showa Denko K. K., Tokyo, Japan) and 1H,1H,2H,2H-perfluoro-1-decanol ( > 97%, Sigma-Aldrich, Tokyo, Japan) were used as received without further purification. 1H,1H,2H,2H-perfluorooctylethyl acrylate ( > 97%) was kindly provided by Daikin Industries, Ltd. (Osaka, Japan). 2,2’-Azobis-isobutyronitrile (AIBN, > 98%, Wako Pure Chemical Corp., Osaka, Japan) was recrystallized from acetone. n-Hexane ( > 98%) and tetrahydrofuran (THF > 98%) were purchased from Wako Pure Chemical Corp. and dried over activated 4A molecular sieves overnight before use. PFA-C8 [Number-average molecular weight (Mn): 7.8 k; polydispersity index: 1.91] was prepared by following the previously reported synthetic method [9].
Synthesis of {2-[(perfluorooctylethyl)carbamate]ethyl}acrylate (FAUr-C8)
2-Isocyanatoethyl acrylate (0.88 g, 6.25 mmol) was added dropwise to a stirred solution of 1H,1H,2H,2H-perfluoro-1-decanol (2.30 g, 6.31 mmol) in anhydrous n-hexane (10 mL) under a blanket of nitrogen gas. The yellowish solution was allowed to stir at 338 K for 12 h. The solvent was removed using an evaporator to give a white cream crude. The crude was then recrystallized from n-hexane to give 1.18 g (37% yield) of the pure monomer as a white solid. The Fourier transform infrared (FT-IR) and proton nuclear magnetic resonance (1H-NMR) spectra, and the peak assignments were shown in Supplementary Information as Figure S1 and Figure S3.
Synthesis of PFAUr-C8
A solution of the FAUr-C8 monomer (1.10 g, 1.82 mmol) and AIBN (16.8 mg, 0.10 mmol) in dry THF (4.5 mL) was refluxed under an argon atmosphere for 24 h. The solution gradually turned to be sticky and phase-separated as the reaction proceeded. The solution was poured into a large excess of methanol. The precipitate was filtrated followed by vacuum-drying at 323 K for 24 h to give 0.83 g (yield: 75%) of the pure polymer as a white powder. The Mn and polydispersity index were 6.3 k and 1.48, respectively.
Film preparation
PFAUr-C8 and PFA-C8 films were prepared by spin coating (2000 r.p.m., 30 s) onto disk-shaped silicon (111) wafers with a 1 inch diameter and 3 mm thickness from the 1.0 wt% polymer solutions in AK-225. The film thickness was determined to be approximately 100 nm by ellipsometry. The films were subjected to thermal annealing at a temperature of (Tm - 5) K for 24 h (Tm is the peak temperature of the first phase transition determined by DSC measurements). The thin films were stable during thermal annealing without dewetting.
Measurements
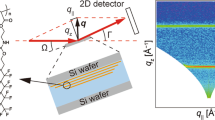
1H-NMR spectra were recorded on a Bruker Advance-400 spectrometer at 400 MHz. Deuterated chloroform (CDCl3) was used as the solvent. The chemical shifts were calibrated with the residual proton signal of CDCl3. FT-IR spectra were obtained with a Spectrum One spectrometer (PerkinElmer) equipped with a temperature-control sample holder in the transmittance mode and recorded by averaging 64 scans at a resolution of 1 cm−1 under nitrogen flow. The FT-IR spectrum for FAUr-C8 was measured at room temperature as KBr pellets. FT-IR spectra for the PFAUr-C8 films on NaCl plates were measured at various temperatures. The PFAUr-C8 AK-225 solution (1 wt %) was cast onto a NaCl plate and then dried under vacuum at ambient temperature for 2 h. The temperature was maintained constant for approximately 15 min before collection of the FT-IR spectra. Gel permeation chromatography was performed using an HLC-8220GPC (Tosoh Corp.) equipped with a refractive index detector. Polystyrene gel column of a PLgel mixed-C (Agilent Technologies, Ltd.) was connected. Measurements were performed at 310 K using 1, 1, 3, 3, 3-hexafluoro-2-propanol (Central Glass Co., Ltd.) with 1 wt% AK-225G (AGC, Inc.) as an eluent at a flow rate of 1.0 mL min−1. Molecular weights were calibrated by poly(methyl methacrylate) (PMMA) standards. DSC measurements were performed by means of a Hitachi High-Tech Science Corporation DSC6220 calorimeter at a scanning rate of 10 K min−1 under nitrogen flow in a temperature range of 173–473 K. A heating/cooling cycle was carried out before collecting the DSC data to erase the thermal history. POM observations were carried out on a Nikon Eclipse LV100 equipped with a LINKAM THMS600 thermostat stage. The powder samples were sandwiched