Abstract
Immunotherapy, as a powerful strategy for cancer treatment, has achieved tremendous efficacy in clinical trials. Despite these advancements, there is much to do in terms of enhancing therapeutic benefits and decreasing the side effects of cancer immunotherapy. Advanced nanobiomaterials, including liposomes, polymers, and silica, play a vital role in the codelivery of drugs and immunomodulators. These nanobiomaterial-based delivery systems could effectively promote antitumor immune responses and simultaneously reduce toxic adverse effects. Furthermore, nanobiomaterials may also combine with each other or with traditional drugs via different mechanisms, thus giving rise to more accurate and efficient tumor treatment. Here, an overview of the latest advancement in these nanobiomaterials used for cancer immunotherapy is given, describing outstanding systems, including lipid-based nanoparticles, polymer-based scaffolds or micelles, inorganic nanosystems, and others.
Similar content being viewed by others
Introduction
Cancer immunotherapy is a promising treatment for cancer that aims to provide treatment more accurately and safely than other traditional therapies [1, 2]. Agents are designed to provoke a robust primary and secondary antitumor immune response by repairing or enhancing natural mechanisms that are evaded or damaged during disease progression, thus inhibiting tumor growth and metastasis [3,4,5].
Approximately a century ago, Coley first used a method to activate the patient’s immune system to help treat tumors [6]. In the immune system, antigen-presenting cells (APCs) continuously eliminate exogenous or endogenous antigens; antigens are taken up and processed to be exposed onto major histocompatibility complexes (MHCs) I or II on the APC surface for further presentation to naive T cells [7, 8]. The three main pathways by which APCs activate T cells are the binding of MHC complexes to T-cell receptors, the presence of costimulatory molecules on the cell surface (CD80 and 86 on APCs binding to CD28 on T cells) and the cytokines that stimulate T cells [9]. T cells can differentiate into two major subpopulations: CD4+ T cells, which can further differentiate into T-helper 1 (Th1) and T-helper 2 (Th2) cells, and CD8+ T cells, which can further differentiate into cytotoxic T lymphocytes (CTLs) to directly kill tumor cells [9, 10]. Both CD8+ T cells and IFN-γ-secreting Th1 CD4+ T cells play a vital role in killing tumors [10, 11] (Fig. 1).
Scheme of the cancer immunotherapy mechanism. After antigens are processed by immature dendritic cells (ImDCs), they are presented to T cells by mature dendritic cells (mDCs) through major histocompatibility complex (MHC) class I or MHC class II complexes binding to CD8+ or CD4+ T cells, separately. Simultaneously, mDCs also express costimulatory molecules and cytokines such as IFN-γ and IL-12 to synergistically stimulate T cells. CD8+ T cells further differentiate into cytotoxic T lymphocytes (CTLs), and CD4+ T cells further differentiate into IFN-γ secreting T-helper 1 (Th1) cells to assist in activating CD8 cells and other innate immune cells, such as natural killer (NK) cells, granulocytes or macrophages, to directly kill tumor cells
In 1986, the US Food and Drug Administration (FDA) approved recombinant versions of the cytokine interferon-α (IFN-α) as the first cancer immunotherapeutic drug for the treatment of hairy cell leukemia; however, IFN-α was replaced because of its short therapeutic duration [12]. Subsequently, recombinant interleukin-2 (IL-2) was approved by the FDA as a cancer immunotherapy drug for the treatment of metastatic renal cancer (in 1992) and metastatic melanoma (in 1998), separately [13]. Although IL-2 initially has a good therapeutic effect in some patients, the use of large doses due to its short half-life results in many immune-related side effects, such as cytokine release syndrome and vascular leakage syndrome [14,15,16]. After a stagnant phase, sipuleucel-T (an autologous dendritic cell (DC) therapy) as the first cancer therapeutic vaccine was approved by the FDA for prostate cancer, which meant tumor immunotherapy had finally made successful progress in the early 21st century. However, production complexities and other issues hindered the clinical translation of sipuleucel-T [17, 18]. Since the cytotoxic T-lymphocyte antigen-4 (CTLA-4)-targeted checkpoint inhibitor ipilimumab was approved for advanced melanoma in 2011 [19], there has been a shift towards novel immunotherapies, including programmed cell death-1 or its ligand monoclonal antibody (aPD1 or aPDL1) [20] and chimeric antigen receptor (CAR) T-cell therapies [21,22,23].
Although these treatments have been developed and approved for clinical use and have achieved some efficacy, many problems regarding safety and effectiveness remain to be solved [14, 24, 25]. In terms of safety, some immunotherapeutic drugs require a large dose for their short half-life, which causes autoimmune side effects in some patients. For example, two syndromes (cytokine release syndrome and vascular leakage syndrome) caused by IL-2 lead to severe and even lethal systemic inflammatory reactions in some patients [25]. In terms of efficacy, current immunotherapy is only effective in some patients, and most immunotherapy is initially used only to treat hematological tumors. Only a few immunotherapies for the treatment of solid tumors are approved because solid tumors have a complex tumor microenvironment (TME) that is a difficult barrier to break through [26].
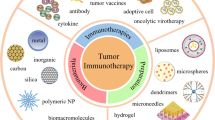
To reduce side effects and improve the accuracy of immunotherapy, novel delivery systems need to be manufactured. In recent years, with the development of nanotechnology, an increasing number of delivery systems have been designed for the local and sustained release of immunotherapeutic drugs in vivo [27,28,29]. Biomaterial-based delivery systems have many advantages in cancer immunotherapy, such as the specific and targeted delivery of biomolecules, high efficacy, low toxicity, and immune-stimulating effects (Table 1) [27, 30, 31]. A great variety of advanced biomaterials can be used for cancer immunotherapy, including liposomes, polymers, silica, and so on (Fig. 2) [2, 32, 33]. Different biomaterials use various means and technologies to play an important role in cancer prevention [34,35,36,37,38]. To achieve precise antitumor effects, these advanced biomaterials with different functions can be used to deliver immunopharmaceuticals to organs or tissues (such as the mucosa or skin) that are rich in immune cells by different routes of administration (for instance, intranasally [39], orally [40], and subcutaneously [84]. However, naked RNA is highly susceptible to degradation by nucleases; therefore, it requires special transfection reagents or delivery techniques to enhance its intracellular delivery [85]. Thus, nucleic acid vaccines can greatly benefit from advanced delivery technologies and materials, which are technical barriers to current nucleic acid vaccines. An effective and safe delivery system is the key to the successful application of nucleic acid vaccines.
Neoantigen vaccines use tumor somatic DNA as antigens to promote the antitumor immune response [86]. These antigens are only expressed in tumor cells and can avoid damage to normal tissues [87]. Advanced materials and delivery systems can improve the stability of these neoantigens and combine multiple vaccine classes to improve the safety and efficacy of cancer vaccines [88,159].
PLGA-based microspheres were found to enable the eradication of prostate carcinoma by codelivering tumor lysates, CpG-ODN, and Poly(I:C), and their capacity to stimulate T cells to produce IFN-γ and granzyme B was significantly enhanced in TRAMP mice [160]. PLGA polymers were also used to codeliver OVA, CpG-ODN, and Poly(I:C); however, the therapeutic effects were weakened, and the production of IFN-γ and the activation of DCs were decreased under chronic stress [161]. Two TLR7/8 agonists have been synthesized and encapsulated in PLGA NPs. This OVA or tumor lysate NP vaccine significantly inhibits tumor growth in B16F10-OVA or renal cell carcinoma by stimulating the CD8+ CTL response [162].
To realize image-guided delivery of immunomodulators, IFN-γ and iron oxide nanocubes were coencapsulated in PLGA microspheres. This delivery system could provide a convenient way of delivering drugs to tumor sites after injection and monitoring the distribution of drugs sequentially [163]. A new combination method that uses PLGA for encapsulating a physical mixture of ovalbumin and hydroxychloroquine promotes CD8+ CTL and memory T-cell immune responses in tumor tissues via the controlled release of OVA and upregulation of MHC-I and CD86 costimulatory molecules in DCs [164]. To achieve the goal of targeting DCs more accurately, Rosalia et al. designed a PLGA-based CD-40-targeted cancer vaccine that showed significant enhancements in delaying tumor growth and extending the survival of tumor-bearing mice by facilitating antigen-specific antitumor CD8+ T-cell responses [165]. Recently, combination immunotherapy has become a particularly promising strategy for tumor treatment, and PLGA has been used to realize the codelivery of antiprogrammed cell death-1 (aPD1) and T-cell agonist (aOX40) agents to simultaneously rather than sequentially elicit the activation of T cells. These dual-immunotherapy NPs increased the ratio of CD8+ to regulatory T cells infiltrating the tumor, thereby promoting therapeutic efficacy in both B16F10 melanoma tumors and 4T1 breast cancers [166].
