Abstract
Peripheral vascular disease (PVD) is an emerging public health burden with a high rate of disability and mortality. Gasdermin D (GSDMD) has been reported to exert pyroptosis and play a critical role in the pathophysiology of many cardiovascular diseases. We ought to determine the role of GSDMD in the regulation of perfusion recovery after hindlimb ischemia (HLI). Our study revealed that GSDMD-mediated pyroptosis occurred in HLI. GSDMD deletion aggravated perfusion recovery and angiogenesis in vitro and in vivo. However, how GSDMD regulates angiogenesis after ischemic injury remains unclear. We then found that GSDMD-mediated pyroptosis exerted the angiogenic capacity in macrophages rather than endothelial cells after HLI. GSDMD deletion led to a lower level of CCL11 in mice serum. GSDMD knockdown in macrophages downregulated the expression and decreased the releasing level of CCL11. Furthermore, recombinant CCL11 improved endothelial functions and angiogenesis, which was attenuated by CCL11 antibody. Taken together, these results demonstrate that GSDMD promotes angiogenesis by releasing CCL11, thereby improving blood flow perfusion recovery after hindlimb ischemic injury. Therefore, CCL11 may be a novel target for prevention and treatment of vascular ischemic diseases.
Similar content being viewed by others
Introduction
Peripheral vascular disease (PVD) is an increasingly serious public health problem, of which the disability and mortality rates are rising with the continuous increase of the aged population, the improving life quality, and the changing diet structure. Critical limb ischemia (CLI) is a kind of PVD with a high incidence rate, caused by tissue ischemia and hypoxia after arterial stenosis or occlusion. CLI results in circulatory disorders, which manifests as intermittent claudication, resting pain, ischemic ulcer, and other symptoms.
Conventional treatments of PVD such as angioplasty, stent implantation, and bypass surgery, have achieved success in treating local ischemic lesions. However, these methods are limited by their aggressiveness [1]. Insufficient neovascularization in ischemic regions is one of the main causes of perfusion impairment and limb dysfunction. Hence, promoting neovascularization is vital for perfusion and functional recovery based on the physiopathology of PVD, and is in urgent need of advanced development [2, 3]. Angiogenesis, the process of new capillaries generated from the originals, is necessary for perfusion and functional recovery in ischemic limbs of PVD patients. The procedure includes degradation of basal membrane, proliferation and migration of endothelial cells, endothelial reconstruction, and formation of new vascular networks [4].
Pyroptosis is a novel form of programmed cell death, which was identified after apoptosis and necrosis. Gasdermin D (GSDMD), a protein of Gasdermin family, is a hub molecule of pyroptosis process [5]. GSDMD has the ability to bind lipid and form protein pores on cell membranes, leading to plasma membrane rupture, cell swelling, release of intracellular substances including IL-1β and IL-18, and eventually cell death [6]. The involvement of GSDMD-mediated pyroptosis in cardiovascular diseases is well-documented [7,8,9]. However, the effects on endothelial function and angiogenesis remain controversial. It is reported that pyroptosis affects angiogenesis by releasing related inflammatory cytokines. IL-1β has been shown to promote angiogenesis in many studies [10,11,12], while the role of IL-18 remains double-sided. Inhibition of Nod-like receptor family pyrin domain-containing protein 3 (NLRP3) inflammasome activation and the release of IL-1β and IL-18 regulates endothelial cell proliferation and migration, further improving retinal neovascularization and alleviating leakage of impaired vessels [13]. On the contrary, a series of studies demonstrated the anti-angiogenic effect in tumor angiogenesis of IL-18 [14, 15]. Thus, there is considerable interest in clarifying the effect of GSDMD in angiogenesis and its underlying mechanisms.
In the present study, we used the GSDMD deficiency (Gsdmd-/-) transgenic mice to reveal the role of GSDMD in angiogenesis after hindlimb ischemia (HLI). Our research provided direct evidence that GSDMD-mediated pyroptosis regulated perfusion recovery after HLI by affecting the production and secretion of cytokines, aiming to identify novel targets in the pyroptosis process after ischemic injury for the prevention and treatment of PVD.
