Abstract
Developmental disorders characterized by small body size have been linked to CDK5RAP2 loss-of-function mutations, but the mechanisms underlying which remain obscure. Here, we demonstrate that knocking down CDK5RAP2 in human fibroblasts triggers premature cell senescence that is recapitulated in Cdk5rap2an/an mouse embryonic fibroblasts and embryos, which exhibit reduced body weight and size, and increased senescence-associated (SA)-β-gal staining compared to Cdk5rap2+/+ and Cdk5rap2+/an embryos. Interestingly, CDK5RAP2-knockdown human fibroblasts show increased p53 Ser15 phosphorylation that does not correlate with activation of p53 kinases, but rather correlates with decreased level of the p53 phosphatase, WIP1. Ectopic WIP1 expression reverses the senescent phenotype in CDK5RAP2-knockdown cells, indicating that senescence in these cells is linked to WIP1 downregulation. CDK5RAP2 interacts with GSK3β, causing increased inhibitory GSK3β Ser9 phosphorylation and inhibiting the activity of GSK3β, which phosphorylates β-catenin, tagging β-catenin for degradation. Thus, loss of CDK5RAP2 decreases GSK3β Ser9 phosphorylation and increases GSK3β activity, reducing nuclear β-catenin, which affects the expression of NF-κB target genes such as WIP1. Consequently, loss of CDK5RAP2 or β-catenin causes WIP1 downregulation. Inhibition of GSK3β activity restores β-catenin and WIP1 levels in CDK5RAP2-knockdown cells, reducing p53 Ser15 phosphorylation and preventing senescence in these cells. Conversely, inhibition of WIP1 activity increases p53 Ser15 phosphorylation and senescence in CDK5RAP2-depleted cells lacking GSK3β activity. These findings indicate that loss of CDK5RAP2 promotes premature cell senescence through GSK3β/β-catenin downregulation of WIP1. Premature cell senescence may contribute to reduced body size associated with CDK5RAP2 loss-of-function.
Similar content being viewed by others
Introduction
CDK5RAP2 was identified based on its ability to interact with the Cdk5 regulatory subunit, p35 [1]. CDK5RAP2 localizes to the centrosome in a dynein-dependent manner [2] and regulates centriole engagement and centrosome cohesion during the cell cycle [3,4,5]. CDK5RAP2 moved into the spotlight as loss-of-function mutations cause primary microcephaly, an autosomal recessive neurodevelopmental disorder characterized by the small brain and cognitive deficit [6, 7]. In fact, CDK5RAP2 is most abundant at the luminal surface of the brain’s ventricular zone, particularly in cells lining the ventricular wall where the neural stem and progenitor cells reside. However, since CDK5RAP2 is also expressed in other tissues, it is not surprising that loss-of-function mutations are further associated with other developmental disorders [8]; e.g., primordial dwarfism [9, 10] and Seckel syndrome [11]. However, the molecular mechanisms by which CDK5RAP2 loss-of-function mutations cause these developmental disorders to remain elusive.
Cellular senescence is a state of stable cell cycle arrest where cells remain viable and metabolically active [12]. It is characterized by increased SA-β-gal activity [13], senescence-associated heterochromatin foci (SAHF) formation [14], and morphological transformation [15]. Senescence is triggered by cellular stresses [16,17,18,19]. For example, activated oncogenes such as H-RASG12V and B-RAFV600E induce senescence by evoking sustained anti-proliferative response [20], which acts as an initial barrier in preventing normal cell transformation into malignant cells. H-RASG12V-induced senescence is associated with DNA damage foci accumulation and p53 kinase activation, suggesting that aberrant oncogene activation induces DNA damage response (DDR) [20, 21]. Phosphorylation of the p53 tumor suppressor protein at Ser15 by p53 kinases such as ATM, Chk1, and/or Chk2 is one of the key events in p53-associated cell senescence [22, 23]. Apparently, p53 Ser15 phosphorylation stabilizes p53 by inhibiting its interaction with Mdm2, an E3 ubiquitin ligase that catalyzes polyubiquitination, subsequently inducing proteasome degradation of p53 [24]. However, p53 Ser15 phosphorylation is also required for p53 activation [25, 26], that blocks cell cycle progression by inducing p21CIP1 expression [27]. Aside from phosphorylation by kinases, p53 is also regulated by phosphatases such as WIP1. In fact, WIP1 has been associated with p53-mediated cell senescence [28, 29]. For example, hematopoietic stem cells (HSC) from WIP1−/− mice exhibit senescent phenotypes, impairing repopulating activity [29]. MEFs [29] and mesenchymal stem cells [30] from WIP1−/− mice also undergo premature senescence. In primary chondrocytes, reduced WIP1 is associated with senescent phenotype, which is reversed by ectopic WIP1 expression [31]. WIP1, which contains an NF-κB binding site in its promoter region, is a downstream gene target of NF-κB [32]. Interestingly, β-catenin associates with NF-κB and induces the expression of NF-κB target genes [33] such as WIP1.