Indocyanine green (ICG) and imiquimod (R837) were coloaded by PLGA to achieve the eradication of preexisting tumors and enhance the antitumor immune response simultaneously. Moreover, with the combination of these particles and anti-CTLA-4, this strategy has been proven to delay tumor growth and extend survival in both 4T1 and CT26 tumor models [167]. PLGA polymers as biodegradable materials can also be combined with photothermal agents. Researchers produced anti-PD-1 peptide (AUNP12) and hollow gold nanoshell coencapsulated PLGA NPs, and these particles facilitated the effective inhibition of primary and distal tumor growth via an increasing percentage of CD8+ CTLs and secretion of IFN-γ [168]. In addition, the coadministration of anti-PD-1 peptide (AUNP12) and hollow gold nanoshell coencapsulated PLGA NPs with CpG has been proven to mediate the maturation of DCs in vitro and enable direct tumor necrosis in bilateral and lung metastatic 4T1 tumor-bearing mice [169]. The antibody-modified PLGA core was used to load the hydrophobic drug imatinib (IMT), which was developed as an inhibitor of tyrosine kinase and then manufactured as IR-780 and IMT codelivery PH-sensitive NPs, which showed a great capacity to stimulate an effective CD8+ T-cell antitumor immune response [170].
Researchers have compared the capacity of synthetic long peptide-based cationic liposomes and PLGA NPs to induce an immune response. They proved that liposomes have advantages over PLGA particles for inducing the T-cell response. However, the mechanism of this phenomenon has not been investigated [171]. In addition, PLGA polymers used to formulate antigen-capturing nanoparticles (AC-NPs) were proven to promote an antitumor immune response and improve the efficacy of αPD-1 immunotherapy. The surfaces of PLGA NPs were modified by different chemical groups to bind tumor antigens; however, although all other AC-NPs except mPEG AC-NPs loaded plenty of proteins, PLGA and Mal AC-NPs showed a higher ability to improve the immunotherapeutic efficacy (Fig. 4) [172].
AC-NPs have the capacity to inhibit distant B16F10 xenografts. a Schematic illustration of cancer immunotherapy promotion by using antigen-capturing nanoparticles (AC-NPs) combined with radiotherapy and αPD-1 treatment. b Average tumor growth curves of abscopal tumors in mice treated with different administrations. c The survival rate of the treated mice in b. Reprinted with permission from [172]
Hydrogels
Hydrogels can serve as antigen storage caverns because of their gelation properties, and they have been used as vectors to coload cytokines, proteins, DNAs, and so on [31, 173,174,175,176,177,178,179]. Alginate microparticle-based injectable gels were reported ~10 years ago and have been used to codeliver mature DCs and chemokines CCL21 and CCL19. The study showed that this hydrogel system could recruit host DCs to the injection site and migrate to local lymph nodes at the same time, thus providing a continual process to initiate the immune response [180]. Researchers have designed a two-step strategy to realize the recruitment of APCs and presentation of antigens via the injection of GM-CSF delivering mPEG−PLGA hydrogels followed by the administration of antigen-loaded vectors, which showed obvious antitumor immunotherapeutic potential [181]. Nanogel particles formed by cholesteryl pullulan showed the good function of delivering and cross-presenting antigens to medullary macrophages. In addition, the study revealed that this vaccine could significantly slow tumor growth with the help of Toll-like receptor agonists [182].
Hyaluronic acid–tyramine-based hydrogels have been used to deliver IFN-α to the injection site and to inhibit tumor proliferation via the coadministration of sorafenib [183]. In addition, a hyaluronic acid-pluronic F-127 hydrogel was used to prepare black phosphorus quantum dot nanovesicles (BPQD-CCNVs), GM-CSF, and LPS coloaded systems. The study showed that the sustained release of GM-CSF and LPS from the injection site could recruit and activate DCs. In addition, NIR irradiation combined with PD-1 antibody could generate a strong antitumor immune response [184].
Mooney and his colleagues manufactured an infection-mimicking system to coload GM-CSF, Toll-like receptor agonists (CpG-ODN) and a tumor lysate to achieve the recruitment and activation of DCs, which promoted a specific and effective antitumor immune response [185, 186]. The same team designed a cryogel-based delivery system to encapsulate GM-CSF and CpG-ODN. This vaccine could be subcutaneously injected into mice and controlled release immunomodulatory factors and cancer antigens, thus provoking strong antitumor T-cell responses and improving the survival rate of B16F10-bearing mice [187].
Yang and coworkers reported that hydrogels formed by phosphatase enzymes had good potency in evoking humoral and cellular immune responses and could be used as protein vaccine adjuvants [188]. Moreover, they also proved that peptide Nap-GFFY hydrogels formed by a very simple process could also provoke a cogent CD8+ T-cell immune response [189]. Chao et al. designed a combination system in which ALG was cross-linked by multivalent cations and 131I-labeled catalase coadministered and jellified in a local tumor site, followed by systemic CTLA-4 injection. This strategy delayed local tumor growth and metastasis [190].
Gu and coworkers designed a therapeutic scaffold formed by a ROS-responsive hydrogel to release gemcitabine (GEM) and an anti-PD-L1 blocking antibody (aPDL1) locally in tumor-bearing mice [191]. This system significantly decreased the level of ROS and the numbers of myeloid-derived suppressor cells and TAMs in the tumor site. Moreover, a 50% survival rate and 30% recurrence rate were observed in the aPDL1-GEM@Gel treatment group on account of primary and memory immune responses. In the same year, this research group also generated another immunotherapeutic gel for postsurgical tumor treatment, which was manufactured by mixing the fibrinogen solution containing anti-CD47 antibody-loaded CaCO3 NPs and thrombin solution in the postsurgical tumor site [192]. This strategy had the potency of preventing local and distant tumors via activating M1-type TAMs and promoting macrophage phagocytosis and antitumor immune responses.
DNA-based supramolecular hydrogels were reported to recruit and activate APCs by releasing a high concentration of CpG, which could serve as a promising method for tumor immunotherapy [193]. Song et al. demonstrated a poly(L-valine) hydrogel for coencapsulating TCL, TLR3 agonist, poly(I:C) that realizes the controlled release of antigens and adjuvants, thus promoting antigen persistence and presentation to enhance the cytotoxic T-lymphocyte immune response against cancer [194]. A tumor-penetrable peptide-based hydrogel was prepared by encapsulating JQ-1 (a BRD4 inhibitor) and ICG coloaded tumor cells (Fig. 5) [195]. This vaccine could evoke a strong patient-specific immune response and prevent recurrence and metastasis of postsurgical tumors by NIR laser-triggered release of tumor-specific antigens and JQ-1.
PVAX immunotherapy for both recurrent and metastatic 4T1 tumors. a Schematic depiction of the manufacture of PVAX for cancer immunotherapy. b Average and individual tumor growth curves of recurrent 4T1 xenografts in mice treated with different formulations. c Survival curves of the mice bearing 4T1 recurrent tumors. d Average tumor growth curves of the distant tumors treated with different formulations. e Tumor-free percentages of the abscopal tumor. Reprinted with permission from [195]
Inorganic biomaterials
Siliceous nanoparticles
Mesoporous silica NPs can be prepared by using organosilane precursors to participate in hydrolysis and condensation reactions [196, 197]. Moreover, the surface of these particles can be modified with various reactive groups for different medical applications [198, 199]. Amino acid-modified silica NPs were reported to promote cytokine production, and silica nanospheres doped with Ca, Mg, and Zn (MS-Ca, MS-Mg, and MS-Zn) showed the capacity to provoke a Th1 anticancer immune response [200, 201]. Both spherical silica NPs and asymmetric mesoporous silica NPs were found to have the potential to activate and mature immune cells [202,203,204,205,206]. In addition, siliceous NPs also play a strong role in vaccine formulations such as the Japanese encephalitis vaccine [207], hepatitis B virus DNA vaccine [208], and oral hepatitis B vaccine [209], as well as in viral vaccine heat resistance [210] and viral inhibition [211]. Mesoporous silica-templated and hollow particles were designed to load antigens and adjuvants that showed a robust lymph node targeting and immune cell-activating capacity [212,213,214,215,216]. Yang et al. achieved the synthesis of dendritic mesoporous organosilica hollow spheres for the first time, which showed a significant potential to provoke an antitumor immune response [217]. Mooney and coworkers reported mesoporous silica rods (MSRs) with a high aspect ratio that spontaneously assembled as a macroporous structure to recruit DCs and generate humoral and cellular immune responses against tumors in the presence of GM-CSF and CpG [218]. They also modified these MSRs with PEG, PEG–RGD, or PEG–RDG groups [219] and PEI (Fig. 6) [220], which promoted immune cell activation and infiltration and may pave the way for cancer vaccination.
The MSR–PEI vaccine inhibits established tumors. a Schematic illustration of PEI and antigen adsorption. b Schematic depiction of the MSR vaccine and MSR–PEI vaccine. Tumor growth (c) and survival rate (d) of mice bearing E7-expressing TC-1 tumors rechallenged with TC-1 cells. e The survival rate of mice bearing E7-expressing TC-1 tumors treated with different formulations. Reprinted with permission from [220]
Iron oxide nanoparticles
Because iron oxide NPs have been approved for human use as MRI contrast agents and their degradation products are good for the body’s iron store, iron oxide NPs have been increasingly used simultaneously for cancer immunotherapy and imaging [221,222,223]. Iron oxide NPs can be modified with many cargos to improve the antitumor immune response, such as heat shock protein 70 (Hsp70) [224], R837 and Poly(I:C) [225], CpG-ODN [226], and ICG [227]. These NPs have the potential to realize the integration of imaging and therapy.
Gold nanoparticles
Photothermal immunotherapy is an effective treatment combining laser photophysical effects with immunoregulation [228,229,230]. Many photothermal biomaterials, such as gold nanorods [231], Prussian blue [232, 233], and NIR photosensitizers [234], have recently been used for cancer immunotherapy. Gold NP labeled melanoma-specific T cells show the potential to be noninvasively imaged by classical X-ray computed tomography, which provides a convenient way to track immune cells in immunotherapy [235]. More interestingly, gold NPs can predict the therapeutic response to immune checkpoint blockade after modification with programmed death-ligand 1 antibody (αPD-L1) [236]. Gold-based NPs may also be combined with adjuvants to promote antitumor immune responses and contribute to cancer immunotherapy [237, 238].