Results
GSDMD-mediated pyroptosis is activated in ischemic hindlimbs
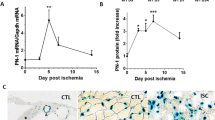
To investigate the involvement of GSDMD-mediated pyroptosis in hindlimb ischemic injury in vivo, we established hindlimb ischemia (HLI) model by ligation of the femoral artery in wild-type (WT) mice. The protein expression levels of ischemic and non-ischemic gastrocnemius muscle tissues at different time points (the 2nd, 5th, 7th, and 14th day post-HLI) were determined by western blot analysis (Fig. 1A) (Supplementary 1). The results showed that the levels of full-length GSDMD (GSDMD-FL) and N-terminus of GSDMD (GSDMD-N) were significantly upregulated in ischemic tissues (Fig. 1B, C). Moreover, the levels of NLRP3, IL-1β, and IL-18 were also markedly upregulated after HLI, among which IL-1β and IL-18 markedly increased at the 5th day post-HLI, then decreased since the 7th day, indicating the inflammatory reaction peaked at the 5th day (Fig. 1D–F). Taken together, these results suggested that GSDMD-mediated pyroptosis might occur in gastrocnemius muscles after HLI. The higher levels of CD31 and VEGF-α in ischemic tissues indicated that angiogenesis occurs after ischemic injury, mediating the recovery of blood flow perfusion after HLI (Fig. 1G, H).
A Western blots and B–H quantitative analysis of GSDMD-FL, GSDMD-N, NLRP3, IL-1β, IL-18, CD31, and VEGF-α protein levels in ischemic gastrocnemius muscles of WT mice compared to non-ischemic controls at the 2nd, 5th, 7th, and 14th day post-HLI. n = 3 for each group; ns represents p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, #p < 0.0001.
Hypoxia stimulates pyroptosis in macrophages
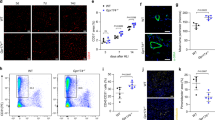
Since both endothelial cells and macrophages participate in angiogenesis, we ought to determine the involvement of GSDMD in different cell types in the process. Therefore, we established a cellular hypoxia model, and normoxia as control to reveal whether hypoxia induces endothelial cell or macrophage pyroptosis in vitro. We extracted proteins of human umbilical vein endothelial cells (HUVECs) at different time points (6, 12, 24, and 48 h) after hypoxia and performed western blot analysis. The results showed that the levels of GSDMD-FL and GSDMD-N had no significant differences between normoxia and hypoxia endothelial cells (Fig. 2A) (Supplementary 1).
A Western blots of GSDMD-FL, GSDMD-N, IL-1β, and IL-18 protein expression levels in normoxia and hypoxia HUVECs. B The relative mRNA levels of GSDMD, NLRP3, IL-1α, IL-1β, IL-18, TNF-α, and HMGB1 of hypoxia THP-1 cells compared to normoxia THP-1 cells. C Western blots of GSDMD-FL and GSDMD-N protein expression levels in normoxia and hypoxia THP-1 cells. D The level of LDH in cell culture supernatants detected by LDH release test. E The level of IL-1β in cell culture supernatants detected by ELISA. n = 3 for each group; ns represents p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, #p < 0.0001.
Then, a 24-h hypoxia intervention was also conducted on adherent human myeloid leukemia mononuclear cells (THP-1 cells) induced by Phorbol 12-Myristate 13-Acetate (PMA), a THP-1 cell differentiation inducer. The quantitative PCR was performed to verify the changes of cytokine mRNA levels in THP-1 cells after hypoxia. The results showed that the mRNA expression of NLRP3, IL-1α, IL-1β, IL-18, and TNF-α were significantly upregulated in hypoxia THP-1 cells (Fig. 2B). On the contrary to that in HUVECs, the protein levels of GSDMD-FL and GSDMD-N were significantly upregulated in hypoxia THP-1 cells (Fig. 2C) (Supplementary 1). We evaluated the degree of pyroptosis by measuring the LDH content in the cell supernatants of THP-1 cells. As we assumed, the LDH releasing level significantly increased after hypoxia in THP-1 cells (Fig. 2D). ELISA test demonstrated a higher level of IL-1β in hypoxia THP-1 cell culture supernatants (Fig. 2E). Collectively, hypoxia-induced GSDMD-mediated pyroptosis in macrophages.