between two glass slides with a 10 µm spacer. The POM images were recorded during the heating and cooling process at 1 K min−1 in the cross-polarized mode with a sensitive color plate at atmospheric pressure. Contact angles for water droplets on the polymer films were measured using a Theta T-200 drop shape analysis system (Biolin Scientific) equipped with a charge-coupled device camera at 295 K. The contact angles were reported as averaged values obtained from over five data. The static contact angles were recorded using a free drop of ultrapure water (2.0 µL) on the film surfaces. The advancing contact angles were measured by injecting 4.0 µL of water. The receding contact angles were measured by withdrawing 8.0 µL water from a droplet (10.0 µL). WAXD measurements were conducted at the BL05XU beamline in the SPring-8 synchrotron research facility (Hyogo, Japan), using a 941 × 1043 pixel PILATUS 1 M with a pixel size of 172 × 172 μm2 (DECTRIS, Switzerland). The distance from the sample to the detector was 338 mm and the X-ray wavelength was 0.10 nm. The powder samples were loaded into 2.0 mm ø quartz capillary tubes (Mark-tubes, Hilgenberg Co., Malsfeld, Germany); then, the tubes were subjected to thermal annealing at (Tm - 5) K for 24 h. WAXD measurements were performed under precise temperature control by using a capillary tube holder equipped with a block heater. GI-WAXD measurements were performed at the BL40B2 and BL03XU beamline in the SPring-8 [19, 20]. The wavelength of the X-ray beam was 0.10 nm and the distance from the sample to the detector was 169 mm. The diffraction patterns were acquired using a charge-integration type detector named “SOPHIAS (RIKEN, Japan),” which has a detector plane of 26.40 mm × 63.0 mm and pixel resolution of 30 μm × 30 μm [21, 22]. A homemade thermostat vacuum sample stage with a polyimide dome was used to avoid radiation damage and scattering from the air. XPS measurements were carried out at the BL12 beamline in the Kyushu Synchrotron Light Research Center (Saga, Japan). The high-resolution C1s XPS spectra were obtained using synchrotron radiation soft X-rays with an energy of 400 eV at room temperature in a vacuum of 1 × 10−9 torr and at a photoelectron take-off angle of 54°.
Results and discussions
Thermal behavior and optical textures
DSC data are listed in Table 1. Typical DSC thermograms for PFAUr-C8 and PFA-C8 in the second heating and cooling scans are shown in Fig. 1. PFAUr-C8 showed two endotherms at 357 K and 416 K with quite different transition enthalpies (12.0 J/g for the first transition, 1.3 J/g for the second transition) in the heating process, whereas PFA-C8 exhibited a single endotherm at 347 K with a relatively large transition enthalpy of 20.6 J/g. The first endothermic peak temperature for PFAUr-C8 was approximately 10 K greater than that for PFA-C8. Both polymers showed exothermic peaks during the cooling scan implying that the transitions are a rapid and thermodynamically reversible process.
To identify the thermal events, POM images were observed (Figs. 2 and 3). In the heating process, PFAUr-C8 showed a birefringent texture at 393 K; then, the optical textures disappeared at 433 K, indicating the appearance of an aligned liquid crystal phase before isotropization (Fig. 2). When the melt was cooled to 413 K (a temperature lower than the second phase transition temperature determined by DSC) and then isothermally held for 2 h, fan-like birefringent textures appeared. Subsequently, the fan-like textures were transformed to batonet-like rod-shaped textures after isothermal holding at 351 K for 8 h. Meanwhile, the PFA-C8 exhibited no birefringent textures in the heating process (Fig. 3). When the melt was cooled to 343 K, numerous birefringent microdomains developed, which then finally filled the field of view, whereas no other significant birefringent texture was observed regardless of the thermal history. These POM observations indicate that the PFAUr-C8 exhibits a phase transition to produce a liquid crystalline ordered structure before isotropization, whereas the PFA-C8 exhibits a single order–disorder phase transition. Moreover, their global ordered structures and alignment are completely different as shown in the optical texture at room temperature.