Others
Microneedles with dimensions of <1 mm can be utilized to pierce the skin to the dermis in a minimally invasive and painless manner [239,240,241]. As a significant research target, microneedles have been used in many aspects, such as the delivery of small molecule and protein drugs and vaccines [242,243,244]. It is an inexpensive and convenient way to use microneedles for various medical applications [245,246,247,248,249]. There are many lymph nodes in the dermis; thus, microneedles achieve direct contact with DCs for antigen uptake and presentation [27]. To decrease the cost of treatment and reduce the dosage-dependent side effect [250,251,252] of immunomodulators, Gu and coworkers prepared biocompatible hyaluronic acid (HA)-based microneedles integrated with pH-sensitive dextran NPs containing aPD1 and glucose oxidase, which realized the substantial release of aPD1 and evoked a strong immune response in B16F10-bearing mice [253]. Meanwhile, the same group designed another microneedle system that combined aPD1 and 1-methyl-DL-tryptophan (1-MT), an inhibitor of IDO, to promote T-cell immunity and reduce immunosuppression [254]. Moreover, a hyaluronic acid-based MN encapsulated B16F10 melanoma whole tumor lysate and GM-CSF were proven to stimulate a robust antitumor immune response through spatiotemporal PTT and immunotherapy (Fig. 7) [255]. In addition to soluble microneedles, hollow microneedles can also be used for vaccine delivery and immunotherapy on account of the controllability and accuracy of the injection progress [256, 257]. Researchers compared four types of NP-loaded OVA and poly(I:C) by using hollow microneedles, which showed that PLGA NPs and liposomes could provoke stronger IgG2a responses [258]. In addition, a digitally controlled hollow microneedle system was used for the injection of liposomes containing an HPV E743–63 synthetic long peptide, thus reducing pain and the dosage of injection [259].
Local immunotherapy for various tumors via microneedles. a Schematic illustration of immunotherapy utilizing microneedles. b Average tumor growth and survival rate of treated C57BL/6J mice in the BP tumor model. c Average tumor growth and survival rate of treated BALB/c mice in the 4T1 tumor model. d Average tumor growth and survival rate of C57BL/6J mice in established BP tumor models. e Average tumor growth and survival rate of BALB/c mice in established 4T1 tumor models. Reprinted with permission from [255]
In recent years, biologically derived nanobiomaterials, such as cancer cell membranes, viral proteins, and DNAs, have started to be used for new cancer nanovaccines [260,261,262,263]. Researchers found that tumor antigens and subcellular particles in the cancer membrane, such as melanoma cells, could be loaded into various NPs, which induce specific cellular and humoral responses, thus preventing tumor growth [157, 264, 265]. Another approach is to form virus-like NPs via the self-assembly of viral proteins [266]. Furthermore, cowpea mosaic virus NPs effectively evoked cytokine secretion and inhibited tumor growth in various models (Fig. 8) [267].
eCPMV immunotherapy for metastatic breast, colon, and ovarian tumors. a Photo and survival rate of mice in a metastatic breast tumor model. b Photo and survival rate of mice in a colon tumor model. c Photo and survival rate of mice with ID8-Defb29/Vegf-A ovarian cancer. Reprinted with permission from [267]
Conclusion
In this review, we have analyzed different strategies of immunotherapy and described advanced biomaterials that may be applied to improve therapeutic potency and reduce adverse effects. Although cancer immunotherapy is advancing at a high speed, the use of biomaterials to manufacture optimal systems for various tumors remains in its nascent stages. It is hoped that the biomaterials described in this review can be more widely and innovatively designed for cancer immunotherapy, thus promoting its efficacy and reducing immune-related side effects. Although preliminary advances have been made in the design of immunotherapy strategies based on biomaterials, many systems, including NPs, micelles, and hydrogels, can be loaded with multiple drugs and selected based on the targets identified in the patient’s biopsy sample. This personalized treatment will be an important and promising research direction in the future.
Change history
03 August 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41401-020-0464-9
References
Quesada JR, Hersh EM, Manning J, Reuben J, Keating M, Schnipper E, et al. Treatment of hairy cell leukemia with recombinant alpha-interferon. Blood. 1986;68:493–7.
Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18:175–96.
Mandal A, Boopathy AV, Lam LKW, Moynihan KD, Welch ME, Bennett NR, et al. Cell and fluid sampling microneedle patches for monitoring skin-resident immunity. Sci Transl Med. 2018;10:eaar2227.
Dellacherie MO, Seo BR, Mooney DJ. Macroscale biomaterials strategies for local immunomodulation. Nat Rev Mater. 2019;4:379–97.
Wang C, Ye Y, Hu Q, Bellotti A, Gu Z. Tailoring biomaterials for cancer immunotherapy: emerging trends and future outlook. Adv Mater. 2017;29:29.
Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin Orthop Relat Res. 1991;262:3–11.
Storni T, Kundig TM, Senti G, Johansen P. Immunity in response to particulate antigen-delivery systems. Adv Drug Deliv Rev. 2005;57:333–55.
De Koker S, Lambrecht BN, Willart MA, van Kooyk Y, Grooten J, Vervaet C, et al. Designing polymeric particles for antigen delivery. Chem Soc Rev. 2011;40:320–39.
Silva JM, Videira M, Gaspar R, Preat V, Florindo HF. Immune system targeting by biodegradable nanoparticles for cancer vaccines. J Control Release. 2013;168:179–99.
Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–62.
Gilboa E. The promise of cancer vaccines. Nat Rev Cancer. 2004;4:401–11.
Ahmed S, Rai KR. Interferon in the treatment of hairy-cell leukemia. Best Pr Res Clin Haematol. 2003;16:69–81.
Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192:5451–8.
Lee S, Margolin K. Cytokines in cancer immunotherapy. Cancers. 2011;3:3856–93.
Alwan LM, Grossmann K, Sageser D, Van Atta J, Agarwal N, Gilreath JA. Comparison of acute toxicity and mortality after two different dosing regimens of high-dose interleukin-2 for patients with metastatic melanoma. Target Oncol. 2014;9:63–71.
Rosenberg SA, Lotze MT, Muul LM, Chang AE, Avis FP, Leitman S, et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987;316:889–97.
Tanimoto T, Hori A, Kami M. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:1966–8.
Graff JN, Chamberlain ED. Sipuleucel-T in the treatment of prostate cancer: an evidence-based review of its place in therapy. Core Evid. 2015;10:1–10.
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23.
Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–5.
Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer. 2016;16:566–81.
Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7:303ra139–303ra139.
June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–5.
Milling L, Zhang Y, Irvine DJ. Delivering safer immunotherapies for cancer. Adv Drug Deliv Rev. 2017;114:79–101.
June CH, Warshauer JT, Bluestone JA. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat Med. 2017;23:540–7.
Menon S, Shin S, Dy G. Advances in cancer immunotherapy in solid tumors. Cancers. 2016;8:106.
Stephan SB, Taber AM, Jileaeva I, Pegues EP, Sentman CL, Stephan MT. Biopolymer implants enhance the efficacy of adoptive T-cell therapy. Nat Biotechnol. 2015;33:97–101.
Ma Y, Lu M, Deng Y, Bai R, Zhang X, Li D, et al. The preparation of C-reactive protein immunosensor based on nano-mimetic enzyme Co3O4. J Biomed Nanotechnol. 2018;14:1169–77.
Zhou Z, Lin H, Li C, Wu Z. Recent progress of fully synthetic carbohydrate-based vaccine using TLR agonist as build-in adjuvant. Chin Chem Lett. 2018;29:19–26.
Fontana F, Liu D, Hirvonen J, Santos HA. Delivery of therapeutics with nanoparticles: what’s new in cancer immunotherapy? Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9:e1421.
Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016;1:1–17.
Sadtler K, Wolf MT, Ganguly S, Moad CA, Chung L, Majumdar S, et al. Divergent immune responses to synthetic and biological scaffolds. Biomaterials 2019;192:405–15.
Kim J, Li WA, Sands W, Mooney DJ. Effect of pore structure of macroporous poly(lactide-co-glycolide) scaffolds on the in vivo enrichment of dendritic cells. ACS Appl Mater Interfaces. 2014;6:8505–12.
Whiteside TL, Demaria S, Rodriguez-Ruiz ME, Zarour HM, Melero I. Emerging opportunities and challenges in cancer immunotherapy. Clin Cancer Res. 2016;22:1845–55.
Lyon JG, Mokarram N, Saxena T, Carroll SL, Bellamkonda RV. Engineering challenges for brain tumor immunotherapy. Adv Drug Deliv Rev. 2017;114:19–32.
Sagiv-Barfi I, Czerwinski DK, Levy S, Alam IS, Mayer AT, Gambhir SS, et al. Eradication of spontaneous malignancy by local immunotherapy. Sci Transl Med. 2018;10:eaan4488.
Lai Y, Wang L, Liu Y, Yang G, Tang C, Deng Y, et al. Immunosensors based on nanomaterials for detection of tumor markers. J Biomed Nanotechnol. 2018;14:44–65.