GSDMD deficiency inhibits perfusion recovery after HLI
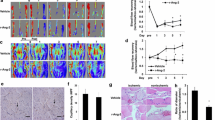
Next, we sought to unravel the contribution of GSDMD to blood flow perfusion recovery after HLI, owing to the upregulation of GSDMD in ischemic gastrocnemius muscles. We built models of HLI in GSDMD deficiency (Gsdmd-/-) mice and their litters (WT). Laser Doppler perfusion imaging of hindlimb blood flow was performed on the 3rd, 7th, 14th, and 21st day post-HLI to evaluate perfusion. The results, expressed in the form of perfusion ratio of ischemic to non-ischemic limb, showed that GSDMD-/- mice group exhibited a lower blood flow perfusion recovery compared to WT mice group with significant differences at the 7th, 14th, and 21st day post-HLI (Fig. 3A, B). The gastrocnemius muscles of both ischemic and non-ischemic limbs were harvested on the 21st day post-HLI for subsequent analyses. Western blot results revealed a lower expression of CD31 and VEGF-α in the ischemic tissues of GSDMD-/- mice compared to controls (Fig. 3C–E) (Supplementary 1), indicating that GSDMD deficiency decreased angiogenesis in vivo. Masson’s trichrome staining results showed that the fibrosis area of Gsdmd-/- mice group was markedly larger than WT mice group (Fig. 3F, G), indicating a wider deposition of collagen fibers in the absence of GSDMD. Combining the results above, the lower blood flow perfusion recovery in Gsdmd-/- mice group is attributed to decreased angiogenesis and aggravated tissue repairment caused by interstitial fibrosis after HLI.
A Representative images of limb perfusion in WT and Gsdmd-/- mice analyzed by Laser Doppler Perfusion Imaging and B quantitative Laser Doppler analysis measured by limb perfusion ratio of ischemic to non-ischemic hindlimbs (n = 5 for each group). C Western blots and D, E quantitative analysis of CD31 and VEGF-α expression levels of gastrocnemius tissues in WT and Gsdmd-/- mice at 21st day post-HLI. F Representative images of Masson staining and G quantitative analysis of fibrosis area in gastrocnemius muscles in WT and Gsdmd-/- mice at 21st day post-HLI. Fibrosis area was measured by Image Pro Plus. Scale bar = 100 μm; n = 3 for each group; ns represents p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, #p < 0.0001.
GSDMD overexpression and knockdown in endothelial cells do not affect endothelial function
To fully verify the effect of GSDMD and pyroptosis in endothelial cells, we generated GSDMD-overexpression (GSDMD-OV) and GSDMD-knockdown (GSDMD-KD) HUVECs by transfecting plasmid DNA and siRNA (Fig. 4A) (Supplementary 1). Next, we performed CCK-8 test. The results revealed that there was no significant difference in cell proliferation in GSDMD-OV HUVECs or GSDMD-KD HUVECs compared to controls (Fig. 4B, C). The wound healing assay also demonstrated that there was no significant difference in endothelial cell migration (Fig. 4D–G) after GSDMD overexpression or GSDMD knockdown.
A Western blot of GSDMD expression level of HUVECs transfected with plasmid DNA (GSDMD-OV) and siRNA (GSDMD-KD). B, C Relative cell proliferation rate of GSDMD-OV and GSDMD-KD HUVECs for 24 h. D, E Representative images and quantification of wound closure of GSDMD-OV and F, G GSDMD-KD HUVECs for 24 h. The value of relative wound closure was measured by ImageJ. Scale bar = 50 μm. n = 3 for each group; ns represents p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, #p < 0.0001.
