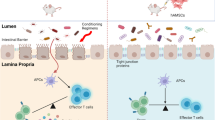
Abstract
Graft-versus-host disease (GVHD), an immunological disorder that arises from donor T cell activation through recognition of host alloantigens, is the major limitation in the application of allogeneic hematopoietic stem cell transplantation (allo-HSCT). Traditional immunosuppressive agents can relieve GVHD, but they induce serious side effects. It is highly required to explore alternative therapeutic strategy. Human amniotic epithelial stem cells (hAESCs) were recently considered as an ideal source for cell therapy with special immune regulatory property. In this study, we evaluated the therapeutic role of hAESCs in the treatment of GVHD, based on our previous developed cGMP-grade hAESCs product. Humanized mouse model of acute GVHD (aGVHD) was established by injection of huPBMCs via the tail vein. For prevention or treatment of aGVHD, hAESCs were injected to the mice on day -1 or on day 7 post-PBMC infusion, respectively. We showed that hAESCs infusion significantly alleviated the disease phenotype, increased the survival rate of aGVHD mice, and ameliorated pathological injuries in aGVHD target organs. We demonstrated that hAESCs directly induced CD4+ T cell polarization, in which Th1 and Th17 subsets were downregulated, and Treg subset was elevated. Correspondingly, the levels of a series of pro-inflammatory cytokines were reduced while the levels of the anti-inflammatory cytokines were upregulated in the presence of hAESCs. We found that hAESCs regulated CD4+ subset polarization in a paracrine mode, in which TGFβ and PGE2 were selectively secreted to mediate Treg elevation and Th1/Th17 inhibition, respectively. In addition, transplanted hAESCs preserved the graft-versus-leukemia (GVL) effect by inhibiting leukemia cell growth. More intriguingly, hAESCs infusion in HSCT patients displayed potential anti-GVHD effect with no safety concerns and confirmed the immunoregulatory mechanisms in the preclinical study. We conclude that hAESCs infusion is a promising therapeutic strategy for post-HSCT GVHD without compromising the GVL effect. The clinical trial was registered at www.clinicaltrials.gov as #NCT03764228.









Similar content being viewed by others
References
Paczesny S. Discovery and validation of graft-versus-host disease biomarkers. Blood. 2013;121:585–94.
Reddy P, Ferrara JLM. Immunobiology of acute graft-versus-host disease. Blood Rev. 2003;17:187–94.
Ferrara JLM, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–61.
Kanakry CG, Fuchs EJ, Luznik L. Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat Rev Clin Oncol. 2016;13:10–24.
Huang X, Liu D. Related HLA-mismatched/haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion: observations of a single Chinese center. Clin Transpl 2011:237–45.
Wang Y, Liu Q, Xu L, Liu K, Zhang X, Ma X, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood. 2015;125:3956–62.
Ferrara JLM, Reddy P. Pathophysiology of graft-versus-host disease. Semin Hematol. 2006;43:3–10.
Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95:2754–9.
Ratanatharathorn V, Nash RA, Przepiorka D, Devine SM, Klein JL, Weisdorf D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92:2303–14.
Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–8.
Macmillan ML, Weisdorf DJ, Wagner JE, Defor TE, Burns LJ, Ramsay NKC, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transpl. 2002;8:387–94.
Martin PJ, Schoch G, Fisher L, Byers V, Anasetti C, Appelbaum FR, et al. A retrospective analysis of therapy for acute graft-versus-host disease: initial treatment. Blood. 1990;76:1464–72.
Deeg HJ. How I treat refractory acute GVHD. Blood. 2007;109:4119–26.
Carpenter PA, Sanders JE. Steroid-refractory graft-vs.-host disease: past, present and future. Pediatr Transpl. 2003;7:19–31.
Highfill SL, Rodriguez PC, Zhou Q, Goetz CA, Koehn BH, Veenstra R, et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010;116:5738–47.
Sockel K, Bornhaeuser M, Mischak-Weissinger E, Trenschel R, Wermke M, Unzicker C, et al. Lenalidomide maintenance after allogeneic HSCT seems to trigger acute graft-versus-host disease in patients with high-risk myelodysplastic syndromes or acute myeloid leukemia and del(5q): results of the LENAMAINT trial. Haematologica. 2012;97:e34–5.
Reshef R, Luger SM, Hexner EO, Loren AW, Frey NV, Nasta SD, et al. Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N Engl J Med. 2012;367:135–45.
Abouelnasr A, Roy J, Cohen S, Kiss T, Lachance S. Defining the role of sirolimus in the management of graft-versus-host disease: from prophylaxis to treatment. Biol Blood Marrow Transpl. 2013;19:12–21.
Brunstein CG, Miller JS, Cao Q, Mckenna DH, Hippen KL, Curtsinger J, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117:1061–70.