Shi K, Xue BX, Liao JF, Qu Y, Qian ZY. Polymeric hydrogels for post-operative adhesion prevention: a review. Mater Express. 2017;7:417–38.
Wu Y, Wei W, Zhou M, Wang Y, Wu J, Ma G, et al. Thermal-sensitive hydrogel as adjuvant-free vaccine delivery system for H5N1 intranasal immunization. Biomaterials. 2012;33:2351–60.
Hu Q, Wu M, Fang C, Cheng C, Zhao M, Fang W, et al. Engineering nanoparticle-coated bacteria as oral DNA vaccines for cancer immunotherapy. Nano Lett. 2015;15:2732–9.
Peng J, Yang Q, **ao Y, Shi K, Liu Q, Hao Y, et al. Tumor microenvironment responsive drug-dye-peptide nanoassembly for enhanced tumor-targeting, penetration, and photo-chemo-immunotherapy. Adv Funct Mater. 2019;29:1900004–1900123.
Katze MG, He Y, Gale M Jr. Viruses and interferon: a fight for supremacy. Nat Rev Immunol. 2002;2:675–87.
Sun T, Yang Y, Luo X, Cheng Y, Zhang M, Wang K, et al. Inhibition of tumor angiogenesis by interferon-gamma by suppression of tumor-associated macrophage differentiation. Oncol Res. 2014;21:227–35.
Cox MA, Harrington LE, Zajac AJ. Cytokines and the inception of CD8 T cell responses. Trends Immunol. 2011;32:180–6.
Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46.
Ben-Sasson SZ, Hu-Li J, Quiel J, Cauchetaux S, Ratner M, Shapira I, et al. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci USA. 2009;106:7119–24.
Yan WL, Shen KY, Tien CY, Chen YA, Liu SJ. Recent progress in GM-CSF-based cancer immunotherapy. Immunotherapy. 2017;9:347–60.
Mehta HM, Malandra M, Corey SJ. G-CSF and GM-CSF in Neutropenia. J Immunol. 2015;195:1341–9.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64.
Munn DH, Bronte V. Immune suppressive mechanisms in the tumor microenvironment. Curr Opin Immunol. 2016;39:1–6.
Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561.
Webb ES, Liu P, Baleeiro R, Lemoine NR, Yuan M, Wang YH. Immune checkpoint inhibitors in cancer therapy. J Biomed Res. 2018;32:317–26.
Ellis PM, Vella ET, Ung YC. Immune checkpoint inhibitors for patients with advanced non-small-cell lung cancer: a systematic review. Clin Lung Cancer. 2017;18:444–59 e1.
Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2:1346–53.
Byun DJ, Wolchok JD, Rosenberg LM, Girotra M. Cancer immunotherapy—immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol. 2017;13:195–207.
Restifo NP, Smyth MJ, Snyder A. Acquired resistance to immunotherapy and future challenges. Nat Rev Cancer. 2016;16:121–6.
Dillman RO. Is there a role for therapeutic cancer vaccines in the age of checkpoint inhibitors? Hum Vaccin Immunother. 2017;13:528–32.
Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80.
Lim WA, June CH. The principles of engineering immune cells to treat cancer. Cell. 2017;168:724–40.
Scholler J, Brady TL, Binder-Scholl G, Hwang WT, Plesa G, Hege KM, et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med. 2012;4:132ra53–132ra53.
Levine BL, Miskin J, Wonnacott K, Keir C. Global manufacturing of CAR T cell therapy. Mol Ther Methods Clin Dev. 2017;4:92–101.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44.
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young Adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–48.
Davila ML, Brentjens RJ. CD19-Targeted CAR T cells as novel cancer immunotherapy for relapsed or refractory B-cell acute lymphoblastic leukemia. Clin Adv Hematol Oncol. 2016;14:802–8.
O’Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9:eaaa0984.
Posey AD Jr, Schwab RD, Boesteanu AC, Steentoft C, Mandel U, Engels B, et al. Engineered CAR T cells targeting the cancer-associated Tn-glycoform of the membrane mucin MUC1 control adenocarcinoma. Immunity. 2016;44:1444–54.
Bailey SR, Nelson MH, Majchrzak K, Bowers JS, Wyatt MM, Smith AS, et al. Human CD26(high) T cells elicit tumor immunity against multiple malignancies via enhanced migration and persistence. Nat Commun. 2017;8:1961.
Fitzgerald JC, Weiss SL, Maude SL, Barrett DM, Lacey SF, Melenhorst JJ, et al. Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit Care Med. 2017;45:e124–e131.
Migliorini D, Dietrich PY, Stupp R, Linette GP, Posey AD Jr, June CH. CAR T-Cell therapies in glioblastoma: a first look. Clin Cancer Res. 2018;24:535–40.
Hege KM, Bergsland EK, Fisher GA, Nemunaitis JJ, Warren RS, McArthur JG, et al. Safety, tumor trafficking and immunogenicity of chimeric antigen receptor (CAR)-T cells specific for TAG-72 in colorectal cancer. J Immunother Cancer. 2017;5:22.
Linnemann C, Heemskerk B, Kvistborg P, Kluin RJ, Bolotin DA, Chen X, et al. High-throughput identification of antigen-specific TCRs by TCR gene capture. Nat Med 2013;19:1534–41.
Cameron BJ, Gerry AB, Dukes J, Harper JV, Kannan V, Bianchi FC, et al. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med. 2013;5:197ra103–197ra103.
Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 2013;122:863–71.
Guo C, Manjili MH, Subjeck JR, Sarkar D, Fisher PB, Wang XY. Therapeutic cancer vaccines: past, present, and future. Adv Cancer Res. 2013;119:421–75.
Mullard A. The cancer vaccine resurgence. Nat Rev Drug Discov. 2016;15:663–5.
Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15.
Garg AD, Coulie PG, Van den Eynde BJ, Agostinis P. Integrating next-generation dendritic cell vaccines into the current cancer immunotherapy landscape. Trends Immunol. 2017;38:577–93.
Schreibelt G, Bol KF, Westdorp H, Wimmers F, Aarntzen EH, Duiveman-de Boer T, et al. Effective clinical responses in metastatic melanoma patients after vaccination with primary myeloid dendritic cells. Clin Cancer Res. 2016;22:2155–66.
Chiang CL, Coukos G, Kandalaft LE. Whole tumor antigen vaccines: where are we? Vaccines. 2015;3:344–72.
Srivatsan S, Patel JM, Bozeman EN, Imasuen IE, He S, Daniels D, et al. Allogeneic tumor cell vaccines: the promise and limitations in clinical trials. Hum Vaccin Immunother. 2014;10:52–63.
Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–79.
Liu MA. DNA vaccines: an historical perspective and view to the future. Immunol Rev. 2011;239:62–84.
Yang B, Jeang J, Yang A, Wu TC, Hung CF. DNA vaccine for cancer immunotherapy. Hum Vaccin Immunother. 2014;10:3153–64.
Schlake T, Thess A, Fotin-Mleczek M, Kallen KJ. Develo** mRNA-vaccine technologies. RNA Biol. 2012;9:1319–30.
Kauffman KJ, Webber MJ, Anderson DG. Materials for non-viral intracellular delivery of messenger RNA therapeutics. J Control Release. 2016;240:227–34.
Li L, Goedegebuure SP, Gillanders WE. Preclinical and clinical development of neoantigen vaccines. Ann Oncol. 2017;28:xii11–xii17.
Lauss M, Donia M, Harbst K, Andersen R, Mitra S, Rosengren F, et al. Mutational and putative neoantigen load predict clinical benefit of adoptive T cell therapy in melanoma. Nat Commun. 2017;8:1738.
Zhu G, Zhang F, Ni Q, Niu G, Chen X. Efficient nanovaccine delivery in cancer immunotherapy. ACS Nano. 2017;11:2387–92.
**a Q, Gong C, Gu F, Wang Z, Hu C, Zhang L, et al. Functionalized multi-walled carbon nanotubes for targeting delivery of immunostimulatory CpG oligonucleotides against prostate cancer. J Biomed Nanotechnol. 2018;14:1613–26.
Men K, Huang R, Zhang X, Zhang R, Zhang Y, He M, et al. Local and systemic delivery of interleukin-12 gene by cationic micelles for cancer immunogene therapy. J Biomed Nanotechnol. 2018;14:1719–30.
Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–77.
Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604.
Breton G, Lee J, Liu K, Nussenzweig MC. Defining human dendritic cell progenitors by multiparametric flow cytometry. Nat Protoc. 2015;10:1407–22.
Schlitzer A, Sivakamasundari V, Chen J, Sumatoh HR, Schreuder J, Lum J, et al. Identification of cDC1- and cDC2-committed DC progenitors reveals early lineage priming at the common DC progenitor stage in the bone marrow. Nat Immunol. 2015;16:718–28.
Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1–22.
Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev. 2010;234:45–54.
Lee J, Breton G, Oliveira TY, Zhou YJ, Aljoufi A, Puhr S, et al. Restricted dendritic cell and monocyte progenitors in human cord blood and bone marrow. J Exp Med. 2015;212:385–99.
Yang W, Zhu G, Wang S, Yu G, Yang Z, Lin L, et al. In situ dendritic cell vaccine for effective cancer immunotherapy. ACS Nano 2019;13:3083–94.
Palucka K, Banchereau J, Mellman I. Designing vaccines based on biology of human dendritic cell subsets. Immunity. 2010;33:464–78.