Inhibition of macrophage pyroptosis damages endothelial cell function
The contribution of macrophages to angiogenesis is well-documented. The observations above prompted us to confirm the role of GSDMD-mediated pyroptosis in macrophages. GSDMD-knockdown (GSDMD-KD) THP-1 cells were established and western blot results showed that the expression of GSDMD-FL, GSDMD-N, IL-1β, and IL-18 were significantly downregulated in GSDMD-KD THP-1 cells (Fig. 5A–E) (Supplementary 1). Meanwhile, GSDMD-KD THP-1 cells had a lower LDH releasing level after 24 h hypoxia than negative control (GSDMD-NC) THP-1 cells (Fig. 5F), and the secretion of IL-1β was also decreased in GSDMD-KD THP-1 cells (Fig. 5G), indicating that knockdown of GSDMD inhibited pyroptosis in macrophages. To further clarify whether macrophage pyroptosis affects endothelial function and angiogenesis, we generated a co-culture system of HUVECs and THP-1 cells. Specifically, the conditioned medium of GSDMD-NC and GSDMD-KD THP-1 cells underwent 24 h normoxia or hypoxia treatment was collected and used for HUVEC stimulation. HUVECs stimulated with condition medium were tested to illustrate endothelial function and angiogenic potential via CCK-8 kits, wound healing assay, and Matrigel tube formation assay. The results of CCK-8 test and wound healing assay indicated that HUVECs stimulated with conditioned medium from normoxia and hypoxia NC THP-1 cells had no significant differences in cell proliferation and migration. Meanwhile, HUVECs stimulated with hypoxia KD THP-1 cells exhibited impaired proliferation and migration compared to those stimulated with conditioned medium from normoxia KD THP-1 cells and normoxia/hypoxia NC THP-1 cells (Fig. 5H–J). In addition, HUVECs treated with the conditioned medium of NC group had a stronger angiogenic capacity (Fig. 5K–L). Therefore, we concluded that inhibition of GSDMD-mediated pyroptosis in macrophages weakened endothelial function and angiogenesis.
A Western blots and B–E quantitative analysis of GSDMD-FL, GSDMD-N, IL-1β, and IL-18 expression levels of GSDMD-NC and GSDMD-KD THP-1 cells after normoxia or hypoxia treatment. F The level of LDH in cell culture supernatants detected by LDH release test. G The level of IL-1β in cell culture supernatants detected by ELISA. H Relative cell proliferation rate of HUVECs stimulated with conditioned medium collected from normoxia/hypoxia GSDMD-NC and GSDMD-KD THP-1 cells for 24 h. I, J Representative images and quantification of wound closure for 24 h of HUVECs stimulated with the conditioned medium. K, L Tube formation assay and quantification in HUVECs stimulated with conditioned medium. The values of relative wound closure and tube length were measured by ImageJ. Scale bar = 50 μm. n = 3 for each group; ns represents p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, #p < 0.0001.
GSDMD deficiency inhibits the expression and secretion level of CCL11 in vivo and in vitro
In vitro observations verified that endothelial function and tube formation were exacerbated by the culture supernatants of GSDMD-KD macrophages. To demonstrate whether a specific cytokine plays a role in angiogenesis after hindlimb ischemia, we measured mice serum cytokine levels using Luminex technology in four groups: WT sham (n = 3), WT HLI (n = 4), KO sham (n = 3), and KO HLI (n = 4) to compare cytokine releasing levels between WT mice and GSDMD-/- mice after HLI (Fig. 6A). Interestingly, we found a lower level of chemokine CC motif ligand 11 (CCL11) after HLI in Gsdmd-/- mice serum than that in WT mice serum (Fig. 6B). We analyzed mRNA and protein expression of CCL11 in GSDMD-NC and GSDMD-KD THP-1 cells. The mRNA level of CCL11 in THP-1 cells was increased after hypoxia, whereas it decreased by the knockdown of GSDMD (Fig. 6C). Western blots results showed that CCL11 protein expression was downregulated in GSDMD-KD THP-1 cells (Fig. 6D, E) (Supplementary 1). Meanwhile, the concentration of CCL11 in culture supernatants was measured by ELISA. Results showed that CCL11 secretion level significantly increased after hypoxia in GSDMD-NC THP-1 cells and decreased after hypoxia in GSDMD-KD THP-1 cells (Fig. 6F). Collectively, these findings suggested that inhibition of pyroptosis in THP-1 cells may reduce the expression and release of CCL11.
A Heatmap for contrasting the mean log10 concentration values of cytokines for WT HLI and KO HLI mice serum. B The concentration of CCL11 in WT sham, WT HLI, KO sham, and KO HLI mice serum. C The relative mRNA level of CCL11 of GSDMD-NC and GSDMD-KD THP-1 cells after normoxia and hypoxia treatment. D Western blots and E quantitative analysis of CCL11 expression level of GSDMD-NC and GSDMD-KD THP-1 cells after normoxia and hypoxia treatment. F The level of CCL11 in cell culture supernatants detected by ELISA. n = 3 for each group; ns represents p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, #p < 0.0001.
