Abstract
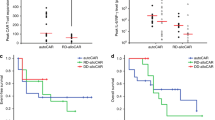
The efficacy and safety of donor-derived anti-CD19 CAR T cells vs DLI for the management of relapsed B-cell acute lymphoblastic leukemia (B-ALL) after allo-hematopoietic stem cell transplantation (HSCT) remain unclear. Thirteen B-ALL patients with relapsed after allo-HSCT and thus were treated with donor-derived anti-CD19 CAR T-cell (study group). Fifteen B-ALL patients relapsed after allo-HSCT and thus were treated with DLI (DLI group). The rates of MRD-negative complete remission (61.5%) in the study group were significantly higher than those in the DLI group (13.3%) (p = 0.02). The complete remission duration in study group and DLI group were median 8.0 months (range, 3–25 months) and 4.4 months (range, 1–25 months; p = 0.026), respectively. The overall survival of patients in the study group was superior to that of the DLI group: 9.5 months (range,3–25 months) versus 5.5 months (range, 1–25 months; p = 0.030). One patient with grade 1 acute graft-versus-host disease (aGVHD) was identified in the study group. While five (33.3%) patients in the DLI group developed grades III–IV aGVHD. Three patients (23.07%) developed grade 3 or 4 cytokine release syndrome in the study group. This study suggested that donor-derived anti-CD19 CAR T-cell therapy is promising, safe, and potentially effective for relapsed B-ALL after allo-HSCT and may be superior to DLI.


Similar content being viewed by others
References
Forman SJ, Rowe JM. The myth of the second remission of acute leukemia in the adult. Blood. 2013;121:1077–82.
Yan CH, Wang JZ, Liu DH, Xu LP, Chen H, Liu KY, et al. Chemotherapy followed by modified donor lymphocyte infusion as a treatment for relapsed acute leukemia after haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion: superior outcomes compared with chemotherapy alone and an analysis of prognostic factors. Eur J Haematol. 2013;91:304–14.
Huang XJ. Immunomodulatory strategies for relapse after haploidentical hematopoietic stem cell transplantation in hematologic malignancy patients. Best Pr Res Clin Haematol. 2011;24:351–8.
Yeh SP, Lin CC, Lin CH, Lo WC, Chen TT, Lo WJ, et al. Second haploidentical peripheral blood stem cell transplantation for treatment of acute leukemia with relapse after first allogeneic peripheral blood stem cell transplantation. Bone Marrow Transpl. 2015;50:1001–3.
Zeidan AM, Forde PM, Symons H, Chen A, Smith BD, Pratz K, et al. HLA-haploidentical donor lymphocyte infusions for patients with relapsed hematologic malignancies after related HLA-haploidentical bone marrow transplantation. Biol Blood Marrow Transpl. 2014;20:314–8.
Haen SP, Groh C, Schumm M, Backert L, Loffler MW, Federmann B, et al. Haploidentical hematopoietic cell transplantation using in vitro T cell depleted grafts as salvage therapy in patients with disease relapse after prior allogeneic transplantation. Ann Hematol. 2017;96:817–27.
Poon LM, Bassett R Jr, Rondon G, Hamdi A, Qazilbash M, Hosing C, et al. Outcomes of second allogeneic hematopoietic stem cell transplantation for patients with acute lymphoblastic leukemia. Bone Marrow Transpl. 2013;48:666–70.
Liga M, Triantafyllou E, Tiniakou M, Lambropoulou P, Karakantza M, Zoumbos NC, et al. High alloreactivity of low-dose prophylactic donor lymphocyte infusion in patients with acute leukemia undergoing allogeneic hematopoietic cell transplantation with an alemtuzumab-containing conditioning regimen. Biol Blood Marrow Transpl. 2013;19:75–81.
Porter DL, Levine BL, Bunin N, Figliola MJ, Bassett R, Olivares S, et al. A phase 1 trial of donor lymphocyte infusions expanded and activated ex vivo via CD3/CD28 costimulation. Blood. 2006;107:1325–31.