Thus, our investigation examines the possibility that WIP1 expression is regulated by β-catenin and that WIP1-associated p53-mediated senescence accounts for phenotypes resulting from CDK5RAP2 loss-of-function. Since β-catenin is phosphorylated by GSK3β that earmarks β-catenin for degradation [34], we further examined whether GSK3β controls our presumed β-catenin-mediated WIP1 expression. Using BJ fibroblasts and Cdk5rap2an/an mice [35] and MEFs, we demonstrate that CDK5RAP2 loss-of-function triggers premature cell senescence. Proliferation defect and senescent phenotypes in CDK5RAP2-depleted BJ cells are recapitulated in Cdk5rap2an/an MEFs and embryos, manifesting as reduced growth rate and reduced embryonic body weight and size, respectively, as well as increased SA-β-gal staining in both MEFs and embryos. Our findings demonstrate that premature cell senescence due to CDK5RAP2 loss-of-function occurs via elevation of GSK3β activity that causes β-catenin-mediated downregulation of WIP1 and subsequent upregulation of p53 Ser15 phosphorylation.
Results
Loss of CDK5RAP2 induces senescence
To investigate how CDK5RAP2 loss-of-function causes proliferation defects associated with developmental disorders, we examined the effect of CDK5RAP2 depletion in BJ fibroblasts by siRNA. As shown in Fig. 1, knocking down CDK5RAP2 using two different siRNAs (#1 and #2) triggers the formation of SAHF as demonstrated by DAPI staining, and SAHF colocalization with heterochromatin protein 1γ (HP1γ), a SAHF marker [36] (Fig. 1B). Senescence induced by activated H-RASG12V oncogene [37] was used as a positive control to detect SAHF and HP1γ staining. To explore the suggestion that CDK5RAP2 depletion causes premature cell senescence, cells transfected with CDK5RAP2 siRNA #2 were monitored for the appearance of SAHF-positive cells and the expression of the senescence-associated biomarkers [36], p21CIP1, and p16INK4a, over 5 days post-transfection. As shown in Fig. 1C, the number of SAHF positive cells increases markedly in the first 3 days after transfection with CDK5RAP2 siRNA #2, reaching peak levels on days 3–5, a period when no or only a modest number of SAHF positive cells was observed in cells transfected with control siRNA. The increase in the number of SAHF positive cells upon CDK5RAP2 depletion coincides with increased expression of p21CIP1 and p16INK4a (Fig. 1D). Together with the noticeable staining for SA-β-gal, another marker of cellular senescence [38], in cells transfected with CDK5RAP2 siRNA #2 (Fig. 1E), our findings indicate that loss of CDK5RAP2 induces premature cellular senescence. To substantiate the occurrence of SA-β-gal positive cells following CDK5RAP2 loss, endogenous CDK5RAP2 was depleted in BJ cells using CDK5RAP2 siRNA #2 then exogenous CDK5RAP2 was overexpressed by infection of lentivirus carrying GFP-CDK5RAP2. As shown in Supplementary Fig. 1, exogenous CDK5RAP2 expression reversed the occurrence of SA-β-gal positive cells, which was clearly increased upon endogenous CDK5RAP2 depletion. Together, these findings indicate that CDK5RAP2 loss promotes premature cell senescence.
A Depletion of CDK5RAP2 in BJ human diploid foreskin fibroblasts. Lysates of cells transfected with CDK5RAP2 siRNA for 3 days were analyzed by SDS-PAGE and immunoblotting for CDK5RAP2 (left panel). Actin blot was used as a loading control. Representative blots are from one of three independent experiments (n = 3) showing similar results. The numbers under the CDK5RAP2 bands represent the ratios of the densitometric levels of CDK5RAP2 vs actin, with values from cells transfected with control siRNA normalized to 1.0. Densitometric analysis of blots was performed using NIH Image J 1.61. B CDK5RAP2 loss causes the formation of SAHF. BJ cells transfected with CDK5RAP2 siRNA for 3 days were stained with DAPI and HP1γ antibody and subjected to microscopic examination. SAHF formation induced by infecting adenovirus carrying H-RASG12V into BJ cells was used as a positive control. Images shown are of single-cell nuclei from control and CDK5RAP2-depleted cells, and cells infected with adenovirus carrying GFP alone or H-RASG12V. Inset images show differences in DAPI and HP1γ staining. C, D BJ cells transfected with CDK5RAP2 siRNA #2 for 5 days were analyzed for SAHF positive cells (C) and by SDS-PAGE and immunoblotting for p21CIP1 and p16INK4a as well as CDK5RAP2 and actin (D). The number of SAHF positive cells was assessed in ~200 cells per treatment group in each of 3 independent experiments (n = 3). *p < 0.01. In D, actin was used as a loading control. Representative blots are from one of three independent experiments (n = 3) showing similar results. Ratios of levels of p21CIP1 and p16INK4a vs. actin and standard deviation for the 3 independent sets of experiments (right panels) were calculated as described for CDK5RAP2 vs actin above, with values from cells transfected with CDK5RAP2 siRNA #2 at day 5 normalized to 1.0. *p < 0.02. E Cells transfected with CDK5RAP2 siRNA #2 or control siRNA were subjected to SA-β-gal staining at days 1, 3, and 5 post-transfection. Representative images (upper panel) are from one of three independent experiments (n = 3) showing similar staining patterns. The number of SA-β-gal positive cells was assessed in ~200 cells per treatment group in each of the 3 independent experiments (n = 3). *p < 0.001.