CCL11 facilitates endothelial cell proliferation, migration, and in vitro angiogenesis
To verify whether the CCL11 released by pyroptotic THP-1 cells affects the endothelial function and angiogenesis in vitro, transwell assay was conducted to demonstrate the chemotaxis of HUVEC toward CCL11. The results showed that there was a dose-dependent migration of HUVECs toward CCL11, indicating that CCL11 released by THP-1 cells can affect endothelial cell function by its chemotaxin essence (Fig. 7A, B). To clarify the effect of CCL11 on angiogenesis and perfusion recovery, the co-cultural system established by HUVECs and hypoxia GSDMD-NC or GSDMD-KD THP-1 cells were further stimulated with recombinant human CCL11 (CCL11) or CCL11 antibody (anti-CCL11) to augment or neutralize the effect of CCL11. Cell function experiments were performed to assess endothelial function. CCK-8 assay showed that anti-CCL11 inhibited cell proliferation of HUVECs stimulated with conditioned medium from hypoxia GSDMD-NC THP-1 cells, while CCL11 repaired weakened cell proliferation of HUVECs stimulated with conditioned medium from hypoxia GSDMD-KD THP-1 cells (Fig. 7C). Wound healing assay revealed that anti-CCL11 aggravated endothelial cell migration induced by hypoxia GSDMD-NC THP-1, while CCL11 rescued impaired cell migration stimulated by hypoxia GSDMD-KD THP-1 cells (Fig. 7D, E). Matrigel tube formation assay showed that anti-CCL11 aggravated in vitro angiogenesis induced by hypoxia GSDMD-NC THP-1 cells, while CCL11 enhanced lower angiogenesis stimulated by hypoxia GSDMD-KD THP-1 cells, determining the pro-angiogenic effect of CCL11 (Fig. 7F, G). The results indicated that CCL11 secreted after 24 h hypoxia treatment facilitated endothelial cell proliferation, migration, and in vitro angiogenesis, while anti-CCL11 inhibited enhanced endothelial function.
A, B Representative images and quantification of chemotaxis of HUVECs toward CCL11 were assessed with transwell assay. There was a dose-dependent increase of chemotaxis toward CCL11. C Relative cell proliferation rate of HUVECs stimulated with conditioned medium collected from hypoxia THP-1 cells, CCL11, and CCL11 antibody (anti-CCL11). D, E Representative images and quantification of wound closure for 24 h of HUVECs stimulated with the conditioned medium, CCL11, and anti-CCL11. F, G Tube formation assay and quantification of tube length in HUVECs stimulated with the conditioned medium, CCL11, and anti-CCL11. The number of cells in transwell assay was counted by Image Pro Plus; the values of relative wound closure and tube length were measured by ImageJ. Scale bar = 50 μm. n = 3 for each group; ns represents p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, #p < 0.0001.
Discussion
In the present study, we provided direct evidence that GSDMD-FL and GSDMD-N were both upregulated in hindlimb ischemic injury. Meanwhile, the cleavage and activation of GSDMD were increased in hypoxia macrophages. The release of LDH, which is widely used as an auxiliary method to detect pyroptosis [16], also increased in the culture supernatants of hypoxia macrophages, indicating that pyroptosis mediated by GSDMD occurred after HLI. Furthermore, in the absence of GSDMD, mice exhibited a hindered perfusion recovery and an aggravated tissue repair after ischemia, which were associated with the inhibition of pyroptosis in injured tissues.
Injury repair and angiogenesis affect the recovery of blood flow perfusion after hindlimb ischemic injury. Macrophages and endothelial cells both play crucial roles in the process of angiogenesis [17]. In the early stage after injury, pro-inflammatory macrophages are mainly involved in the local inflammatory reaction, which promotes the recruitment of neutrophils by secreting pro-inflammatory cytokines. Neutrophils and macrophages then phagocytize the necrotic cell fragments and mediate the degradation of the basal membrane. In the late stage, anti-inflammatory macrophages constitute the majority of its population in injured tissues, inhibiting the inflammation and regulating cell functions of smooth muscle cells (SMC) and endothelial cells [18, 19] as well as the formation and reconstruction of new vascular networks [20,21,22].