Roddie C, Peggs KS. Donor lymphocyte infusion following allogeneic hematopoietic stem cell transplantation. Expert Opin Biol Ther. 2011;11:473–87.
Davila ML, Brentjens RJ. CD19-Targeted CAR T cells as novel cancer immunotherapy for relapsed or refractory B-cell acute lymphoblastic leukemia. Clin Adv Hematol Oncol. 2016;14:802–8.
Kebriaei P, Singh H, Huls MH, Figliola MJ, Bassett R, Olivares S, et al. Phase I trials using Slee** Beauty to generate CD19-specific CAR T cells. J Clin Invest. 2016;126:3363–76.
Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–30.
Park JH, Geyer MB, Brentjens RJ. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood. 2016;127:3312–20.
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–17.
Lacey SFZZLB. Sustained remissions with CD19-specific chimeric antigen receptor (CAR)-modified T cells in children with relapsed/refractory ALL. J Clin Oncol. 2016;34:3011.
Brudno JN, Somerville RP, Shi V, Rose JJ, Halverson DC, Fowler DH, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol. 2016;34:1112–21.
Kochenderfer JN, Rosenberg SA. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol. 2013;10:267–76.
Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–95.
Ferrara JL LJRP. Graft-versus-host disease. Lancet. 2009;373:1550–61.
Sullivan KM, Shulman HM, Storb R, Weiden PL, Witherspoon RP, McDonald GB, et al. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood. 1981;57:267–76.
Chang YJ, Huang XJ. Donor lymphocyte infusions for relapse after allogeneic transplantation: when, if and for whom? Blood Rev. 2013;27:55–62.
Chalandon Y, Passweg JR, Schmid C, Olavarria E, Dazzi F, Simula MP, et al. Outcome of patients develo** GVHD after DLI given to treat CML relapse: a study by the Chronic Leukemia Working Party of the EBMT. Bone Marrow Transpl. 2010;45:558–64.
Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112:4371–83.
Collins RJ, Goldstein S, Giralt S, Levine J, Porter D, Drobyski W, et al. Donor leukocyte infusions in acute lymphocytic leukemia. Bone Marrow Transpl. 2000;26:511–6.
Shiobara S, Nakao S, Ueda M, Yamazaki H, Takahashi S, Asano S, et al. Donor leukocyte infusion for Japanese patients with relapsed leukemia after allogeneic bone marrow transplantation: lower incidence of acute graft-versus-host disease and improved outcome. Bone Marrow Transpl. 2000;26:769–74.
Chang X, Zang X, **a CQ. New strategies of DLI in the management of relapse of hematological malignancies after allogeneic hematopoietic SCT. Bone Marrow Transpl. 2016;51:324–32.
Wang T, Gao L, Hu X, Liu B, Chen J, Zhang WP, et al. Chimeric antigen receptor-modified donor lymphocyte infusion improves the survival of acute lymphoblastic leukemia patients with relapsed diseases after allogeneic hematopoietic stem cell transplantation. J Immunother. 2019;42:81–8.
Chen Y, Cheng Y, Suo P, Yan CH, Wang Y, Chen Y, et al. Donor-derived CD19-targeted T cell infusion induces minimal residual disease-negative remission in relapsed B-cell acute lymphoblastic leukaemia with no response to donor lymphocyte infusions after haploidentical haematopoietic stem cell transplantation. Br J Haematol. 2017;179:598–605.
Jiang H, Li C, Yin P, Guo T, Liu L, **a LH, et al. Anti-CD19 chimeric antigen receptor-modified T-cell therapy bridging to allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia: an open-label pragmatic clinical trial. Am J Hematol. 2019;94:1113–22.
Kochenderfer JN, Dudley ME, Carpenter RO, Hardy NM, Mato AR, Hickstein DD, et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122:4129–39.
Teachey DT, Lacey SF, Shaw PA, Melenhorst JJ, Maude SL, Frey N, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6:664–79.
Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–28.
Gardner RLKAC. Decreased rates of severe CRS seen with early intervention strategies for CD19 CAR-T cell toxicity management. Blood. 2016;128:612.
Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019;34:45–55.