Ordered structures for PFAUr-C8 and PFA-C8 were further studied by means of WAXD. WAXD patterns for the powders showed isotropic Debye–Sheller rings. The circular averaged WAXD profiles at various temperatures are shown in Fig. 4. Diffraction peaks were observed at q = 1.55, 3.09, 4.63, 6.16, and 12.34 nm−1 for the PFAUr-C8 and q = 1.92, 3.86, 5.77, 9.61, and 12.42 nm−1 for the PFA-C8 at 303 K. In the small-angle region, the diffraction peaks appeared with nearly identical intervals indicating a periodic lamellar structure. The side chain length of the PFAUr-C8 [CH2CHCOO(CH2)2NHCO(CH2)2(CF2)7CF3] and the PFA-C8 [CH2CHCOO(CH2)2(CF2)7CF3] were calculated to be 1.81 nm and 1.39 nm, respectively, on the basis of density functional theory (DFT) calculation using the B3LYP/6-31G* functional and basis set. The d-spacings were calculated from the first diffraction peak to be 4.06 nm for the PFAUr-C8 and 3.27 nm for the PFA-C8, which are approximately consistent with the thickness of a bilayer composed of poly(acrylate) backbone and a pair of Rf side chains. In the wide-angle region, a relatively broad diffraction peak was observed at q = 12.34 nm−1 for the PFAUr-C8 and q = 12.42 nm−1 for the PFA-C8. The peak was assigned to the lateral translational distance for the Rf groups arranged in a distorted two-dimensional hexagonal lattice. Therefore, both PFAUr-C8 and PFA-C8 formed a hexatic smectic-B phase at room temperature. The diffraction peak from the hexatic order was relatively weak in the PFAUr-C8, with the full-width at half maximum broader than that of the PFA-C8, indicating that the lateral translational order of the Rf groups in PFAUr-C8 is more distorted than that in PFA-C8.
The PFA-C8 underwent an abrupt phase transition. All diffraction peaks weakened at 353 K and disappeared at 363 K simultaneously, whereas a broad amorphous hallo appeared instead, indicating that the collapse of the bilayer lamellar structure and the disordering of the lateral hexatic translational order were synchronous. The layered lamellar structure of Rf groups was no longer present without the lateral translational order. Therefore, the phase transition was assignable to a bilayered hexatic smectic-B to isotropic transition. Meanwhile, PFAUr-C8 showed complex structure transitions during heating. As mentioned above, the PFAUr-C8 forms a bilayered hexatic smectic-B phase at 303 K similar to PFA-C8. A small shoulder peak appeared at q = 1.72 nm−1 when the temperature was raised to 353 K, indicating the onset of the phase transition. At 363 K, a temperature above the first endothermic peak temperature in DSC, three new peaks appeared at q = 1.72, 3.44 and 5.15 nm−1 accompanied by a sharp drop in the diffraction peak intensities at q = 1.55, 3.09, 4.63, and 12.13 nm−1. The long period for the lamellar structure decreased from 4.19 to 3.66 nm through the transition, whereas the layer spacing was approximately identical to twice the side chain length. In addition, the diffraction assigned to the lateral translational distance of the Rf groups simultaneously disappeared. Thus, the PFAUr-C8 exhibits a phase transition from the hexatic smectic-B to the partially interdigitated bilayered smectic-A. Although the two phases co-existed at 363 K, the smectic-B phase completely disappeared at 373 K. The diffraction peaks assigned to the lamellar structure shifted to higher q-values with increasing temperature and then finally disappeared at 453 K, indicating isotropization. The mean lamellar period decreased with increasing temperature by thermal activation followed by progressive interdigitation. It was quite interesting that the lamellar structure remained even at 443 K, approximately 30 K higher than the endothermic peak temperature of 416 K in the DSC. The distorted lamellar structure remains as a mesophase at temperatures well above the second endothermic temperature.