Pyroptosis and the involvement of GSDMD were first found in macrophages [23, 24]. In recent years, studies have shown that pyroptosis can also be triggered by different stimulations in many other types of cells, such as endothelial cells [9, 25] and cardiomyocytes [7, 8]. Intriguingly, our study showed that GSDMD-mediated pyroptosis in endothelial cells had no significant effects on endothelial function and angiogenesis, while the inhibition of macrophage pyroptosis impaired endothelial function and in vitro angiogenesis, which was determined by the co-cultural incubation of THP-1 cells and HUVECs. Endothelial dysfunction and impaired angiogenesis stimulated by GSDMD-knockdown macrophage supernatants indicated that intracellular substances and cytokines released by pyroptotic macrophages stimulate endothelial cells and further regulate endothelial function and angiogenesis [26].
GSDMD-mediated pyroptosis exerts its biological effects by the release and activation of IL-1β and IL-18. IL-1β reportedly promotes angiogenesis, while the effect of IL-18 remains controversial. We observed that GSDMD deletion in mice led to aggravated perfusion recovery as well as downregulation of GSDMD in macrophages caused the inhibition of tube formation. Combining our findings and the evidence above, the effects of IL-1β and IL-18 cannot fully explain the decreased angiogenesis and perfusion recovery. To clarify the involvement of multiple cytokines, we here performed a Luminex analysis. The results showed a lower level of CC motif ligand 11 (CCL11) in the serum of GSDMD deficiency mice who underwent HLI, demonstrating that the effect of pyroptosis on angiogenesis and perfusion recovery after ischemia may be caused by CCL11.
CCL11, also known as Eotaxin, is a member of CC chemokine family with a CC domain on N-terminal. The biological effects of CCL11 are closely associated with its chemotaxis of recruiting eosinophils in inflammatory sites during allergic reactions [27, 28]. CCL11 can be produced by immune cells [29, 30] and a wide range of other cell types like epithelial cells, fibroblasts, and smooth muscle cells in allergic tissues [27, The data supporting the findings of the study are available from the corresponding author upon reasonable request. Tu C, Das S, Baker AB, Zoldan J, Suggs LJ. Nanoscale strategies: treatment for peripheral vascular disease and critical limb ischemia. ACS Nano. 2015;9:3436–52. Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, et al. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115:2119–27. Annex BH, Cooke JP. New directions in therapeutic angiogenesis and arteriogenesis in peripheral arterial disease. Circ Res. 2021;128:1944–57. Nowak-Sliwinska P, Alitalo K, Allen E, Anisimov A, Aplin AC, Auerbach R, et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis. 2018;21:425–532. Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42:245–54. Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–8. Shi H, Gao Y, Dong Z, Yang J, Gao R, Li X, et al. GSDMD-mediated cardiomyocyte pyroptosis promotes myocardial I/R injury. Circ Res. 2021;129:383–96. Wen L, Wang M, Luo P, Meng X, Zhao M. Melatonin exerts cardioprotective effects by inhibiting NLRP3 inflammasome-induced pyroptosis in mice following myocardial infarction. Oxid Med Cell Longev. 2021;2021:5387799. Wang Y, Guan X, Gao CL, Ruan W, Zhao S, Kai G, et al. Medioresinol as a novel PGC-1alpha activator prevents pyroptosis of endothelial cells in ischemic stroke through PPARalpha-GOT1 axis. Pharm Res. 2021;169:105640. Lee JG, Heur M. Interleukin-1beta enhances cell migration through AP-1 and NF-kappaB pathway-dependent FGF2 expression in human corneal endothelial cells. Biol Cell. 2013;105:175–89. Mantsounga CS, Lee C, Neverson J, Sharma S, Healy A, Berus JM, et al. Macrophage IL-1beta promotes arteriogenesis by autocrine STAT3- and NF-kappaB-mediated transcription of pro-angiogenic VEGF-A. Cell Rep. 2022;38:110309. Huang J, Li Y, Jiang Z, Wu L, Liu Y, Ma S, et