Karaki S, Anson M, Tran T, Giusti D, Blanc C, Oudard S, et al. Is there still room for cancer vaccines at the era of checkpoint inhibitors. Vaccines. 2016;4:37.
Sabado RL, Balan S, Bhardwaj N. Dendritic cell-based immunotherapy. Cell Res. 2017;27:74–95.
Le Moignic A, Malard V, Benvegnu T, Lemiegre L, Berchel M, Jaffres PA, et al. Preclinical evaluation of mRNA trimannosylated lipopolyplexes as therapeutic cancer vaccines targeting dendritic cells. J Control Release. 2018;278:110–21.
Botto C, Augello G, Amore E, Emma MR, Azzolina A, Cavallaro G, et al. Cationic solid lipid nanoparticles as non viral vectors for the inhibition of hepatocellular carcinoma growth by RNA interference. J Biomed Nanotechnol. 2018;14:1009–16.
Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–60.
**ng H, Hwang K, Lu Y. Recent developments of liposomes as nanocarriers for theranostic applications. Theranostics. 2016;6:1336–52.
Pattni BS, Chupin VV, Torchilin VP. New developments in liposomal drug delivery. Chem Rev. 2015;115:10938–66.
Lai C, Duan S, Ye F, Hou X, Li X, Zhao J, et al. The enhanced antitumor-specific immune response with mannose- and CpG-ODN-coated liposomes delivering TRP2 peptide. Theranostics. 2018;8:1723–39.
Yuba E, Harada A, Sakanishi Y, Watarai S, Kono K. A liposome-based antigen delivery system using pH-sensitive fusogenic polymers for cancer immunotherapy. Biomaterials. 2013;34:3042–52.
Yuba E, Yamaguchi A, Yoshizaki Y, Harada A, Kono K. Bioactive polysaccharide-based pH-sensitive polymers for cytoplasmic delivery of antigen and activation of antigen-specific immunity. Biomaterials. 2017;120:32–45.
Yuba E, Kanda Y, Yoshizaki Y, Teranishi R, Harada A, Sugiura K, et al. pH-sensitive polymer-liposome-based antigen delivery systems potentiated with interferon-gamma gene lipoplex for efficient cancer immunotherapy. Biomaterials. 2015;67:214–24.
Yoshizaki Y, Yuba E, Sakaguchi N, Koiwai K, Harada A, Kono K. pH-sensitive polymer-modified liposome-based immunity-inducing system: effects of inclusion of cationic lipid and CpG-DNA. Biomaterials. 2017;141:272–83.
Zheng Y, Tang L, Mabardi L, Kumari S, Irvine DJ. Enhancing adoptive cell therapy of cancer through targeted delivery of small-molecule immunomodulators to internalizing or noninternalizing receptors. ACS Nano. 2017;11:3089–100.
Barenholz Y. Doxil(R)–the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160:117–34.
Grosso JF, Jure-Kunkel MN. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immun. 2013;13:5.
Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974–82.
Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9:325–38.
Nikpoor AR, Tavakkol-Afshari J, Sadri K, Jalali SA, Jaafari MR. Improved tumor accumulation and therapeutic efficacy of CTLA-4-blocking antibody using liposome-encapsulated antibody: In vitro and in vivo studies. Nanomedicine. 2017;13:2671–82.
Miura N, Akita H, Tateshita N, Nakamura T, Harashima H. Modifying antigen-encapsulating liposomes with KALA facilitates MHC class I antigen presentation and enhances anti-tumor effects. Mol Ther. 2017;25:1003–13.
Guo L, Yan DD, Yang D, Li Y, Wang X, Zalewski O, et al. Combinatorial photothermal and immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles. ACS Nano. 2014;8:5670–81.
Wang C, Xu L, Liang C, **ang J, Peng R, Liu Z. Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Adv Mater. 2014;26:8154–62.
Li L, Yang S, Song L, Zeng Y, He T, Wang N, et al. An endogenous vaccine based on fluorophores and multivalent immunoadjuvants regulates tumor micro-environment for synergistic photothermal and immunotherapy. Theranostics. 2018;8:860–73.
Liang R, **e J, Li J, Wang K, Liu L, Gao Y, et al. Liposomes-coated gold nanocages with antigens and adjuvants targeted delivery to dendritic cells for enhancing antitumor immune response. Biomaterials. 2017;149:41–50.
Huang J, Zhang X, Wu Z, Wu Y, Wu X, Wang Y, et al. Preparation and biocompatibility evaluation of PEG-PLL/RGD-PEG-DSPE/Phospholipid/CaP nanoparticles. J Biomed Nanotechnol. 2018;14:98–113.
Liu L, Wang Y, Miao L, Liu Q, Musetti S, Li J, et al. Combination immunotherapy of MUC1 mRNA nano-vaccine and CTLA-4 blockade effectively inhibits growth of triple negative breast cancer. Mol Ther. 2018;26:45–55.
Kang T, Huang Y, Zhu Q, Cheng H, Pei Y, Feng J, et al. Necroptotic cancer cells-mimicry nanovaccine boosts anti-tumor immunity with tailored immune-stimulatory modality. Biomaterials. 2018;164:80–97.
Huo M, Zhao Y, Satterlee AB, Wang Y, Xu Y, Huang L. Tumor-targeted delivery of sunitinib base enhances vaccine therapy for advanced melanoma by remodeling the tumor microenvironment. J Control Release. 2017;245:81–94.
Letchford K, Burt H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: micelles, nanospheres, nanocapsules and polymersomes. Eur J Pharm Biopharm. 2007;65:259–69.
Liu T, Liu Z, Chen J, ** R, Bai Y, Zhou Y, et al. Redox-responsive supramolecular micelles for targeted imaging and drug delivery to tumor. J Biomed Nanotechnol. 2018;14:1107–16.
Zhan X, Tran KK, Shen H. Effect of the poly(ethylene glycol) (PEG) density on the access and uptake of particles by antigen-presenting cells (APCs) after subcutaneous administration. Mol Pharmacol. 2012;9:3442–51.
Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159–64.
Zhuang Y, Ma Y, Wang C, Hai L, Yan C, Zhang Y, et al. PEGylated cationic liposomes robustly augment vaccine-induced immune responses: role of lymphatic trafficking and biodistribution. J Control Release. 2012;159:135–42.
Jiang L, Liang Y, Huo Q, Pu Y, Lu W, Han S, et al. Viral capsids mimicking based on pH-sensitive biodegradable polymeric micelles for efficient anticancer drug delivery. J Biomed Nanotechnol. 2018;14:1409–19.
Zeng Q, Jiang H, Wang T, Zhang Z, Gong T, Sun X. Cationic micelle delivery of Trp2 peptide for efficient lymphatic draining and enhanced cytotoxic T-lymphocyte responses. J Control Release. 2015;200:1–12.
Kudo S, Nagasaki Y. A novel nitric oxide-based anticancer therapeutics by macrophage-targeted poly(l-arginine)-based nanoparticles. J Control Release. 2015;217:256–62.
Liu L, He H, Liang R, Yi H, Meng X, Chen Z, et al. ROS-inducing micelles sensitize tumor-associated macrophages to TLR3 stimulation for potent immunotherapy. Biomacromolecules. 2018;19:2146–55.
Kumar A, Sirohi VK, Anum F, Singh PK, Gupta K, Gupta D, et al. Enhanced apoptosis, survivin down-regulation and assisted immunochemotherapy by curcumin loaded amphiphilic mixed micelles for subjugating endometrial cancer. Nanomedicine. 2017;13:1953–63.
Lu Y, Miao L, Wang Y, Xu Z, Zhao Y, Shen Y, et al. Curcumin micelles remodel tumor microenvironment and enhance vaccine activity in an advanced melanoma model. Mol Ther. 2016;24:364–74.
Ishii S, Kaneko J, Nagasaki Y. Development of a long-acting, protein-loaded, redox-active, injectable gel formed by a polyion complex for local protein therapeutics. Biomaterials. 2016;84:210–8.
Cui L, Osada K, Imaizumi A, Kataoka K, Nakano K. Feasibility of a subcutaneously administered block/homo-mixed polyplex micelle as a carrier for DNA vaccination in a mouse tumor model. J Control Release. 2015;206:220–31.
Li H, Li Y, Wang X, Hou Y, Hong X, Gong T, et al. Rational design of polymeric hybrid micelles to overcome lymphatic and intracellular delivery barriers in cancer immunotherapy. Theranostics. 2017;7:4383–98.
Luo Z, Wang C, Yi H, Li P, Pan H, Liu L, et al. Nanovaccine loaded with poly I:C and STAT3 siRNA robustly elicits anti-tumor immune responses through modulating tumor-associated dendritic cells in vivo. Biomaterials. 2015;38:50–60.
Liu L, Yi H, Wang C, He H, Li P, Pan H, et al. Integrated nanovaccine with microRNA-148a inhibition reprograms tumor-associated dendritic cells by modulating miR-148a/DNMT1/SOCS1 axis. J Immunol. 2016;197:1231–41.
Li C, Zhang X, Chen Q, Zhang J, Li W, Hu H, et al. Synthetic polymeric mixed micelles targeting lymph nodes trigger enhanced cellular and humoral immune responses. ACS Appl Mater Interfaces. 2018;10:2874–89.
Hamdy S, Haddadi A, Hung RW, Lavasanifar A. Targeting dendritic cells with nano-particulate PLGA cancer vaccine formulations. Adv Drug Deliv Rev. 2011;63:943–55.
Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 1997;28:5–24.
Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161:505–22.
Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B. 2010;75:1–18.
Fonseca C, Simões S, Gaspar R. Paclitaxel-loaded PLGA nanoparticles: preparation, physicochemical characterization and in vitro anti-tumoral activity. J Control Release. 2002;83:273–86.
Derakhshandeh K, Erfan M, Dadashzadeh S. Encapsulation of 9-nitrocamptothecin, a novel anticancer drug, in biodegradable nanoparticles: factorial design, characterization and release kinetics. Eur J Pharm Biopharm. 2007;66:34–41.
Mittal G, Sahana DK, Bhardwaj V, Ravi Kumar MNV. Estradiol loaded PLGA nanoparticles for oral administration: Effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivo. J Control Release. 2007;119:77–85.
Clawson C, Huang CT, Futalan D, Seible DM, Saenz R, Larsson M, et al. Delivery of a peptide via poly(D,L-lactic-co-glycolic) acid nanoparticles enhances its dendritic cell-stimulatory capacity. Nanomedicine. 2010;6:651–61.
Prasad S, Cody V, Saucier-Sawyer JK, Saltzman WM, Sasaki CT, Edelson RL, et al. Polymer nanoparticles containing tumor lysates as antigen delivery vehicles for dendritic cell-based antitumor immunotherapy. Nanomedicine. 2011;7:1–10.
Cruz LJ, Tacken PJ, Fokkink R, Joosten B, Stuart MC, Albericio F, et al. Targeted PLGA nano- but not microparticles specifically deliver antigen to human dendritic cells via DC-SIGN in vitro. J Control Release. 2010;144:118–26.
Niu L, Chu LY, Burton SA, Hansen KJ, Panyam J. Intradermal delivery of vaccine nanoparticles using hollow microneedle array generates enhanced and balanced immune response. J Control Release. 2019;294:268–78.
Kim H, Sehgal D, Kucaba TA, Ferguson DM, Griffith TS, Panyam J. Acidic pH-responsive polymer nanoparticles as a TLR7/8 agonist delivery platform for cancer immunotherapy. Nanoscale. 2018;10:20851–62.
Jia C, Yang T, Liu Y, Zhu A, Yin F, Wang Y, et al. A novel human papillomavirus 16 L1 pentamer-loaded hybrid particles vaccine system: influence of size on immune responses. ACS Appl Mater Interfaces. 2018;10:35745–59.
Fang RH, Hu CM, Luk BT, Gao W, Copp JA, Tai Y, et al. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014;14:2181–8.
Kosmides AK, Meyer RA, Hickey JW, Aje K, Cheung KN, Green JJ, et al. Biomimetic biodegradable artificial antigen presenting cells synergize with PD-1 blockade to treat melanoma. Biomaterials. 2017;118:16–26.
Guo Y, Wang D, Song Q, Wu T, Zhuang X, Bao Y, et al. Erythrocyte membrane-enveloped polymeric nanoparticles as nanovaccine for induction of antitumor immunity against melanoma. ACS Nano. 2015;9:6918–33.
Mueller M, Reichardt W, Koerner J, Groettrup M. Coencapsulation of tumor lysate and CpG-ODN in PLGA-microspheres enables successful immunotherapy of prostate carcinoma in TRAMP mice. J Control Release. 2012;162:159–66.
Sommershof A, Scheuermann L, Koerner J, Groettrup M. Chronic stress suppresses anti-tumor TCD8+ responses and tumor regression following cancer immunotherapy in a mouse model of melanoma. Brain Behav Immun. 2017;65:140–9.
Kim H, Niu L, Larson P, Kucaba TA, Murphy KA, James BR, et al. Polymeric nanoparticles encapsulating novel TLR7/8 agonists as immunostimulatory adjuvants for enhanced cancer immunotherapy. Biomaterials. 2018;164:38–53.
Park W, Gordon AC, Cho S, Huang X, Harris KR, Larson AC, et al. Immunomodulatory magnetic microspheres for augmenting tumor-specific infiltration of natural killer (NK) cells. ACS Appl Mater Interfaces. 2017;9:13819–24.
Liu J, Liu X, Han Y, Zhang J, Liu D, Ma G, et al. Nanovaccine incorporated with hydroxychloroquine enhances antigen cross-presentation and promotes antitumor immune responses. ACS Appl Mater Interfaces. 2018;10:30983–93.
Rosalia RA, Cruz LJ, van Duikeren S, Tromp AT, Silva AL, Jiskoot W, et al. CD40-targeted dendritic cell delivery of PLGA-nanoparticle vaccines induce potent anti-tumor responses. Biomaterials. 2015;40:88–97.
Mi Y, Smith CC, Yang F, Qi Y, Roche KC, Serody JS, et al. A dual immunotherapy nanoparticle improves T-cell activation and cancer immunotherapy. Adv Mater. 2018;30:e1706098.
Chen Q, Xu L, Liang C, Wang C, Peng R, Liu Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun. 2016;7:13193.
Luo L, Yang J, Zhu C, Jiang M, Guo X, Li W, et al. Sustained release of anti-PD-1 peptide for perdurable immunotherapy together with photothermal ablation against primary and distant tumors. J Control Release. 2018;278:87–99.
Luo L, Zhu C, Yin H, Jiang M, Zhang J, Qin B, et al. Laser immunotherapy in combination with perdurable PD-1 blocking for the treatment of metastatic tumors. ACS Nano. 2018;12:7647–62.
Ou W, Jiang L, Thapa RK, Soe ZC, Poudel K, Chang JH, et al. Combination of NIR therapy and regulatory T cell modulation using layer-by-layer hybrid nanoparticles for effective cancer photoimmunotherapy. Theranostics. 2018;8:4574–90.
Varypataki EM, Silva AL, Barnier-Quer C, Collin N, Ossendorp F, Jiskoot W. Synthetic long peptide-based vaccine formulations for induction of cell mediated immunity: a comparative study of cationic liposomes and PLGA nanoparticles. J Control Release. 2016;226:98–106.
Min Y, Roche KC, Tian S, Eblan MJ, McKinnon KP, Caster JM, et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat Nanotechnol. 2017;12:877–82.
Koshy ST, Mooney DJ. Biomaterials for enhancing anti-cancer immunity. Curr Opin Biotechnol. 2016;40:1–8.
Thomann-Harwood LJ, Kaeuper P, Rossi N, Milona P, Herrmann B, McCullough KC. Nanogel vaccines targeting dendritic cells: contributions of the surface decoration and vaccine cargo on cell targeting and activation. J Control Release. 2013;166:95–105.
Singh A, Peppas NA. Hydrogels and scaffolds for immunomodulation. Adv Mater. 2014;26:6530–41.
Ren C, Gao Y, Liu J, Zhang Y, Pu G, Yang L, et al. Anticancer supramolecular hydrogel of D/L-peptide with enhanced stability and bioactivity. J Biomed Nanotechnol. 2018;14:1125–34.
Song Q, Zhang R, Lei L, Li X. Self-assembly of succinated paclitaxel into supramolecular hydrogel for local cancer chemotherapy. J Biomed Nanotechnol. 2018;14:1471–6.
Zhou YF, Li XY. Self-assembled small molecular weight hydrogels of prodrugs. Chin Chem Lett. 2017;28:1835–40.
Wu DD, **e X, Kadi AA, Zhang Y. Photosensitive peptide hydrogels as smart materials for applications. Chin Chem Lett. 2018;29:1098–104.
Hori Y, Winans AM, Huang CC, Horrigan EM, Irvine DJ. Injectable dendritic cell-carrying alginate gels for immunization and immunotherapy. Biomaterials. 2008;29:3671–82.
Liu Y, **ao L, Joo KI, Hu B, Fang J, Wang P. In situ modulation of dendritic cells by injectable thermosensitive hydrogels for cancer vaccines in mice. Biomacromolecules. 2014;15:3836–45.
Muraoka D, Harada N, Hayashi T, Tahara Y, Momose F, Sawada SI, et al. Nanogel-based immunologically stealth vaccine targets macrophages in the medulla of lymph node and induces potent antitumor immunity. ACS Nano. 2014;8:9209–18.
Ueda K, Akiba J, Ogasawara S, Todoroki K, Nakayama M, Sumi A, et al. Growth inhibitory effect of an injectable hyaluronic acid-tyramine hydrogels incorporating human natural interferon-alpha and sorafenib on renal cell carcinoma cells. Acta Biomater. 2016;29:103–11.
Ye X, Liang X, Chen Q, Miao Q, Chen X, Zhang X, et al. Surgical tumor-derived personalized photothermal vaccine formulation for cancer immunotherapy. ACS Nano. 2019;13:2956–68.
Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ. Infection-mimicking materials to program dendritic cells in situ. Nat Mater. 2009;8:151–8.
Ali OA, Emerich D, Dranoff G, Mooney DJ. In situ regulation of DC subsets and T cells mediates tumor regression in mice. Sci Transl Med. 2009;1:8ra19–8ra19.
Bencherif SA, Warren Sands R, Ali OA, Li WA, Lewin SA, Braschler TM, et al. Injectable cryogel-based whole-cell cancer vaccines. Nat Commun. 2015;6:7556.
Wang H, Luo Z, Wang Y, He T, Yang C, Ren C, et al. Enzyme-catalyzed formation of supramolecular hydrogels as promising vaccine adjuvants. Adv Funct Mater. 2016;26:1822–9.