Increased levels of SAHF, as well as p21CIP1 and p16INK4a, that also inhibit cell cycle Cdks, upon loss of CDK5RAP2, led us to test whether CDK5RAP2 loss affects cell proliferation. Indeed, we found that CDK5RAP2-depleted cells proliferate at a slower rate compared to control cells (Supplementary Fig. 2A). Flow cytometry showed an increased population of cells at G0/G1, but a reduced population of cells at S in CDK5RAP2-depleted cells compared to control cells (Supplementary Fig. 2B). In addition, a reduced number of Ki-67 positive cells was observed in CDK5RAP2-depleted cells compared to control cells (Supplementary Fig. 2C). Altogether, these findings indicate that CDK5RAP2 loss triggers senescence-associated proliferation arrest.
Reduced body weight and size of Cdk5rap2 an/an embryos are associated with increased senescence and reduced proliferation in corresponding MEFs
The availability of Hertwig’s anemia (an) mutant mice (Cdk5rap2an/an) that carry exon 4 inversions in the Cdk5rap2 gene, allowed us to test whether such Cdk5rap2an/an mutation affects body weight and size, and whether the senescence-associated phenotypes observed in CDK5RAP2-deficient BJ cells are also exhibited in Cdk5rap2an/an embryos and MEFs. Since Cdk5rap2an/an embryos are likely to die in late embryonic stages, we isolated and compared body weights of embryonic day 17.5 (E17.5) Cdk5rap2+/+, Cdk5rap2+/an, and Cdk5rap2an/an embryos from pregnant Cdk5rap2+/an mice. As shown in Fig. 2A, E17.5 Cdk5rap2an/an embryos weigh less than the Cdk5rap2+/+ and Cdk5rap2+/an embryos. A similar pattern was noted for average weights of E12.5 to E17.5 Cdk5rap2+/+, Cdk5rap2+/an, and Cdk5rap2an/an embryos (Fig. 2A, right panel). We then tested whether Cdk5rap2an/an embryos with reduced body weight exhibit increased senescence compared with Cdk5rap2+/an and Cdk5rap2+/+ embryos. To do so, E12.5-E14.5 littermate embryos were subjected to whole-mount SA-β-gal staining. As shown in Fig. 2B, Cdk5rap2an/an embryos with reduced body weight display increased SA-β-gal staining compared to Cdk5rap2+/+ and Cdk5rap2+/an embryos. Next, MEFs isolated from E12.5 Cdk5rap2+/+, Cdk5rap2+/an and Cdk5rap2an/an embryos were examined for the appearance of SA-β-gal positive cells. Figure 2C (left panel) shows that the number of SA-β-gal positive cells in Cdk5rap2an/an MEFs is remarkably greater than those in Cdk5rap2+/+ and Cdk5rap2+/an MEFs. In addition, Cdk5rap2an/an MEFs exhibit decreased proliferation (Fig. 2D) compared to Cdk5rap2+/+ and Cdk5rap2+/an MEFs. Thus, the senescence-associated phenotypes that we observed in CDK5RAP2-depleted BJ cells arerecapitulated in Cdk5rap2an/an embryos and ex vivo MEFs.
A Average weights of isolated E17.5 (left panel) and E12.5 to E17.5 (right panel). Cdk5rap2+/+, Cdk5rap2+/an, and Cdk5rap2an/an embryos from different litters are shown. B SA-β-gal staining of E12.5-E14.5 Cdk5rap2an/an and Cdk5rap2+/an and Cdk5rap2+/+ littermate embryos was performed as described in Materials and Methods. C MEFs isolated from Cdk5rap2+/+, Cdk5rap2+/an, and Cdk5rap2an/an embryos were subjected to SA-β-gal staining. Representative images (left panel) are from one of three independent experiments (n = 3) showing similar staining patterns. The number of SA-β-gal positive cells was assessed in ~200 cells per treatment group in each of the 3 independent experiments (n = 3). *p = 0.0002. D Growth of MEFs obtained from Cdk5rap2+/+, Cdk5rap2+/an, and Cdk5rap2an/an embryos were analyzed by cell viability assay.