al. IL-1β promotes hypoxic vascular endothelial cell proliferation through the miR-24-3p/NKAP/NF-κB axis. Biosci Rep. 2022;42:BSR20212062. Sui A, Chen X, Shen J, Demetriades AM, Yao Y, Yao Y, et al. Inhibiting the NLRP3 inflammasome with MCC950 ameliorates retinal neovascularization and leakage by reversing the IL-1beta/IL-18 activation pattern in an oxygen-induced ischemic retinopathy mouse model. Cell Death Dis. 2020;11:901. Coughlin CM, Salhany KE, Wysocka M, Aruga E, Kurzawa H, Chang AE, et al. Interleukin-12 and interleukin-18 synergistically induce murine tumor regression which involves inhibition of angiogenesis. J Clin Invest. 1998;101:1441–52. Majewski S, Marczak M, Mlynarczyk B, Benninghoff B, Jablonska S. Imiquimod is a strong inhibitor of tumor cell-induced angiogenesis. Int J Dermatol. 2005;44:14–9. He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015;25:1285–98. Cooke JP, Meng S. Vascular regeneration in peripheral artery disease. Arterioscler Thromb Vasc Biol. 2020;40:1627–34. Wong BW, Marsch E, Treps L, Baes M, Carmeliet P. Endothelial cell metabolism in health and disease: impact of hypoxia. EMBO J. 2017;36:2187–203. Lee HW, Xu Y, He L, Choi W, Gonzalez D, ** SW, et al. Role of venous endothelial cells in developmental and pathologic angiogenesis. Circulation. 2021;144:1308–22. Chazaud B. Inflammation and skeletal muscle regeneration: leave it to the macrophages! Trends Immunol. 2020;41:481–92. Gurevich DB, Severn CE, Twomey C, Greenhough A, Cash J, Toye AM, et al. Live imaging of wound angiogenesis reveals macrophage orchestrated vessel sprouting and regression. EMBO J. 2018;37:e97786. Fung E, Helisch A. Macrophages in collateral arteriogenesis. Front Physiol. 2012;3:353. Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–5. Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–42. Chen H, Lu Y, Cao Z, Ma Q, Pi H, Fang Y, et al. Cadmium induces NLRP3 inflammasome-dependent pyroptosis in vascular endothelial cells. Toxicol Lett. 2016;246:7–16. Hong H, Tian XY. The role of macrophages in vascular repair and regeneration after ischemic injury. Int J Mol Sci. 2020;21:6328. Polosukhina D, Singh K, Asim M, Barry DP, Allaman MM, Hardbower DM, et al. CCL11 exacerbates colitis and inflammation-associated colon tumorigenesis. Oncogene. 2021;40:6540–6. Hanazawa T, Antuni JD, Kharitonov SA, Barnes PJ. Intranasal administration of eotaxin increases nasal eosinophils and nitric oxide in patients with allergic rhinitis. J Allergy Clin Immunol. 2000;105:58–64. Lampinen M, Waddell A, Ahrens R, Carlson M, Hogan SP. CD14+CD33+ myeloid cell-CCL11-eosinophil signature in ulcerative colitis. J Leukoc Biol. 2013;94:1061–70. Crapster-Pregont M, Yeo J, Sanchez RL, Kuperman DA. Dendritic cells and alveolar macrophages mediate IL-13-induced airway inflammation and chemokine production. J Allergy Clin Immunol. 2012;129:1621–7.e3. Lv J, **ong Y, Li W, Cui X, Cheng X, Leng Q, et al. IL-37 inhibits IL-4/IL-13-induced CCL11 production and lung eosinophilia in murine allergic asthma. Allergy. 2018;73:1642–52. Nickel W, Rabouille C. Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol. 2009;10:148–55. Chen W, Chen S, Yan C, Zhang Y, Zhang R, Chen M, et al. Allergen protease-activated stress granule assembly and gasdermin D fragmentation control interleukin-33 secretion. Nat Immunol. 2022;23:1021–30. Yamagishi R, Kamachi F, Nakamura M, Yamazaki S, Kamiya T, Takasugi M, et al. Gasdermin D-mediated release of IL-33 from senescent hepatic stellate cells promotes obesity-associated hepatocellular carcinoma. Sci Immunol. 2022;7:eabl7209. de Carvalho Ribeiro M, Szabo G. Role of the inflammasome in liver disease. Annu Rev Pathol. 2022;17:345–65. Xu B, Jiang M, Chu Y, Wang W, Chen D, Li X, et al. Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice. J Hepatol. 2018;68:773–82. Chalouhi N, Points L, Pierce GL, Ballas Z, Jabbour P, Hasan D. Localized increase of chemokines in the lumen of human cerebral aneurysms. Stroke. 