Abstract
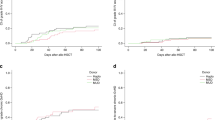
Despite novel cellular and immunomodulatory therapies, allogeneic hematopoietic stem cell transplantation (HSCT) remains a treatment option for lymphoid malignancies. Post-transplant cyclophosphamide (PTCY) is increasingly employed for graft vs. host disease (GVHD) prophylaxis. This study aims to evaluate the safety and efficacy of PTCY in reduce intensity (RIC) HSCT for patients with lymphoid neoplasms compared to sirolimus with tacrolimus (SIR/TAC). The primary endpoint was to compare grade III-IV acute GVHD, severe chronic GVHD, and relapse-free survival (GRFS) between the two GVHD prophylaxis strategies. This study, conducted from January 2012 to December 2020, included 171 consecutive patients (82 in the PTCY and 89 in the SIR/TAC group). Results revealed a significantly decreased incidence of moderate and severe forms of chronic GVHD in PTCY cohort (5.8% [95% CI, 1.8 to 13.1]) versus the SIR/TAC cohort (39.6% [95% CI, 29.3 to 49.7] (p < 0.001)). Other outcomes, including GRFS (PTCY [45.9% (95% CI, 35.8–58.7)] and SIR/TAC groups [36.8% (95% CI, 28–48.4)], (p = 0.72)), non-relapse mortality (NRM), relapse and overall survival (OS) were similar in both groups. Interestingly, the failure to achieve GRFS was mainly attributed to GVHD in the SIR/TAC group, while disease relapse was the primary reason in the PTCY cohort.



Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Snowden JA, Sánchez-ortega I, Corbacioglu S, Basak GW, Chabannon C, Camara R, et al. Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022. 2022;(May).
Cieri N, Maurer K, Wu CJ. 60 years young: The evolving role of allogeneic hematopoietic stem cell transplantation in cancer immunotherapy. Cancer Res. 2021;81:4373–84.
Perez-Simón JA, Martino R, Parody R, Cabrero M, Lopez-Corral L, Valcarcel D, et al. The combination of sirolimus plus tacrolimus improves outcome after reduced-intensity conditioning, unrelated donor hematopoietic stem cell transplantation compared with cyclosporine plus mycofenolate. Haematologica. 2013;98:526–32.
Bailén R, Kwon M, Pascual-Cascón MJ, Ferrà C, Sanz J, Gallardo-Morillo A, et al. Post-transplant cyclophosphamide for GVHD prophylaxis compared to ATG-based prophylaxis in unrelated donor transplantation. Ann Hematol. 2021;100:541–53. http://springer.longhoe.net/10.1007/s00277-020-04317-7.
Mehta RohteshS, Saliba RimaM, Chen Julianne, Rondon Gabriela, Hammerstrom AimeeE, Alousi Amin, et al. Post-transplantation cyclophosphamide versus conventional graft-versus-host disease prophylaxis in mismatched unrelated donor haematopoietic cell transplantation. Br J Haematol. 2016;173:444–55.
Maurer K, Ho VT, Inyang E, Cutler CS, Koreth J, Shapiro RM, et al. Posttransplant cyclophosphamide vs tacrolimus-based GVHD prophylaxis: lower incidence of relapse and chronic GVHD. Blood Adv. 2023;7:3903–15. https://doi.org/10.1182/bloodadvances.2023009791.
McCurdy SR, Luznik L. Relapse after allogeneic transplantation with post-transplant cyclophosphamide: Shattering myths and evolving insight. Blood Rev. 2023:101093. https://doi.org/10.1016/j.blre.2023.101093
Mussetti A, Kanate AS, Wang T, He M, Hamadani M, Finel H, et al. Haploidentical versus matched unrelated donor transplants using post-transplantation cyclophosphamide for lymphomas. Transpl Cell Ther. 2023;29:184.e1–184.e9.
Bolaños-Meade J, Hamadani M, Wu J, Al Malki MM, Martens MJ, Runaas L, et al. Post-transplantation cyclophosphamide-based graft-versus-host disease prophylaxis. N. Engl J Med. 2023;388:2338–48.