Cell senescence due to loss of CDK5RAP2 is linked to increased p53 Ser15 phosphorylation via WIP1 downregulation
p53 plays a key role in triggering SA-β-gal expression. It is phosphorylated at Ser15 by ATM, Chk1, or Chk2 [22,23,24]. Upon DNA damage and presence of other stressors that induce cellular senescence [25, Cell culture BJ cells were cultured in Eagle’s minimal essential medium (EMEM, Lonza) containing 10% fetal bovine serum (FBS, GIBCO), and 50 U/ml penicillin and 50 mg/ml streptomycin (Invitrogen, Carlsbad). HEK293 human embryonic kidney cells were cultured in Dulbecco’s modified Eagle medium (DMEM, Invitrogen), containing 10% FBS, 50 U/ml penicillin and 50 mg/ml streptomycin. Cells were maintained at 37 °C in a 5% CO2 humidified incubator. After recovering from cryopreservation, BJ cells were used for up to 10 additional population doublings to maintains many characteristics of normal primary cells. Cells were tested for mycoplasma contamination. Primary MEFs were isolated from E12.5 Cdk5rap2+/+, Cdk5rap2+/an and Cdk5rap2an/an embryos as described previously [53]. Briefly, embryos were washed with 1x PBS, decapitated and eviscerated then washed again with PBS. Embryos were minced using sterile forceps and placed in 3–5 ml of 0.05% trypsin-EDTA, pipetted up and down to get cells into suspension and incubated at 37 °C for 5 min. Cell suspensions were transferred to tubes containing MEF medium (DMEM-high glucose supplemented with 10% FBS, 50 U/ml penicillin and 50 mg/ml streptomycin (Invitrogen, Carlsbad), and 2 mM GlutaMAX) then centrifuged at 1000 rpm for 5 min. Cell pellets were resuspended in fresh media and plated in 10 cm cell culture dishes. Primary MEFs were cultured in DMEM supplemented with 10% FBS and 50 U/ml penicillin and 50 mg/ml streptomycin (Invitrogen, Carlsbad) under hypoxic condition (5% O2 and 5% CO2 incubator). All experiments were performed in passage P2-P7 MEFs. Cells cultured ~18 h and at about 60% confluency were transfected using Lipofectamine 2000 (Invitrogen) in serum-free medium, which was replaced with complete medium 5 h post-transfection. Cells were harvested at different time points as indicated. siRNA target sequences are: control, CGUACGCGGAAUACUUCGAUU; CDK5RAP2 #1, GGACGUGUUGCUUCAGAAAUU; CDK5RAP2 #2, GAGUCAGCCUUCUGCUAAAUU; WIP1, CCAAUGAAGAUGAGUUAUAUU; GSK3β, AGGAGACCACGACCUGUUAAUU; β-catenin, CTCGGGATGTTCACAACCGAA. Adenovirus infection was carried out at a MOI of 50–100. Media was then replaced with EMEM containing 10% FBS 24 h post infection and cells were incubated until the indicated time. We have previously tested and established the specificity of CDK5RAP2 siRNA #1 and #2 effects in BJ cells [54]. Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol, and transcribed into cDNA using high-capacity cDNA reverse transcription kit (Thermo Fisher, Waltham, MA). Real-time qRT-PCR was performed using power SYBR® green PCR master mix (Thermo Fisher, Waltham, MA), and an Applied Biosystems 7500 real-time PCR machine using a standard protocol. The PCR conditions were 35 cycles at 94 °C for 20 s, 60 °C for 20 s and 72 °C for 35 s. The primer sets used were: WIP1-F, GGGAGTGATGGACTTTGGAA; WIP1-R, CAAGATTGTCCATGCTCACC; GAPDH-F, GGAGCGAGATCCCTCCAAAAT; GAPDH-R, GGCTGTTGTCATACTTCTCATGG. GAPDH was used for normalization. Cell lysates (50 μg) were resolved by SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted for CDK5RAP2, p21CIP1, p16INK4a, p53, phospho-p53, Ras, histone, GSK3β, phosphoSer9-GSK3β, ATM, phosphoSer1981-ATM, Chk1, phosphoSer345-Chk1, Chk2, phosphoThr68-Chk2, β-catenin, WIP1 and actin. Following incubation with HRP-conjugated anti-rabbit or anti-mouse secondary antibody, immunoreactive bands were detected using the ECL reagent (GE Healthcare, Little Chalfont, Buckinghamshire, UK). Western blot images were obtained using the ChemiDoc™ Imager (Bio-Rad) set at optimal exposure. No enhancements were performed. Cells transfected with CDK5RAP2 or control siRNA on coverslips were fixed with 4% paraformaldehyde/PBS for 10 min, permeabilized using 0.1% Triton X-100/PBS for 10 min, then blocked in 2% BSA/PBS for 1 h at room temperature. Coverslips incubated with the indicated primary antibody for 1 h followed by 20 min incubation with secondary antibodies were washed with 1x PBS, counterstained with DAPI, and mounted on glass slides using ProLong™ Diamond