Fantini MC, Monteleone G. Update on the therapeutic efficacy of tregs in IBD: thumbs up or thumbs down? Inflamm Bowel Dis. 2017;23:1682–8.
Velaga S, Ukena SN, Höpting M, Ivanyi P, Borchers S, Mischak-Weissinger E, et al. Reconstitution and phenotype of tregs in CMV reactivating patients following allogeneic hematopoietic stem cell transplantation. Immunol Invest. 2013;42:18–35.
Whangbo JS, Antin JH, Koreth J. The role of regulatory T cells in graft-versus-host disease management. Expert Rev Hematol. 2020;13:141–54.
Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–41.
Galipeau J. The mesenchymal stromal cells dilemma-does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy. 2013;15:2–8.
Ning H, Yang F, Jiang M, Hu L, Feng K, Zhang J, et al. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia. 2008;22:593–9.
Uhlin M, Sairafi D, Berglund S, Thunberg S, Gertow J, Ringden O, et al. Mesenchymal stem cells inhibit thymic reconstitution after allogeneic cord blood transplantation. Stem Cells Dev. 2012;21:1409–17.
Wassmer C, Berishvili E. Immunomodulatory properties of amniotic membrane derivatives and their potential in regenerative medicine. Curr Diab Rep. 2020;20:31.
Magatti M, Caruso M, De Munari S, Vertua E, De D, Manuelpillai U, et al. Human amniotic membrane-derived mesenchymal and epithelial cells exert different effects on monocyte-derived dendritic cell differentiation and function. Cell Transpl. 2015;24:1733–52.
Cargnoni A, Farigu S, Cotti Piccinelli E, Bonassi Signoroni P, Romele P, Vanosi G, et al. Effect of human amniotic epithelial cells on pro-fibrogenic resident hepatic cells in a rat model of liver fibrosis. J Cell Mol Med. 2018;22:1202–13.
Tan JL, Lau SN, Leaw B, Nguyen HPT, Salamonsen LA, Saad MI, et al. Amnion epithelial cell-derived exosomes restrict lung injury and enhance endogenous lung repair. Stem Cells Transl Med. 2018;7:180–96.
Vosdoganes P, Wallace EM, Chan ST, Acharya R, Moss TJM, Lim R. Human amnion epithelial cells repair established lung injury. Cell Transpl. 2013;22:1337–49.
Tan B, Yuan W, Li J, Yang P, Ge Z, Liu J, et al. Therapeutic effect of human amniotic epithelial cells in murine models of Hashimoto’s thyroiditis and systemic lupus erythematosus. Cytotherapy. 2018;20:1247–58.
Li J, Qiu C, Zhang Z, Yuan W, Ge Z, Tan B, et al. Subretinal transplantation of human amniotic epithelial cells in the treatment of autoimmune uveitis in rats. Cell Transpl. 2018;27:1504–14.
Bai X, Liu J, Yuan W, Liu Y, Li W, Cao S, et al. Therapeutic effect of human amniotic epithelial cells in rat models of intrauterine adhesions. Cell Transpl. 2020;29:2138901199.
Yang P, Yuan W, Liu J, Li J, Tan B, Qiu C, et al. Biological characterization of human amniotic epithelial cells in a serum-free system and their safety evaluation. Acta Pharmacol Sin. 2018;39:1305–16.
Kubo M, Sonoda Y, Muramatsu R, Usui M. Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci. 2001;42:1539–46.
Hori J, Wang M, Kamiya K, Takahashi H, Sakuragawa N. Immunological characteristics of amniotic epithelium. Cornea. 2006;25:S53–8.
Akle CA, Adinolfi M, Welsh KI, Leibowitz S, Mccoll I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981;2:1003–5.
Cooke KR, Kobzik L, Martin TR, Brewer J, Delmonte JJ, Crawford JM, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230–9.
Polchert D, Sobinsky J, Douglas G, Kidd M, Moadsiri A, Reina E, et al. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008;38:1745–55.
Qin X, Li G, Qin Y, Wang Y, Wang F, Liu D, et al. Quantitative chimerism: an independent acute leukemia prognosis indicator following allogeneic hematopoietic SCT. Bone Marrow Transpl. 2014;49:1269–77.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transpl. 2015;21:389–401.
Blazar BR, Macdonald KPA, Hill GR. Immune regulatory cell infusion for graft-versus-host disease prevention and therapy. Blood. 2018;131:2651–60.
Grégoire C, Ritacco C, Hannon M, Seidel L, Delens L, Belle L, et al. Comparison of mesenchymal stromal cells from different origins for the treatment of graft-vs.-host-disease in a humanized mouse model. Front Immunol. 2019;10:619.
Duffy MM, Ritter T, Ceredig R, Griffin MD. Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res Ther. 2011;2:34.