2013;44:2594–7. Jones GT, Phillips LV, Williams MJ, van Rij AM, Kabir TD. Two C-C family chemokines, eotaxin and RANTES, are novel independent plasma biomarkers for abdominal aortic aneurysm. J Am Heart Assoc. 2016;5:e002993. Ilatovskaya DV, Pitts C, Clayton J, Domondon M, Troncoso M, Pippin S, et al. CD8(+) T-cells negatively regulate inflammation post-myocardial infarction. Am J Physiol Heart Circ Physiol. 2019;317:H581–96. Haley KJ, Lilly CM, Yang JH, Feng Y, Kennedy SP, Turi TG, et al. Overexpression of eotaxin and the CCR3 receptor in human atherosclerosis: using genomic technology to identify a potential novel pathway of vascular inflammation. Circulation. 2000;102:2185–9. Kodali RB, Kim WJH, Galaria II, Miller C, Schecter AD, Lira SA, et al. CCL11 (Eotaxin) induces CCR3-dependent smooth muscle cell migration. Arterioscler Thromb Vasc Biol. 2004;24:1211–6. Asosingh K, Vasanji A, Tipton A, Queisser K, Wanner N, Janocha A, et al. Eotaxin-rich proangiogenic hematopoietic progenitor cells and CCR3+ endothelium in the atopic asthmatic response. J Immunol. 2016;196:2377–87. Ying S, Robinson DS, Meng Q, Rottman J, Kennedy R, Ringler DJ, et al. Enhanced expression of eotaxin and CCR3 mRNA and protein in atopic asthma. Association with airway hyperresponsiveness and predominant co-localization of eotaxin mRNA to bronchial epithelial and endothelial cells. Eur J Immunol. 1997;27:3507–16. Li Y, Li L, Wadley R, Reddel SW, Qi JC, Archis C, et al. Mast cells/basophils in the peripheral blood of allergic individuals who are HIV-1 susceptible due to their surface expression of CD4 and the chemokine receptors CCR3, CCR5, and CXCR4. Blood. 2001;97:3484–90. Elovic A, Wong DT, Weller PF, Matossian K, Galli SJ. Expression of transforming growth factors-alpha and beta 1 messenger RNA and product by eosinophils in nasal polyps. J Allergy Clin Immunol. 1994;93:864–9. Muramatsu M, Katada J, Hayashi I, Majima M. Chymase as a proangiogenic factor. A possible involvement of chymase-angiotensin-dependent pathway in the hamster sponge angiogenesis model. J Biol Chem. 2000;275:5545–52. Salcedo R, Young HA, Ponce ML, Ward JM, Kleinman HK, Murphy WJ, et al. Eotaxin (CCL11) induces in vivo angiogenic responses by human CCR3+ endothelial cells. J Immunol. 2001;166:7571–8. Takeda A, Baffi JZ, Kleinman ME, Cho WG, Nozaki M, Yamada K, et al. CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nature. 2009;460:225–30. Jamaluddin MS, Wang X, Wang H, Rafael C, Yao Q, Chen C. Eotaxin increases monolayer permeability of human coronary artery endothelial cells. Arterioscler Thromb Vasc Biol. 2009;29:2146–52. Wang H, Wittchen ES, Jiang Y, Ambati B, Grossniklaus HE, Hartnett ME. Upregulation of CCR3 by age-related stresses promotes choroidal endothelial cell migration via VEGF-dependent and -independent signaling. Invest Ophthalmol Vis Sci. 2011;52:8271–7. This work was supported by the National Natural Science Foundation (Grant numbers 82170340, 82000333, 82200376, and 82070320). JG and FZ designed the program. YW, YG and RG operated the cell and animal experiments. JZ and JW conducted the data collection and the analysis was performed by YQ and SH. JY and JC conducted the supervision of the experiments. YW produced the original draft writing, which was checked by YG, HS and JC. All the authors have confirmed the submission of this manuscript. The authors declare no competing interests. Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Wang, Y., Gao, Y., Shi, H. et al. CCL11 released by GSDMD-mediated macrophage pyroptosis regulates angiogenesis after hindlimb ischemia.
Cell Death Discov. 10, 294 (2024). https://doi.org/10.1038/s41420-023-01764-9 Received: Revised: Accepted: Published: DOI: https://doi.org/10.1038/s41420-023-01764-9Data availability
References
Funding
Author information
Authors and Affiliations
Contributions
Corresponding authors
Ethics declarations
Competing interests
Additional information
Supplementary information
Rights and permissions
About this article
Cite this article