Antifade Mountant (P36961, Invitrogen, Carlsbad, CA, USA). Images were captured using a Zeiss Axiovert 200 microscope. BJ cells transfected with CDK5RAP2 siRNA #2 were analyzed for Ki-67 positive cells 3 days post-transfection. Cells were fixed using 3% paraformaldehyde in PBS (pH 6.0) and stained with 1 mg/ml 5-bromo-4-chloro-indolyl-β-D-galactopyranoside (X-gal) solution containing 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 150 mM NaCl, and 2 mM MgCl2 in PBS (pH 6.0) for 16–20 h at 37 °C. For whole-mount SA-β-gal staining, E13.5 embryo littermates from Cdk5rap2+/an heterozygous crosses were isolated in ice-cold PBS. Tails were cut and used for genoty** as described above. Embryos were then fixed in 4% paraformaldehyde in PBS (pH 7.0) overnight at 4 °C, stained with 1 mg/ml 5-bromo-4-chloro-indolyl-β-D-galactopyranoside (X-gal) in 0.2 M citric acid/sodium phosphate buffer (pH 6.0), containing 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 150 mM NaCl and 2 mM MgCl2, for 6 h at 37 °C. Images were taken under a dissecting microscope. Representative images are from one of three independent experiments (n = 3) showing similar staining patterns. BJ cells transfected with CDK5RAP2 siRNA #2 or control siRNA were seeded into 96-well plates at 5000 cells per well 24 h post transfection, then analyzed for cell viability at days 0, 2, and 4 using Cell Counting Kit-8 (CCK-8, Do**do). For analysis of cell cycle distribution, cells transfected with CDK5RAP2 siRNA #2 or control siRNA were fixed using cold 2% formaldehyde and stained with 7-AAD (5 μg/μl) then subjected to flow cytometry. HEK293 cells transfected with Myc-tagged CDK5RAP2 or Flag-tagged GSK3β or both for 48 h were lysed in ice-cold lysis buffer containing 50 mM Tris/pH 8.0, 150 mM NaCl, 1% NP-40, 10 mM EDTA, 5% glycerol, 1 mM phenylmethylsulfonylflouride (PMSF), 10 µg/ml aprotinin, and 10 µg/ml leupeptin. Lysates were then clarified by centrifuged at 13,000 rpm for 25 min at 4 °C. For immunoprecipitation under denaturing condition, 1% SDS was added to the lysis buffer. Lysates were pre-cleared by adding normal mouse or rabbit IgG + sepharose A/G beads and incubating at 4 °C for 2 h followed by centrifugation at 14,000 rpm for 10 min. One-tenth of the lysates from each sample was retained and used to assess input or total cell lysate in western blots. Pre-cleared cell lysates were subjected to immunoprecipitation using Myc or Flag antibody conjugated to protein A/G agarose beads. After incubating the mixture overnight at 4 °C, immunoprecipitates were washed with lysis buffer three times at 4 °C. GSK3β activity was measured using the GSK-3β activity assay kit (Sigma, ON, Canada) following the manufacturer’s protocol. Briefly, cells transfected with CDK5RAP2 or control siRNA were lysed in ice-cold lysis buffer containing 50 mM Tris/pH 8.0, 150 mM NaCl, 1% NP-40, 10 mM EDTA, 5% glycerol, 1 mM phenylmethylsulfonylflouride (PMSF), 10 µg/ml aprotinin and 10 µg/ml leupeptin. Cell lysates (300 µg) were incubated with 2 µl of anti-GSK-3β and 30 µl EZview Red protein-G affinity gel beads at 4 °C for 3 h. The beads were recovered by centrifugation at 8000 × g for 30 sec and washing with 500 µl of ice-cold lysis buffer. The immunoprecipitates were mixed with 20 µl of reaction mixture containing 25 μCi γ32P-ATP and 5 µl of GSK-3β substrate solution, incubated at 37 °C for 30 m, then spotted onto P81 phosphate cellulose membranes. Membranes were washed 4 times with 0.5% phosphoric acid and once with acetone. Counting of incorporated radioactivity was performed using a Beckman scintillation counter. GST-CDK5RAP2 was cloned into pFastBac vectors from which baculovirus was generated according to the Bac-to-Bac® baculovirus protein expression system (Thermo Fisher, Waltham, MA). Sf9 insect cells were infected with P2 baculovirus carrying GST-CDK5RAP2 for 24 h. GST-CDK5RAP2 was purified by affinity chromatography using a glutathione (GSH)-conjugated agarose column (Sigma-Aldrich, St. Louis, MO). Sf9 cell lysates expressing GST-CDK5RAP2 were incubated with GSH-agarose for 1 h at 4 ˚C and washed with 1x PBS containing 1% Triton X-100. Bound proteins were eluted with 10 mM reduced GSH in 50 mM Tris elution buffer (pH 8). GST-CDK5RAP2 (1 μmole) bound to glutathione-agarose beads was mixed with GSK3β (2 μg) at 4 °C for 2 h. The pulled-down complex