Teachey DT, Bishop MR, Maloney DG, Grupp SA. Toxicity management after chimeric antigen receptor T cell therapy: one size does not fit ‘ALL’. Nat Rev Clin Oncol. 2018;15:218.
Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62.
Tamamyan GN, Kantarjian HM, Ning J, Jain P, Sasaki K, McClain KL, et al. Malignancy-associated hemophagocytic lymphohistiocytosis in adults: relation to hemophagocytosis, characteristics, and outcomes. Cancer-Am Cancer Soc. 2016;122:2857–66.
Schram AM, Berliner N. How I treat hemophagocytic lymphohistiocytosis in the adult patient. Blood. 2015;125:2908–14.
Wang J, Hu Y, Huang H. Acute lymphoblastic leukemia relapse after CD19-targeted chimeric antigen receptor T cell therapy. J Leukoc Biol. 2017;102:1347–56.
Shalabi H, Kraft IL, Wang HW, Yuan CM, Yates B, Delbrook C, et al. Sequential loss of tumor surface antigens following chimeric antigen receptor T-cell therapies in diffuse large B-cell lymphoma. Haematologica. 2018;103:e215–e218.
Maude SL, Teachey DT, Porter DL, Grupp SA. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood. 2015;125:4017–23.
Jia H, Wang Z, Wang Y, Liu Y, Dai H, Tong C, et al. Haploidentical CD19/CD22 bispecific CAR-T cells induced MRD-negative remission in a patient with relapsed and refractory adult B-ALL after haploidentical hematopoietic stem cell transplantation. J Hematol Oncol. 2019;12:57.
Fry TJ, Shah NN, Orentas RJ, Stevenson MS, Yuan CM, Ramakrishna S, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24:20–8.
Acknowledgements
We would like to thank to all members of the study team, the patients and their families, and Uni CAR Technology Co., Ltd, Shanghai, China.
Funding
This work was jointly supported by the National Natural Science Foundation of Jiangsu Province [SBE2018740700], the Project of Invigorating Health Care through Science, Technology and Education, Jiangsu Provincial Key Medical Center (YXZXA2016002), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
JZ, HQ, XT, and DW contributed to the conception and design of the study. YH, MM, CL, and CF provided clinical patients. JH, JZ, XW, and LZ collected and analyzed the clinical data. JH and JZ wrote the manuscript. Xz, JZ, Sc, and HQ provided writing advice. Xz, JZ, and HQ revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics
This study was approved by the Medical Ethics Committee of The First Affiliated Hospital of Soochow University (IRB ID:2015070). The patients and donors provided their written informed consent according to the Declaration of Helsinki. The study was registered at https://www.clinicalTrials.gov as NCT03275493.
Consent for publication
Informed consent for publication was agreed and is available for review by the editor
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hua, J., Zhang, J., zhang, X. et al. Donor-derived anti-CD19 CAR T cells compared with donor lymphocyte infusion for recurrent B-ALL after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 56, 1056–1064 (2021). https://doi.org/10.1038/s41409-020-01140-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-01140-6
- Springer Nature Limited
This article is cited by
-
Prophylactic donor-derived CD19 CAR-T cell infusion for preventing relapse in high-risk B-ALL after allogeneic hematopoietic stem cell transplantation
Leukemia (2024)
-
Allogeneic BCMA-targeting CAR T cells in relapsed/refractory multiple myeloma: phase 1 UNIVERSAL trial interim results
Nature Medicine (2023)
-
Donor-derived CAR-T therapy improves the survival of relapsed B-ALL after allogeneic transplantation compared with donor lymphocyte infusion
Human Cell (2023)
-
Donor-derived CD19 CAR-T Cells versus Chemotherapy Plus Donor Lymphocyte Infusion for Treatment of Recurrent CD19-positive B-ALL After Allogeneic Hematopoietic Stem Cell Transplantation
Current Medical Science (2023)
-
Efficacy and safety of CD19 CAR-T cell therapy for acute lymphoblastic leukemia patients relapsed after allogeneic hematopoietic stem cell transplantation
International Journal of Hematology (2022)