Luo Z, Wu Q, Yang C, Wang H, He T, Wang Y, et al. A powerful CD8(+) T-cell stimulating D-tetra-peptide hydrogel as a very promising vaccine adjuvant. Adv Mater. 2017;29:1601776.
Chao Y, Xu L, Liang C, Feng L, Xu J, Dong Z, et al. Combined local immunostimulatory radioisotope therapy and systemic immune checkpoint blockade imparts potent antitumour responses. Nat Biomed Eng. 2018;2:611–21.
Wang C, Wang J, Zhang X, Yu S, Wen D, Hu Q, et al. In situ formed reactive oxygen species-responsive scaffold with gemcitabine and checkpoint inhibitor for combination therapy. Sci Transl Med. 2018;10:eaan3682.
Chen Q, Wang C, Zhang X, Chen G, Hu Q, Li H, et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat Nanotechnol. 2019;14:89–97.
Shao Y, Sun ZY, Wang Y, Zhang BD, Liu D, Li YM. Designable immune therapeutical vaccine system based on DNA supramolecular hydrogels. ACS Appl Mater Interfaces. 2018;10:9310–4.
Song H, Huang P, Niu J, Shi G, Zhang C, Kong D, et al. Injectable polypeptide hydrogel for dual-delivery of antigen and TLR3 agonist to modulate dendritic cells in vivo and enhance potent cytotoxic T-lymphocyte response against melanoma. Biomaterials. 2018;159:119–29.
Wang T, Wang D, Yu H, Feng B, Zhou F, Zhang H, et al. A cancer vaccine-mediated postoperative immunotherapy for recurrent and metastatic tumors. Nat Commun. 2018;9:1532.
Li Z, Barnes JC, Bosoy A, Stoddart JF, Zink JI. Mesoporous silica nanoparticles in biomedical applications. Chem Soc Rev. 2012;41:2590–605.
Han P, Liu TC, Ji XW, Tang SK. Morphology-controlled synthesis of mesoporous silica with co-template of surfactant P123 and ionic liquid Dmim Cl. Chin Chem Lett. 2018;29:1305–9.
Zhou L, Huang J, Yu B, Liu Y, You T. A novel electrochemiluminescence immunosensor for the analysis of HIV-1 p24 antigen based on P-RGO@Au@Ru-SiO2 composite. ACS Appl Mater Interfaces. 2015;7:24438–45.
Gao WX, Hu YL, Xu L, Liu MC, Wu HY, He B. Dual pH and glucose sensitive gel gated mesoporous silica nanoparticles for drug delivery. Chin Chem Lett. 2018;29:1795–8.
Wang X, Li X, Ito A, Sogo Y, Watanabe Y, Tsuji NM, et al. Biodegradable metal ion-doped mesoporous silica nanospheres stimulate anticancer Th1 immune response in vivo. ACS Appl Mater Interfaces. 2017;9:43538–44.
Kakizawa Y, Lee JS, Bell B, Fahmy TM. Precise manipulation of biophysical particle parameters enables control of proinflammatory cytokine production in presence of TLR 3 and 4 ligands. Acta Biomater. 2017;57:136–45.
Vallhov H, Gabrielsson S, Stromme M, Scheynius A, Garcia-Bennett AE. Mesoporous silica particles induce size dependent effects on human dendritic cells. Nano Lett. 2007;7:3576–82.
Abbaraju PL, Meka AK, Song H, Yang Y, Jambhrunkar M, Zhang J, et al. Asymmetric silica nanoparticles with tunable head-tail structures enhance hemocompatibility and maturation of immune cells. J Am Chem Soc. 2017;139:6321–8.
An M, Yu C, ** J, Reyes J, Mao G, Wei WZ, et al. Induction of necrotic cell death and activation of STING in the tumor microenvironment via cationic silica nanoparticles leading to enhanced antitumor immunity. Nanoscale. 2018;10:9311–9.
Lu Y, Yang Y, Gu Z, Zhang J, Song H, **ang G, et al. Glutathione-depletion mesoporous organosilica nanoparticles as a self-adjuvant and Co-delivery platform for enhanced cancer immunotherapy. Biomaterials. 2018;175:82–92.
An M, Li M, ** J, Liu H. Silica nanoparticle as a lymph node targeting platform for vaccine delivery. ACS Appl Mater Interfaces. 2017;9:23466–75.
Wang G, Zhou H, Nian QG, Yang Y, Qin CF, Tang R. Robust vaccine formulation produced by assembling a hybrid coating of polyethyleneimine-silica. Chem Sci. 2016;7:1753–9.
Wang J, Zhu R, Gao B, Wu B, Li K, Sun X, et al. The enhanced immune response of hepatitis B virus DNA vaccine using SiO2@LDH nanoparticles as an adjuvant. Biomaterials. 2014;35:466–78.
Scaramuzzi K, Tanaka GD, Neto FM, Garcia PR, Gabrili JJ, Oliveira DC, et al. Nanostructured SBA-15 silica: an effective protective vehicle to oral hepatitis B vaccine immunization. Nanomedicine. 2016;12:2241–50.
Wang G, Wang HJ, Zhou H, Nian QG, Song Z, Deng YQ, et al. Hydrated silica exterior produced by biomimetic silicification confers viral vaccine heat-resistance. ACS Nano. 2015;9:799–808.
de Souza ESJM, Hanchuk TD, Santos MI, Kobarg J, Bajgelman MC, Cardoso MB. Viral inhibition mechanism mediated by surface-modified silica nanoparticles. ACS Appl Mater Interfaces. 2016;8:16564–72.
Kempe K, **ang SD, Wilson P, Rahim MA, Ju Y, Whittaker MR, et al. Engineered hydrogen-bonded glycopolymer capsules and their interactions with antigen presenting cells. ACS Appl Mater Interfaces. 2017;9:6444–52.
De Koker S, Cui J, Vanparijs N, Albertazzi L, Grooten J, Caruso F, et al. Engineering polymer hydrogel nanoparticles for lymph node-targeted delivery. Angew Chem Int Ed Engl. 2016;55:1334–9.
Wang X, Li X, Ito A, Yoshiyuki K, Sogo Y, Watanabe Y, et al. Hollow structure improved anti-cancer immunity of mesoporous silica nanospheres in vivo. Small. 2016;12:3510–5.
Cui J, De Rose R, Best JP, Johnston AP, Alcantara S, Liang K, et al. Mechanically tunable, self-adjuvanting nanoengineered polypeptide particles. Adv Mater. 2013;25:3468–72.
Gause KT, Yan Y, Cui J, O’Brien-Simpson NM, Lenzo JC, Reynolds EC, et al. Physicochemical and immunological assessment of engineered pure protein particles with different redox states. ACS Nano. 2015;9:2433–44.
Yang Y, Lu Y, Abbaraju PL, Zhang J, Zhang M, **ang G, et al. Multi-shelled dendritic mesoporous organosilica hollow spheres: roles of composition and architecture in cancer immunotherapy. Angew Chem Int Ed. 2017;56:8446–50.
Kim J, Li WA, Choi Y, Lewin SA, Verbeke CS, Dranoff G, et al. Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nat Biotechnol. 2015;33:64–72.
Li WA, Lu BY, Gu L, Choi Y, Kim J, Mooney DJ. The effect of surface modification of mesoporous silica micro-rod scaffold on immune cell activation and infiltration. Biomaterials. 2016;83:249–56.
Li AW, Sobral MC, Badrinath S, Choi Y, Graveline A, Stafford AG, et al. A facile approach to enhance antigen response for personalized cancer vaccination. Nat Mater. 2018;17:528–34.
Levy M, Luciani N, Alloyeau D, Elgrabli D, Deveaux V, Pechoux C, et al. Long term in vivo biotransformation of iron oxide nanoparticles. Biomaterials. 2011;32:3988–99.
Anselmo AC, Mitragotri S. A review of clinical translation of inorganic nanoparticles. AAPS J 2015;17:1041–54.
Hauksdottir HL, Webster TJ. Selenium and iron oxide nanocomposites for magnetically-targeted anti-cancer applications. J Biomed Nanotechnol. 2018;14:510–25.
Shevtsov MA, Nikolaev BP, Yakovleva LY, Parr MA, Marchenko YY, Eliseev I, et al. 70-kDa heat shock protein coated magnetic nanocarriers as a nanovaccine for induction of anti-tumor immune response in experimental glioma. J Control Release. 2015;220:329–40.
Bocanegra Gondan AI, Ruiz-de-Angulo A, Zabaleta A, Gomez Blanco N, Cobaleda-Siles BM, Garcia-Granda MJ, et al. Effective cancer immunotherapy in mice by polyIC-imiquimod complexes and engineered magnetic nanoparticles. Biomaterials. 2018;170:95–115.
Ruiz-de-Angulo A, Zabaleta A, Gomez-Vallejo V, Llop J, Mareque-Rivas JC. Microdosed lipid-coated (67)Ga-magnetite enhances antigen-specific immunity by image tracked delivery of antigen and CpG to lymph nodes. ACS Nano. 2016;10:1602–18.
** H, Qian Y, Dai Y, Qiao S, Huang C, Lu L, et al. Magnetic enrichment of dendritic cell vaccine in lymph node with fluorescent-magnetic nanoparticles enhanced cancer immunotherapy. Theranostics. 2016;6:2000–14.
Chen WR, Adams RL, Carubelli R, Nordquist RE. Laser-photosensitizer assisted immunotherapy: a novel modality for cancer treatment. Cancer Lett. 1997;115:25–30.