was washed 4 times with ice-cold GST lysis buffer (20 mM Tris-HCl (pH 8.0), 200 mM NaCl, 1 mM EDTA (pH 8.0), 0.5% NP-40, 2 µg/µl aprotinin, 1 µg/µl leupeptin, 0.7 µg/ml pepstatin and 25 µg/ml PMSF) by centrifugation at 2500 rpm for 10 min and analyzed by SDS-PAGE and immunoblotting for GST and GSK3β. Using genomic DNAs isolated from HEK293 cells as a template, the WIP1 promoter region was amplified by PCR using the primer set: ACATTTTCTTGAGCTGATTTTGCTT (WIP1-F1) and TCGGAGAAGACGCTCACTCC (WIP1-R1). The promoter region for WIP1-ΔκB was generated by PCR using two different sets of primers: ACATTTTCTTGAGCTGATTTTGCTT (WIP1-F1) and GTTTAAAAAGCACtta accgtcagct (WIP1-R2), and ACCGAGACTGTGCagctgacggttaaGTGCTT (WIP1-F2) and TCGGAGAAGACGC TCACTCC (WIP1-R1), with overlap** fragments (lowercase letters) in WIP1-R2 and WIP1-F2. Two PCR products were annealed and used as templates for subsequent 8 fusion PCR cycles [55]. PCR products were then purified using the GeneJET PCR Purification Kit (Thermo Fisher, Waltham, MA) and used as templates for PCR amplification using the WIP1-F1 and WIP1-R2 primer set. Generated WIP1 and WIP1-ΔκB promoter PCR products were cloned into pGL3-basic luciferase reporter vector (Promega, Madison, WI, USA) using XhoI and BglII restriction enzyme sites and designated as pGL3-WIP1 and pGL3-WIP1-ΔκB, respectively. Successful cloning was confirmed by DNA sequencing. To measure luciferase activity, HEK293 cells were co-transfected with the indicated siRNA, pGL3-WIP1 or pGL3-WIP1-ΔκB and pTK-Renilla luciferase vector using lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Cell extracts were prepared 48 h after transfection and luciferase activity was measured using the Dual-Luciferase Reporter assay system (Promega, Madison, WI, USA). Renilla luciferase served as an internal control for normalization. Student’s t-test (unpaired, two-sided) or one or two-way analysis of variance (ANOVA) was used. Significance was set at p < 0.05.Isolation of primary MEFs
Plasmid/siRNA transfection and adenovirus infection
RNA extraction and real-time qRT-PCR
Western blot analysis
Immunofluorescence microscopy
Senescence-associated β-galactosidase staining
Cell viability and cell cycle analyses
Immunoprecipitation
Measurement of GSK3β activity
Expression and purification of GST- CDK5RAP2
GST pull-down assay
Generation of promoter constructs of wt WIP1 (pGL3-WIP1) and WIP1 with NF-κB binding site deletion (pGL3-WIP1-ΔκB)
Luciferase assay
Statistical analysis
References
Ching YP, Qi Z, Wang JH. Cloning of three novel neuronal Cdk5 activator binding proteins. Gene. 2000;242:285–94.
Jia Y, Fong KW, Choi YK, See SS, Qi RZ. Dynamic recruitment of CDK5RAP2 to centrosomes requires its association with dynein. PloS one. 2013;8:e68523.
Graser S, Stierhof YD, Nigg EA. Cep68 and Cep215 (Cdk5rap2) are required for centrosome cohesion. J Cell Sci. 2007;120:4321–31.
Kim S, Rhee K. Importance of the CEP215-pericentrin interaction for centrosome maturation during mitosis. PloS One. 2014;9:e87016.
Hanafusa H, Kedashiro S, Tezuka M, Funatsu M, Usami S, Toyoshima F, et al. PLK1-dependent activation of LRRK1 regulates spindle orientation by phosphorylating CDK5RAP2. Nat Cell Biol. 2015;17:1024–35.
Bond J, Roberts E, Springell K, Lizarraga SB, Scott S, Higgins J, et al. A centrosomal mechanism involving CDK5RAP2 and CENPJ controls brain size. Nat Genet. 2005;37:353–5.
Rosales JL, Rattner JB, Lee KY. The primary microcephaly 3 (MCPH3) interacting protein, p35 and its catalytic subunit, Cdk5, are centrosomal proteins. Cell Cycle. 2010;9:618–20.
Nasser H, Vera L, Elmaleh-Berges M, Steindl K, Letard P, Teissier N, et al. CDK5RAP2 primary microcephaly is associated with hypothalamic, retinal and cochlear developmental defects. J Med Genet. 2020;57:389–99.
Chavali PL, Chandrasekaran G, Barr AR, Tatrai P, Taylor C, Papachristou EK, et al. A CEP215-HSET complex links centrosomes with spindle poles and drives centrosome clustering in cancer. Nat Commun. 2016;7:11005.
Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–9.
Yigit G, Brown KE, Kayserili H, Pohl E, Caliebe A, Zahnleiter D, et al. Mutations in CDK5RAP2 cause Seckel syndrome. Mol Genet Genom Med. 2015;3:467–80.
Ogrodnik M, Salmonowicz H, Jurk D, Passos JF. Expansion and cell-cycle arrest: Common denominators of cellular Senescence. Trends Biochemical Sci. 2019;44:996–1008.