García-Cadenas I, Esquirol A, Bosch-Vilaseca A, Awol R, Novelli S, Saavedra S, et al. Patterns of infection and infectious-related mortality in patients receiving post-transplant high dose cyclophosphamide as graft-versus-host-disease prophylaxis: impact of HLA donor matching. Bone Marrow Transpl. 2021;56:818–27. https://doi.org/10.1038/s41409-020-01092-x.
Ruggeri A, Labopin M, Ciceri F, Mohty M, Nagler A. Definition of GvHD-free, relapse-free survival for registry-based studies: An ALWP-EBMT analysis on patients with AML in remission. Bone Marrow Transpl. 2016;51:610–1.
Jagasia MH, Greinix HT, Arora M, Williams KM, Cheng G, Kerr H, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease. Biol Blood Marrow Transpl. 2015;21:389–401.
Copelan E, Casper JT, Carter SL, van Burik JAH, Hurd D, Mendizabal AM, et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biol Blood Marrow Transpl. 2007;13:1469–76.
Sathish N, Wu CA. Let’s flip: an approach to understand median follow-up by the reverse Kaplan-Meier estimator from a statistical programmer’s perspective. PharmaSUG. 2019;1:5.
Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–79.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46:399–424.
Cutler C, Logan B, Nakamura R, Johnston L, Choi S, Porter D, et al. Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood. 2014;124:1372–7.
Ho VT, Aldridge J, Kim HT, Cutler C, Koreth J, Antin JH, et al. Comparison of Tacrolimus and Sirolimus (Tac/Sir) versus Tacrolimus, Sirolimus, and mini-methotrexate (Tac/Sir/MTX) as acute graft-versus-host disease prophylaxis after reduced-intensity conditioning allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transpl. 2009;15:844–50.
Khimani F, Kim J, Chen L, Dean E, Rizk V, Betts B, et al. Predictors of overall survival among patients treated with sirolimus/tacrolimus vs methotrexate/tacrolimus for GvHD prevention. Bone Marrow Transpl. 2017;52:1003–9. https://doi.org/10.1038/bmt.2017.63.
Kanakry CG, O’Donnell PV, Furlong T, De Lima MJ, Wei W, Medeot M, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. 2014;32:3497–505.
Broers AEC, de Jong CN, Bakunina K, Hazenberg MD, van Marwijk Kooy M, de Groot MR, et al. Posttransplant cyclophosphamide for prevention of graft-versus-host disease: results of the prospective randomized HOVON-96 trial. Blood Adv. 2022;6:3378–85.
Mehta RS, Saliba RM, Rondon G, Al-Atrash G, Bashir Q, Hosing CM, et al. Post-transplantation cyclophosphamide versus tacrolimus and methotrexate graft-versus-host disease prophylaxis for HLA-matched donor transplantation. Transpl Cell Ther. 2022;28:695.e1–695.e10. https://doi.org/10.1016/j.jtct.2022.07.021.
Holtan SG, Hamadani M, WU J, AL Malki MM, Runaas L, Elmariah H, et al. Post-transplant cyclophosphamide, tacrolimus, and mycophenolate mofetil as the new standard for graft-versus-host disease (GVHD) prophylaxis in reduced intensity conditioning: results from phase III BMT CTN 1703. Blood. 2022;140:LBA-4–LBA-4. https://doi.org/10.1182/blood-2022-171463.
García-Cadenas I, Redondo S, Esquirol A, Portos JM, Novelli S, Saavedra S, et al. Successful outcome in patients with myelofibrosis undergoing allogeneic donor hematopoietic cell transplantation using reduced doses of post-transplantation cyclophosphamide: challenges and review of the literature. Transpl Cell Ther. 2023;29:1–6.
McAdams MJ, Hyder M, Dimitrova D, Sadler JL, McKeown C, Steinberg SM, et al. Phase I/II study of reduced dosing of post-transplantation cyclophosphamide (PTCy) after HLA-haploidentical bone marrow transplantation. Blood. 2021;138:101–101.
García-Cadenas I, Awol R, Esquirol A, Saavedra S, Bosch-Vilaseca A, Novelli S, et al. Incorporating posttransplant cyclophosphamide-based prophylaxis as standard-of-care outside the haploidentical setting: challenges and review of the literature. Bone Marrow Transpl. 2020;55:1041–9. https://doi.org/10.1038/s41409-019-0771-2.