Poly(fluoroalkyl acrylate)s produce a highly anisotropic ordered structure in thin films because of the spontaneous segregation of CF3 groups to the surface. It should be noted that the aggregation structures in polymer films, especially the local structures at the interfaces, are different from those in the bulk. Figure 5 shows the typical GI-WAXD patterns, in-plane and out-of-plane line profiles for PFAUr-C8 and PFA-C8 spin-coated films at various temperatures. The first diffraction peak in the out-of-plane profile overlapped with the edge of the beam stop. Both PFAUr-C8 and PFA-C8 thin films showed a highly anisotropic and hexatic smectic-B structure in which the Rf groups were aligned normal to the substrate. For PFA-C8, the out-of-plane peaks and in-plane peaks disappeared simultaneously at 363 K. For the PFAUr-C8 film, the peaks in the out-of-plane direction shifted to a higher scattering vector at 363 K, and the out-of-plane peak remained even at 423 K. These characteristics are totally consistent with the powder WAXD results; therefore, the ordered structure transition from the hexatic smectic-B to the partially interdigitated smectic-A occurs identically in the thin films.
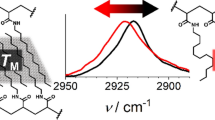
WAXD results for PFAUr-C8 and PFA-C8 in both powder and thin film form are fully consistent with the DSC and POM results. A schematic representation of the phase transitions in PFAUr-C8 and PFA-C8 is shown in Scheme 2. PFAUr-C8 exhibits a bilayered hexatic smectic-B to the partially interdigitated bilayered smectic-A transition, whereas PFA-C8 shows a direct transition from the hexatic smectic-B to isotropic phase. In addition, PFAUr-C8 shows a higher phase transition temperature than PFA-C8. PFAUr-C8 retains a layered lamellar structure even without the hexatic translational order of the Rf groups. This contrast is undoubtedly attributed to the carbamate linker and it is plausible to claim that the carbamate linker enhances the thermal stability of the layered lamellar structure. We have to recognize that PFAUr-C8 has more methylene groups in the side chains than PFA-C8. Extension of the methylene chain length in the side chains generally disturbs the alignment of the fluorinated chains [13]. Thus, the carbamate group is responsible for the remarkable improvement in the thermal stability of lateral translational order and the lamellar structure of the Rf groups. As reported by Corpart et al. [16], polyacrylates with –COO(CH2)2N(CH3)SO2(CH2)2(CF2)nF side chains (n = 6, 8) also retain the lamellar structures at a temperature much higher than the smectic to isotropic phase transition temperature. They explained the survival of the ordered mesophase by the dipole–dipole interactions between polar N-methylsulfonamide groups. In PFAUr-C8, the carbamate groups may bind to each other with dipolar cohesive interactions to retain the layered lamellar structure even above the isotropization temperature. The aggregation state of the carbamate linkers was studied by temperature-controlled FT-IR spectroscopy (Fig. 6 and Figure S2). At 333 K, the PAUr-C8 film shows strong carbonyl stretching vibrations at 1726 cm−1 and a weak shoulder peak at approximately 1705 cm−1. Hydrogen-bonded and free carbamate groups exhibit carbonyl stretching absorption peaks at 1705 cm−1 and 1741 cm−1, respectively [23, 24]. The intermediate wave number for the carbonyl stretching absorption peak (1726 cm−1) suggests disordered dipolar interactions in the ester and carbamate groups. The shoulder peak intensity remained before the first phase transition, whereas it was significantly reduced after the first phase transition. The peak shift indicates the breakage of the hydrogen-bonding network across the structure transition from hexatic smectic-B to partially interdigitated smectic-A. In addition, the main peak at 1726 cm−1 shifted to 1735 cm−1 after the second phase transition. The peak shift indicates the weakening of the cohesive interactions in both the carbamate and ester groups through the breakage of the layered arrangement of PFAUr-C8 chains. These results indicate that the thermal stability of the layered smectic structure is derived from the dipolar interactions of the carbamate linkages.