Cai B, Ma W, Bi C, Yang F, Zhang L, Han Z, et al. Long noncoding RNA H19 mediates melatonin inhibition of premature senescence of c-kit(+) cardiac progenitor cells by promoting miR-675. J Pineal Res. 2016;61:82–95.
Lee JS, Mo Y, Gan H, Burgess RJ, Baker DJ, van Deursen JM, et al. Pak2 kinase promotes cellular senescence and organismal aging. Proc Natl Acad Sci USA. 2019;116:13311–9.
Rajarajacholan UK, Riabowol K. Aging with ING: A comparative study of different forms of stress induced premature senescence. Oncotarget. 2015;6:34118–27.
De Meyer T, Nawrot T, Bekaert S, De Buyzere ML, Rietzschel ER, Andres V. Telomere Length as Cardiovascular Aging Biomarker: JACC Review Topic of the Week. J Am Coll Cardiol. 2018;72:805–13.
Zhu H, Blake S, Kusuma FK, Pearson RB, Kang J, Chan KT. Oncogene-induced senescence: From biology to therapy. Mech Ageing Development. 2020;187:111229.
Gu Y, Han J, Jiang C, Zhang Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res Rev. 2020;59:101036.
Ou HL, Schumacher B. DNA damage responses and p53 in the aging process. Blood. 2018;131:488–95.
Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24:2463–79.
Mallette FA, Gaumont-Leclerc MF, Ferbeyre G. The DNA damage signaling pathway is a critical mediator of oncogene-induced senescence. Genes Dev. 2007;21:43–48.
Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–7.
Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–42.
Kruse JP, Gu W. Modes of p53 regulation. Cell. 2009;137:609–22.
Catalano A, Rodilossi S, Caprari P, Coppola V, Procopio A. 5-Lipoxygenase regulates senescence-like growth arrest by promoting ROS-dependent p53 activation. EMBO J. 2005;24:170–9.
Lin W, Zhao Z, Ni Z, Zhao Y, Du W, Chen S. IFI16 restoration in hepatocellular carcinoma induces tumour inhibition via activation of p53 signals and inflammasome. Cell Prolif. 2017;50:e12392.
Georgakilas AG, Martin OA, Bonner WM. p21: A two-faced genome guardian. Trends Mol Med. 2017;23:310–9.
Sakai H, Fujigaki H, Mazur SJ, Appella E. Wild-type p53-induced phosphatase 1 (Wip1) forestalls cellular premature senescence at physiological oxygen levels by regulating DNA damage response signaling during DNA replication. Cell Cycle. 2014;13:1015–29.
Chen Z, Yi W, Morita Y, Wang H, Cong Y, Liu JP, et al. Wip1 deficiency impairs haematopoietic stem cell function via p53 and mTORC1 pathways. Nat Commun. 2015;6:6808.
Tang Y, Liu L, Sheng M, **ong K, Huang L, Gao Q, et al. Wip1 knockout inhibits the proliferation and enhances the migration of bone marrow mesenchymal stem cells. Exp Cell Res. 2015;334:310–22.
Cha BH, Lee JS, Kim SW, Cha HJ, Lee SH. The modulation of the oxidative stress response in chondrocytes by Wip1 and its effect on senescence and dedifferentiation during in vitro expansion. Biomaterials. 2013;34:2380–8.
Lowe JM, Cha H, Yang Q, Fornace AJ Jr. Nuclear factor-kappaB (NF-kappaB) is a novel positive transcriptional regulator of the oncogenic Wip1 phosphatase. J Biol Chem. 2010;285:5249–57.
Ma B, Hottiger MO. Crosstalk between Wnt/beta-Catenin and NF-kappaB Signaling Pathway during Inflammation. Front Immunol. 2016;7:378.
Blankesteijn WM, van de Schans VA, ter Horst P, Smits JF. The Wnt/frizzled/GSK-3 beta pathway: A novel therapeutic target for cardiac hypertrophy. Trends Pharmacol Sci. 2008;29:175–80.
Lizarraga SB, Margossian SP, Harris MH, Campagna DR, Han AP, Blevins S, et al. Cdk5rap2 regulates centrosome function and chromosome segregation in neuronal progenitors. Development. 2010;137:1907–17.
Kilic Eren M, Tabor V. The role of hypoxia inducible factor-1 alpha in bypassing oncogene-induced senescence. PloS One. 2014;9:e101064.
Aird KM, Zhang R. Detection of senescence-associated heterochromatin foci (SAHF). Methods Mol Biol. 2013;965:185–96.
Severino J, Allen RG, Balin S, Balin A, Cristofalo VJ. Is beta-galactosidase staining a marker of senescence in vitro and in vivo? Exp Cell Res. 2000;257:162–71.
Zhang Y, Zhang Y, Zhong C, **ao F. Cr(VI) induces premature senescence through ROS-mediated p53 pathway in L-02 hepatocytes. Sci Rep. 2016;6:34578.