Mao M, Qian Y, Sun J. Morphine suppresses T helper lymphocyte differentiation to Th1 type through PI3K/AKT pathway. Inflammation. 2016;39:813–21.
Li Y, Fan H, Han X, Sun J, Ni M, Zhang L, et al. PR-957 suppresses Th1 and Th17 cell differentiation via inactivating PI3k/AKT pathway in Alzheimer’s disease. Neuroscience. 2023;510:82–94.
Pesce B, Ribeiro CH, Larrondo M, Ramos V, Soto L, Catalan D, et al. TNF-alpha affects signature cytokines of Th1 and Th17 T cell subsets through differential actions on TNFR1 and TNFR2. Int J Mol Sci. 2022;23:9306.
Zhu H, Yang F, Tang B, Li XM, Chu YN, Liu YL, et al. Mesenchymal stem cells attenuated PLGA-induced inflammatory responses by inhibiting host DC maturation and function. Biomaterials. 2015;53:688–98.
Onuora S. Immunology: Hippo signalling influences T cell fate. Nat Rev Rheumatol. 2017;13:389.
Hu Y, Chen G, Huang J, Li Z, Li Z, **e Y, et al. The calcium channel inhibitor nimodipine shapes the uveitogenicT cells and protects mice from experimental autoimmune uveitis through the p38-MAPK signaling pathway. J Immunol. 2021;207:2933–43.
Podojil JR, Miller SD. Cross-linking of CD80 on CD4+ T cells activates a calcium-dependent signaling pathway. J Immunol. 2009;182:766–73.
Liu J, Chang YJ, Yan CH, Xu LP, Jiang ZF, Zhang XH, et al. Poor CMV-specific CD8+ T central memory subset recovery at early stage post-HSCT associates with refractory and recurrent CMV reactivation. J Infect. 2016;73:261–70.
Song JY, Kang HJ, Ju HM, Park A, Park H, Hong JS, et al. Umbilical cord-derived mesenchymal stem cell extracts ameliorate atopic dermatitis in mice by reducing the T cell responses. Sci Rep. 2019;9:6623.
Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–50.
Acknowledgements
We thank Dr. She-long Zhang and Dr. Fang-liang Huang (Equipment and Technology Service Platform, College of Life Sciences, Zhejiang University) for their excellent technical support with microscopy and flow cytometry. This work was supported by the Zhejiang Provincial Key R&D Program of China (2022C03097), the National Key R&D Program of China (2017YFA0104500, 2018YFA0800504), the National Natural Science Foundation of China (82300296, 81621001, 91839104, 81770444 and 81600354), Project of Health Collaborative Innovation of Guangzhou City (201704020214), the Zhejiang Provincial Natural Science Foundation of China (LZ20H020002), the Medical and Health Science and Technology Program of Health Commission of Zhejiang Province, China (2021KY633) and Fundamental Research Funds for the Central Universities of China.
Author information
Authors and Affiliations
Contributions
PJY, XYZ, LHG, JYL, XJH, and LYY designed research; PJY, XYZ, YHK, JL, YYZ, ZDW, WXY, BT, QL, XYR, and YNS performed research; PJY, XYZ, YHK, ZG, JYL, Chen Qiu, YQJ, Cong Qiu, XYR, and YNS analyzed and interpreted data; PJY, XYZ, YHK, JYL, XJH, and LYY drafted and revised manuscript; JYL, XJH and LYY approved the manuscript submission.
Corresponding authors
Ethics declarations
Competing interests
LYY, PJY, WXY, JYL, LHG, JL authored a China patent (No. ZL 2019 1 0310688.6) held by Zhejiang University and Shanghai iCELL Biotechnology Co. Ltd for the “Use of hAESCs for the treatment of GVHD”. WXY and QL are employees of Shanghai iCELL Biotechnology Co. Ltd. The remaining authors declare no competing financial interests.
Ethics approval and consent to participate
The hAESCs were derived from placenta amniotic membrane of unrelated, unmatched healthy donors with informed patient consent approved by the Institutional Patients and Ethics Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine (Ethics Code: 2020-799). All animal studies were approved by the Institutional Animal Care and Use Committee of Zhejiang University (ZJU20210127) and adhered to the Guide for the Care and Use of Laboratory Animals. The clinical trial was approved by the institutional review board of Peking University People’s Hospital (2018PHD006-01) and conducted under an investigational new stem cells application from the National Health Commission of China. All patients and donors provided written informed consent according to the Declaration of Helsinki. The trial was registered at www.clinicaltrials.gov as #NCT03764228.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Pj., Zhao, Xy., Kou, Yh. et al. Human amniotic epithelial stem cell is a cell therapy candidate for preventing acute graft-versus-host disease. Acta Pharmacol Sin (2024). https://doi.org/10.1038/s41401-024-01283-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41401-024-01283-y
- Springer Nature Singapore Pte Ltd.