Oltolini C, Greco R, Galli L, Clerici D, Lorentino F, Xue E, et al. Infections after allogenic transplant with post-transplant cyclophosphamide: impact of donor HLA matching. Biol Blood Marrow Transpl. 2020;26:1179–88.
Mehta RS, Saliba RM, Ghanem S, Alousi AM, Rondon G, Anderlini P, et al. Haploidentical versus matched unrelated versus matched sibling donor hematopoietic cell transplantation with post-transplantation cyclophosphamide. Transpl Cell Ther. 2022;28:395.e1–395.e11. https://doi.org/10.1016/j.jtct.2022.04.020.
Mikulska M, Bartalucci C, Raiola AM, Oltolini C. Does PTCY increase the risk of infections? Blood Rev. 2023:101092. https://doi.org/10.1016/j.blre.2023.101092.
McCurdy SR, Luznik L. Immune reconstitution after T-cell replete HLA-haploidentical transplantation. Semin Hematol. 2019;56:221–6.
Aldiwani M, Tharakan T, Al-Hassani A, Gibbons N, Pavlu J, Hrouda D. BK Virus Associated Haemorrhagic Cystitis. A systematic review of current prevention and treatment strategies. Int J Surg. 2019;63:34–42. https://doi.org/10.1016/j.ijsu.2019.01.019.
Salas MQ, Prem S, Atenafu EG, Datt Law A, Lam W, Al-Shaibani Z, et al. Dual T-cell depletion with ATG and PTCy for peripheral blood reduced intensity conditioning allo-HSCT results in very low rates of GVHD. Bone Marrow Transpl. 2020;55:1773–83. https://doi.org/10.1038/s41409-020-0813-9.
Mohyuddin GR, Roller J, Shune L, Lin T, Dias A, Ganguly S, et al. Epstein-Barr viremia and post-transplant lymphoproliferative disorders in patients undergoing haploidentical stem cell transplantation with post-transplant cyclophosphamide. Hematol Oncol Stem Cell Ther. 2019;12:171–3. https://doi.org/10.1016/j.hemonc.2018.11.002.
Imus PH, Tsai HL, DeZern AE, Jerde K, Swinnen LJ, Bolaños-Meade J, et al. Thrombotic Microangiopathy after Post-Transplantation Cyclophosphamide-Based Graft-versus-Host Disease Prophylaxis. Biol Blood Marrow Transpl. 2020;26:2306–10.
Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125:1333–8.
Acknowledgements
M.L.F. is PhD candidate at Universidad Autònoma de Barcelona. This work is submitted in partial fulfillment of the requirement for the PhD.
Author information
Authors and Affiliations
Consortia
Contributions
M.L.F. designed the research, analyzed results, made the figures and wrote the paper. I.G. C., P.B and D.V. designed the research; V.N. performed statistical analysis and made the figures; K.M., A.P.M., I.S.B., C.F.C, L.B.M., R.B., E.R., R. P.P, A.E. and A.G.M., collected data. And all authors contributed to the manuscript review and approved the final version for submission.
Corresponding author
Ethics declarations
Competing interests
R.B.A.: Speaker: Pfizer, Gilead. Travel: Jazz, Pfizer, Gilead, AstraZeneca. Funding: Jazz. M.K. Gilead, Novartis, BMS and Pfizer. All other authors declare no competing financial interests. The current affiliation for CF is Division of Hematology, Hospital del Mar, Barcelona, Spain.
Consent to participate
All transplantation centers are required to obtain written informed consent from all patients before data registration with the EBMT in accordance with the 1975 Helsinki Declaration.
Ethical approval
Institutional Review and Privacy Board of University Hospital Vall d´Hebron granted approval for this retrospective review (reference code HEM-TIO-2017-01).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fox, M.L., García-Cadenas, I., Navarro, V. et al. Post-transplant cyclophosphamide compared to sirolimus/tacrolimus in reduced intensity conditioning transplants for patients with lymphoid malignancies. Bone Marrow Transplant (2024). https://doi.org/10.1038/s41409-024-02322-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41409-024-02322-2
- Springer Nature Limited