Wettability of the PFAUr-C8 film
The water repellency of PFAUr-C8 films was evaluated by water droplet contact angle measurements (Table 2). PFAUr-C8 exhibited a striking water repellency. The large receding contact angle and small contact angle hysteresis (Δθ, Δθ = θa − θr) indicate that the surface reorganization through attachment of water is unfavorable. The water wettability for PFAUr-C8 films is approximately identical to PFA-C8 films [11]. As the PFAUr-C8 produces anisotropic lamellar structures with highly packed lateral order for the Rf groups at ambient temperature, the CF3 arrays are stable under water. Moreover, the water repellency for PFAUr-C8 films hardly depends on the thermal history, suggesting that the ordered structure is produced immediately in the coating process.
The water repellency is further supported by the chemical composition at the outermost surface. XPS measurements with synchrotron radiation soft X-rays were applied to determine the outermost chemical composition. The XPS probing depth is defined as 3λsinθ, where λ is the inelastic free path and θ is the take-off angle. The XPS probing depth is usually approximately 10 nm in the case of Al Kα (1486.6 eV) and/or Mg Kα (1253.6 eV) characteristic X-rays [25, 26]. Low-energy soft X-rays excite photoelectrons with much lower kinetic energy and much shorter inelastic free path, hence limiting the XPS probing depth to yield XPS signals from the outermost surface. The probing depth for perfluoroalkyl chains with 400 eV soft X-rays at a take-off angle of 54° is calculated to be approximately 1.3 nm [27, 28]. The single bilayer thickness of PFAUr-C8 determined by the GI-WAXD measurements is approximately 4.05 nm and the fully extended –CH2CHCOO(CH2)2NHCO(CH2)2(CF2)7CF3 side chain length is calculated to be 1.81 nm. Figure 7 shows the C1s XPS spectra for the PFAUr-C8 films measured with 400 eV soft X-rays at a take-off angle of 54°. The high-resolution C1s peaks were deconvoluted into five components (–CF3, –CF2–, –COO–/–CONH, –CH2O–/–CH2NH–, and aliphatic carbon) using a least-square curve fitting program. The C1s binding energies obtained from the curve-fitted spectra agreed with the literature values [29, 30]. The area percentage of fluorinated carbons (–CF3 and –CF2) was 0.66 for PFAUr-C8 films regardless of the thermal annealing, which is higher than the theoretical value of 8/16 = 0.50. The theoretical value was calculated from the number of carbons in the FAUr-C8 monomer. The CF3/CF2 ratio was 0.21 and 0.18 for the non-annealed and annealed PFAUr-C8 films, respectively, which is higher than the theoretical value of 1/7 = 0.14. The deviations indicate that the Rf groups are preferentially segregated to the surface and that the CF3 moieties cover the outermost surface. The Rf localization at the surface is common in polymers with long Rf side chains and has been well understood in terms of surface free energy [31]. The almost identical XPS spectra for the annealed and non-annealed PFAUr-C8 films imply a negligible effect of thermal annealing on the outermost chemical structure.
Conclusions
PFAUr-C8 exhibited polymorphic ordered structures, with the phase transitions of bilayered hexatic smectic-B to partially interdigitated smectic-A followed by isotropic phase transitions in the heating process verified. PFAUr-C8 maintains a stacked lamellar structure for the Rf side chains without lateral translational order, whereas PFA-C8 immediately turns into an isotropic melt after the disordering of the lateral order. The cohesive dipolar interactions in the carbamate linkers induce the thermal stability of the lamellar structure. PFAUr-C8 exhibits superior water repellency similar to PFA-C8. The well-ordered anisotropic hexatic smectic-B structure of PFAUr-C8 induces the wetting performances. Considering the significant impact of the carbamate group on the molecular aggregation structure, this new linker may have the potential to improve the liquid repellency of polyacrylates with shorter Rf side chains ( < 7).
References
Volkov VV, Platé NA, Takahara A, Kajiyama T, Amaya N, Murata Y. Aggregation state and mesophase structure of comb-shaped polymers with fluorocarbon side groups. Polymer. 1992;33:1316–20.
Pittman AG, Ludwig BA. Effect of polymer crystallinity on the wetting properties of certain fluoroalkyl acrylates. J Polym Sci A-1 Polym Chem. 1969;7:3053–66.