Li X, Li N, Sidlauskas K, Li H, Zhang C, Peng M, et al. Doxorubicin-loaded dextran-modified goldmag nanoparticles for targeting hepatocellular carcinoma. J Biomed Nanotechnol. 2018;14:1135–46.
Tian Y, Wang X, Zhao S, Liao X, Younis MR, Wang S, et al. JQ1-loaded polydopamine nanoplatform inhibit c-MYC/PD-L1 to enhance photothermal therapy for triple-negative breast cancer. ACS Appl Mater Interfaces. 2019;11:46626–36.
Li D, Zhang M, Xu F, Chen Y, Chen B, Chang Y, et al. Biomimetic albumin-modified gold nanorods for photothermo-chemotherapy and macrophage polarization modulation. Acta Pharm Sin B. 2018;8:74–84.
Burga RA, Patel S, Bollard CM, Y Cruz CR, Fernandes R. Conjugating Prussian blue nanoparticles onto antigen-specific T cells as a combined nanoimmunotherapy. Nanomedicine. 2016;11:1759–67.
Cano-Mejia J, Burga RA, Sweeney EE, Fisher JP, Bollard CM, Sandler AD, et al. Prussian blue nanoparticle-based photothermal therapy combined with checkpoint inhibition for photothermal immunotherapy of neuroblastoma. Nanomedicine. 2017;13:771–81.
**g H, Weidensteiner C, Reichardt W, Gaedicke S, Zhu X, Grosu AL, et al. Imaging and selective elimination of glioblastoma stem cells with theranostic near-infrared-labeled CD133-specific antibodies. Theranostics. 2016;6:862–74.
Meir R, Shamalov K, Betzer O, Motiei M, Horovitz-Fried M, Yehuda R, et al. Nanomedicine for cancer immunotherapy: tracking cancer-specific T-cells in vivo with gold nanoparticles and CT imaging. ACS Nano. 2015;9:6363–72.
Meir R, Shamalov K, Sadan T, Motiei M, Yaari G, Cohen CJ, et al. Fast image-guided stratification using anti-programmed death ligand 1 gold nanoparticles for cancer immunotherapy. ACS Nano. 2017;11:11127–34.
Ma Y, Qiao SL, Wang Y, Lin YX, An HW, Wu XC, et al. Nanoantagonists with nanophase-segregated surfaces for improved cancer immunotherapy. Biomaterials. 2018;156:248–57.
Yata T, Takahashi Y, Tan M, Nakatsuji H, Ohtsuki S, Murakami T, et al. DNA nanotechnology-based composite-type gold nanoparticle-immunostimulatory DNA hydrogel for tumor photothermal immunotherapy. Biomaterials. 2017;146:136–45.
Gupta J, Park SS, Bondy B, Felner EI, Prausnitz MR. Infusion pressure and pain during microneedle injection into skin of human subjects. Biomaterials. 2011;32:6823–31.
van der Maaden K, Jiskoot W, Bouwstra J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J Control Release. 2012;161:645–55.
Li X, Xu Q, Zhang P, Zhao X, Wang Y. Cutaneous microenvironment responsive microneedle patch for rapid gene release to treat subdermal tumor. J Control Release. 2019;314:72–80.
Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547–68.
Ita K. Dissolving microneedles for transdermal drug delivery: advances and challenges. Biomed Pharmacother. 2017;93:1116–27.
Ma G, Wu C. Microneedle, bio-microneedle and bio-inspired microneedle: a review. J Control Release. 2017;251:11–23.
**e X, Pascual C, Lieu C, Oh S, Wang J, Zou B, et al. Analgesic microneedle patch for neuropathic pain therapy. ACS Nano. 2017;11:395–406.
Zhang Y, Yu J, Wang J, Hanne NJ, Cui Z, Qian C, et al. Thrombin-responsive transcutaneous patch for auto-anticoagulant regulation. Adv Mater. 2017;29:1604043.
Bhatnagar S, Dave K, Venuganti VVK. Microneedles in the clinic. J Control Release. 2017;260:164–82.
Yu J, Qian C, Zhang Y, Cui Z, Zhu Y, Shen Q, et al. Hypoxia and H2O2 dual-sensitive vesicles for enhanced glucose-responsive insulin delivery. Nano Lett. 2017;17:733–9.
Kim NW, Kim SY, Lee JE, Yin Y, Lee JH, Lim SY, et al. Enhanced cancer vaccination by in situ nanomicelle-generating dissolving microneedles. ACS Nano. 2018;12:9702–13.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54.
Chapman AP. PEGylated antibodies and antibody fragments for improved therapy: a review. Adv Drug Deliv Rev. 2002;54:531–45.
Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 2014;13:655–72.
Wang C, Ye Y, Hochu GM, Sadeghifar H, Gu Z. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody. Nano Lett. 2016;16:2334–40.
Ye Y, Wang J, Hu Q, Hochu GM, **n H, Wang C, et al. Synergistic transcutaneous immunotherapy enhances antitumor immune responses through delivery of checkpoint inhibitors. ACS Nano. 2016;10:8956–63.
Ye Y, Wang C, Zhang X, Hu Q, Zhang Y, Liu Q, et al. A melanin-mediated cancer immunotherapy patch. Sci Immunol. 2017;2:eaan5692.
van der Maaden K, Trietsch SJ, Kraan H, Varypataki EM, Romeijn S, Zwier R, et al. Novel hollow microneedle technology for depth-controlled microinjection-mediated dermal vaccination: a study with polio vaccine in rats. Pharmacol Res. 2014;31:1846–54.
Schipper P, van der Maaden K, Romeijn S, Oomens C, Kersten G, Jiskoot W, et al. Determination of depth-dependent intradermal immunogenicity of adjuvanted inactivated polio vaccine delivered by microinjections via hollow microneedles. Pharmacol Res. 2016;33:2269–79.
Du G, Hathout RM, Nasr M, Nejadnik MR, Tu J, Koning RI, et al. Intradermal vaccination with hollow microneedles: a comparative study of various protein antigen and adjuvant encapsulated nanoparticles. J Control Release. 2017;266:109–18.
van der Maaden K, Heuts J, Camps M, Pontier M, Terwisscha van Scheltinga A, Jiskoot W, et al. Hollow microneedle-mediated micro-injections of a liposomal HPV E743-63 synthetic long peptide vaccine for efficient induction of cytotoxic and T-helper responses. J Control Release. 2018;269:347–54.
Miao L, Li J, Liu Q, Feng R, Das M, Lin CM, et al. Transient and local expression of chemokine and immune checkpoint traps to treat pancreatic cancer. ACS Nano. 2017;11:8690–706.
Mohri K, Nishikawa M, Takahashi N, Shiomi T, Matsuoka N, Ogawa K, et al. Design and development of nanosized DNA assemblies in polypod-like structures as efficient vehicles for immunostimulatory CpG motifs to immune cells. ACS Nano. 2012;6:5931–40.
Fang RH, Kroll AV, Zhang L. Nanoparticle-based manipulation of antigen-presenting cells for cancer immunotherapy. Small. 2015;11:5483–96.
Petersburg JR, Shen J, Csizmar CM, Murphy KA, Spanier J, Gabrielse K, et al. Eradication of established tumors by chemically self-assembled nanoring labeled T cells. ACS Nano. 2018;12:6563–76.
Tian X, Zhu M, Tian Y, Ramm GA, Zhao Y, Nie G. A membrane vesicle-based dual vaccine against melanoma and Lewis lung carcinoma. Biomaterials. 2012;33:6147–54.
Cheung AS, Koshy ST, Stafford AG, Bastings MM, Mooney DJ. Adjuvant-loaded subcellular vesicles derived from disrupted cancer cells for cancer vaccination. Small. 2016;12:2321–33.
Murray AA, Wang C, Fiering S, Steinmetz NF. In situ vaccination with cowpea vs tobacco mosaic virus against melanoma. Mol Pharmacol. 2018;15:3700–16.
Lizotte PH, Wen AM, Sheen MR, Fields J, Rojanasopondist P, Steinmetz NF, et al. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat Nanotechnol. 2016;11:295–303.
Acknowledgements
The authors would like to thank the following funding sources: The National Science Fund for Distinguished Young Scholars (31525009), the National Natural Science Foundation of China (31930067, 31800797, and 31771096), the National Key Research and Development Program of China (2017YFC1103502), the China Postdoctoral Science Foundation (2018M631094 and 2019M653410), and the 1·3·5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYGD18002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, F., Shi, K., Jia, Yp. et al. Advanced biomaterials for cancer immunotherapy. Acta Pharmacol Sin 41, 911–927 (2020). https://doi.org/10.1038/s41401-020-0372-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-020-0372-z
- Springer Nature Singapore Pte Ltd.
Keywords
This article is cited by
-
Reprogramming the tumor microenvironment with biotechnology
Biomaterials Research (2023)
-
Improved Targeting of Therapeutics by Nanocarrier-Based Delivery in Cancer Immunotherapy and Their Future Perspectives
BioNanoScience (2023)
-
Engineered nanomedicines block the PD-1/PD-L1 axis for potentiated cancer immunotherapy
Acta Pharmacologica Sinica (2022)
-
Integrative insights and clinical applications of single-cell sequencing in cancer immunotherapy
Cellular and Molecular Life Sciences (2022)
-
Nanomedicine and cancer immunotherapy
Acta Pharmacologica Sinica (2020)