Feng Y, **a Y, Yu G, Shu X, Ge H, Zeng K. et al.Cleavage of GSK-3beta by calpain counteracts the inhibitory effect of Ser9 phosphorylation on GSK-3beta activity induced by H(2)O(2). J Neurochem. 2013;126:234–42.
Shimizu I, Minamino T. Cellular senescence in cardiac diseases. J Cardiol. 2019;74:313–9.
Park JS, Lee MK, Rosales JL, Lee KY. Primary microcephaly 3 (MCPH3): revisiting two critical mutations. Cell Cycle. 2011;10:1331–3.
Barrera JA, Kao LR, Hammer RE, Seemann J, Fuchs JL, Megraw TL. CDK5RAP2 regulates centriole engagement and cohesion in mice. Developmental Cell. 2010;18:913–26.
Tang X, Zheng D, Hu P, Zeng Z, Li M, Tucker L, et al. Glycogen synthase kinase 3 beta inhibits microRNA-183-96-182 cluster via the beta-Catenin/TCF/LEF-1 pathway in gastric cancer cells. Nucleic Acids Res. 2014;42:2988–98.
Rouillard AD, Gundersen GW, Fernandez NF, Wang Z, Monteiro CD, McDermott MG, et al. The harmonizome: a collection of processed datasets gathered to serve and mine knowledge about genes and proteins. J Biol databases Curation. 2016, 2016. https://doi.org/10.1093/database/baw100.
Fumoto K, Hoogenraad CC, Kikuchi A. GSK-3beta-regulated interaction of BICD with dynein is involved in microtubule anchorage at centrosome. EMBO J. 2006;25:5670–82.
Yoshino Y, Ishioka C. Inhibition of glycogen synthase kinase-3 beta induces apoptosis and mitotic catastrophe by disrupting centrosome regulation in cancer cells. Sci Rep. 2015;5:13249.
Fong KW, Choi YK, Rattner JB, Qi RZ. CDK5RAP2 is a pericentriolar protein that functions in centrosomal attachment of the gamma-tubulin ring complex. Mol Biol cell. 2008;19:115–25.
Wang Z, Wu T, Shi L, Zhang L, Zheng W, Qu JY, et al. Conserved motif of CDK5RAP2 mediates its localization to centrosomes and the Golgi complex. J Biol Chem. 2010;285:22658–65.
Leonard MK, Hill NT, Bubulya PA, Kadakia MP. The PTEN-Akt pathway impacts the integrity and composition of mitotic centrosomes. Cell Cycle. 2013;12:1406–15.
Wilkinson MB, Dias C, Magida J, Mazei-Robison M, Lobo M, Kennedy P, et al. A novel role of the WNT-dishevelled-GSK3beta signaling cascade in the mouse nucleus accumbens in a social defeat model of depression. J Neurosci: Off J Soc Neurosci. 2011;31:9084–92.
Aschenbach WG, Ho RC, Sakamoto K, Fujii N, Li Y, Kim YB, et al. Regulation of dishevelled and beta-catenin in rat skeletal muscle: an alternative exercise-induced GSK-3beta signaling pathway. Am J Physiol Endocrinol Metab. 2006;291:E152–8.
NavaneethaKrishnan S, Rosales JL, Lee KY. mPTP opening caused by Cdk5 loss is due to increased mitochondrial Ca(2+) uptake. Oncogene. 2020;39:2797–806.
Wang X, Rosales JL, Gao X, Lee KY. Centromeric chromatin integrity is compromised by loss of Cdk5rap2, a transcriptional activator of CENP-A. Biomed. Pharmacother. 2021;138:111463.
**ong AS, Yao QH, Peng RH, Duan H, Li X, Fan HQ, et al. PCR-based accurate synthesis of long DNA sequences. Nat Protoc. 2006;1:791–7.
Acknowledgements
We thank Tara Beattie and Karl Riabowol at the University of Calgary for providing us BJ cells, and adenovirus carrying Ras V12 (Adeno-Ras V12), and control virus carrying GFP alone, respectively. This work was supported in part by a grant from the NSERC (RGPIN/06270-2019) to KYL.
Author information
Authors and Affiliations
Contributions
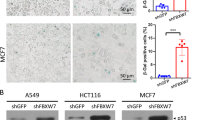
XW performed most of the experiments and drafted the manuscript. PS performed the experiments described in Fig. 2, and Supplementary Fig. 4. ZS performed the experiments for data presented in Fig. 7. XG served as a cotutelle supervisor at Harbin Medical University. JLR and KYL contributed to the analysis and interpretation of data, provided constructive comments, and critically revised the paper for important intellectual content, and wrote the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
All animal studies conformed to regulatory standards and were approved by the University of Calgary Health Sciences Animal Care Committee.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Professor Daniel Aberdam
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, X., Sipila, P., Si, Z. et al. CDK5RAP2 loss-of-function causes premature cell senescence via the GSK3β/β-catenin-WIP1 pathway. Cell Death Dis 13, 9 (2022). https://doi.org/10.1038/s41419-021-04457-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-021-04457-2
- Springer Nature Limited