Smith DW, Lacono ST, Lyer SS. Handbook of fluoropolymer science and technology. 1st ed. Hoboken, New Jersey, USA: John Wiley & Sons, Inc.; 2014.
Shibasaki Y, Zhu Z, Fukuda K. Molecular organization of comb-like polymers containing long fluorocarbon chains in the thin films formed by the LB and lift-up methods. Mol Cryst Liq Cryst Sci Technol Sect A. 1999;337:165–8.
Hasegawa T. Physicochemical nature of perfluoroalkyl compounds induced by fluorine. Chem Rec. 2017;17:903–17.
Banerjee S, Tawade BV, Ladmiral V, Dupuy LX, MacDonald MP, Améduri B. Poly(fluoroacrylate)s with tunable surface hydrophobicity via radical copolymerization of 2, 2, 2-trifluoroethyl α-fluoroacrylate and 2-(trifluoromethyl) acrylic acid. Polym Chem. 2017;8:1978–88.
Oikawa Y, Saito T, Yamada S, Sugiya M, Sawada H. Preparation and surface property of fluoroalkyl end-capped vinyltrimethoxysilane oligomer/talc composite-encapsulated organic compounds: application for the separation of oil and water. ACS Appl Mater Interfaces. 2015;7:13782–93.
Ishige R, Shinohara T, White KL, Meskini A, Raihane M, Takahara A, et al. Unique difference in transition temperature of two similar fluorinated side chain polymers forming hexatic smectic phase: poly{2-(perfluorooctyl) ethyl acrylate} and poly{2-(perfluorooctyl) ethyl vinyl ether}. Macromolecules. 2014;47:3860–70.
Honda K, Yakabe H, Koga T, Sasaki S, Sakata O, Otsuka H, et al. Molecular aggregation structure of poly(fluoroalkyl acrylate) thin films evaluated by synchrotron-sourced grazing-incidence X-ray diffraction. Chem Lett. 2005;34:1024–5.
Roussel F, Saidi S, Guittard F, Geribaldi S. Thermophysical properties of fluorinated acrylate homopolymers: mixing and phase separation. Eur Phys J E. 2002;8:283–8.
Honda K, Morita M, Otsuka H, Takahara A. Molecular aggregation structure and surface properties of poly (fluoroalkyl acrylate) thin films. Macromolecules. 2005;38:5699–705.
Honda K, Morita M, Sakata O, Sasaki S, Takahara A. Effect of surface molecular aggregation state and surface molecular motion on wetting behavior of water on poly(fluoroalkyl methacrylate) thin films. Macromolecules. 2010;43:454–60.
Honda K, Yamamoto I, Morita M, Yamaguchi H, Arita H, Ishige R, et al. Effect of α-substituents on molecular motion and wetting behaviors of poly(fluoroalkyl acrylate) thin films with short fluoroalkyl side chains. Polymer. 2014;55:6303–8.
Ramharack R, Nguyen TH. Fluoropolymers of very low surface energies. J Polym Sci Part C Polym Lett. 1987;25:93–98.
Saïdi S, Guittard F, Guimon C, Géribaldi S. Fluorinated comblike homopolymers: the effect of spacer lengths on surface properties. J Polym Sci A. 2005;43:3737–47.
Corpart JM, Girault S, Juhué D. Structure and surface properties of liquid crystalline fluoroalkyl polyacrylates: role of the spacer. Langmuir. 2001;17:7237–44.
Lindstron AB, Strynar MJ, Libelo EL. Polyfluorinated compoundsː past, present, and future. Environ Sci Technol. 2011;45:7954–61.
Wang Z, Dewitt JC, Higgins CP, Cousins IT. A never-ending story of per- and polyfluoroalkyl substances (PFASs). Environ Sci Technol. 2017;51:2508–18.
Ogawa H, Masunaga H, Sasaki S, Goto S, Tanaka T, Seike T, et al. Experimental station for multiscale surface structural analyses of soft-material films at SPring-8 via a GISWAX/GIXD/XR-integrated system. Polym J. 2013;45:109–16.
Masunaga H, Ogawa H, Takano T, Sasaki S, Goto S, Tanaka T, et al. Multipurpose softmaterial SAXS/WAXS/GISAXS beamline at SPring-8. Polym J. 2011;43:471–7.
Hatsui T, Omodani M, Kudo T, Kobayashi K, Imamura T, Ohmoto T, et al. A direct-detection X-ray CMOS image sensor with 500 µm thick high resistivity silicon. Proc. Int. Image Sensor Workshop. 2013, 3.05.
Hatsui T, Graafsma H. X-ray imaging detectors for synchrotron and XFEL sources. IUCr J. 2015;2:371–83.
Wang H, Aubuchon SR, Thompson DG, Osborn JC, Marsh AL, Nichols WR, et al. Temperature-dependent dynamic mechanical analysis−Fourier transform infrared study of a poly(ester urethane) copolymer. Macromolecules. 2002;35:8794–801.
Brunette CM, Hsu SL, MacKnight WJ. Hydrogen-bonding properties of hard-segment model compounds in polyurethane block copolymers. Macromolecules. 1982;15:71–77.
Kassis CM, Steehler JK, Betts DE, Guan ZB, Romack TJ, Desimone JM, et al. XPS studies of fluorinated acrylate polymers and block copolymers with polystyrene. Macromolecules. 1996;29:3247–54.
Heide, PVD. X-ray photoelectron spectroscopy. 1st ed. Hoboken, New Jersey, USA: John Wiley & Sons, Inc.; 2012.
Nojima S, Shinohara T, Higaki Y, Ishige R, Ohishi T, Kobayashi D, et al. Precise characterization of outermost surface of crystalline-crystalline diblock copolymer thin films using synchrotron radiation soft X-ray photoelectron spectroscopy. Polym J. 2014;46:637–40.
Shinohara T, Higaki Y, Nojima S, Masunaga H, Ogawa H, Okamoto Y, et al. Molecular aggregation states and wetting behavior of a poly{2-(perfluorooctyl) ethyl acrylate} brush-immobilized nano-imprinted surface. Polymer. 2015;69:10–16.
Saïdi S, Guittard F, Guimon C, Géribaldi S. Synthesis and characterization of copolymers based on styrene and partially fluorinated acrylates. Eur Polym J. 2006;42:702–10.
Cecchet F, Pilling M, Hevesi L, Schergna S, Wong JKY, Clarkson GJ, et al. Grafting of benzylic amide macrocycles onto acid-terminated self-assembled monolayers studied by XPS, RAIRS, and contact angle measurements. J Phys Chem B. 2003;107:10863–72.
Thünemann AF, Lieske A, Paulke BR. Low surface energy coatings from waterborne nano-dispersions of polymer complexes. Adv Mater. 1999;11:321–4.
Acknowledgements
YL wishes to acknowledge the financial support from the China Scholarship Council (Grant Number 201608525036), Guizhou Science and Technical Foundation (Grant Number 20152124), Guizhou Oversea Talent Project (Qianren **angmu Zizhu Hetong Number 201414), and the Science Foundation of Guizhou Normal College (Guishiyuan **angmu Zizhu Hetong Number 14BS014). This work was supported by the Photon and Quantum Basic Research Coordinated Development Program of the Ministry of Education, Culture, Sports, Science and Technology, Japan. This work was supported by ImPACT Program of Council for Science, Technology, and Innovation (Cabinet Office, Government of Japan). This work was performed under the Cooperative Research Program “Network Joint Research Center for Materials and Devices”. This work was also supported by the MEXT Project “Integrated Research Consortium on Chemical Sciences.” The powder WAXD and GIXD experiments were performed at BL05XU, BL40B2, and BL03XU in SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal Numbers 2017B1086 and 2018A1177). We gratefully acknowledge Dr. Kazuki Mita and Mr. Kiminori Uchida (Mitsui Chemicals, Inc.) for kindly giving us the opportunity to perform thin film WAXD measurements at the BL03XU. XPS measurement was performed at BL12 in the Kyushu Synchrotron Light Research Center (Proposal Number 1612115F and 1707063F).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Liu, Y., Higaki, Y., Mukai, M. et al. Smectic ordered structure and water repellency of a poly(fluoroalkyl acrylate) with a carbamate linker. Polym J 51, 189–198 (2019). https://doi.org/10.1038/s41428-018-0139-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-018-0139-2
- Springer Nature Limited