Abstract
Ordinary sensations from inside the body are important causes and consequences of our affective states and behaviour, yet the roles of neurotransmitters in interoceptive processing have been unclear. With a within-subjects design, this experiment tested the impacts of acute increases of endogenous extracellular serotonin on the neural processing of attended internal sensations and the links of these effects to anxiety using a selective serotonin reuptake inhibitor (SSRI) (20 mg citalopram) and a placebo. Twenty-one healthy volunteers (fourteen female, mean age 23.9) completed the Visceral Interoceptive Attention (VIA) task while undergoing functional magnetic resonance imaging (fMRI) with each treatment. The VIA task required focused attention on the heart, stomach, or visual sensation. The relative neural interoceptive responses to heart sensation [heart minus visual attention] (heart-IR) and stomach sensation [stomach minus visual attention] (stomach-IR) were compared between treatments. Visual attention subtraction controlled for the general effects of citalopram on sensory processing. Citalopram was associated with lower interoceptive processing in viscerosensory (the stomach-IR of bilateral posterior insular cortex) and integrative/affective (the stomach-IR and heart-IR of bilateral amygdala) components of interoceptive neural pathways. In anterior insular cortex, citalopram reductions of heart-IR depended on anxiety levels, removing a previously known association between anxiety and the region’s response to attended heart sensation observed with placebo. Preliminary post hoc analysis indicated that citalopram effects on the stomach-IR of the amygdalae corresponded to acute anxiety changes. This direct evidence of general and anxiety-linked serotonergic influence on neural interoceptive processes advances our understanding of interoception, its regulation, and anxiety.
Similar content being viewed by others
Introduction
If asked how one feels, it is natural to turn attention to the body. This involves interoception—the sensing and processing of internal physiological states [1,2,3]. The influence of ordinary interoception ranges from basic regulatory reflexes that maintain life with homoeostasis to the conscious affective experiences of hunger, arousal, anxiety, and self that can impact mental health [2, 4,5,6,7]. Yet, little is known about the pharmacology of ordinary interoception or how this pharmacology determines and is determined by affective states. Here, we studied the impact of acute changes of serotonin on ordinary interoception and how this influence relates to anxiety.
Serotonin is recognised for its ability to acutely modulate exteroceptive sensory processing, such as hearing and vision, according to the animal’s needs and environment. In this context, increased serotonin activity is proposed to have a nuanced tempering effect on the flow of sensory information [8, 9].
Serotonin has also been shown to modulate interoception when sensations are aversive and exogenous, such as when a balloon or acid is applied within the digestive tract [10,11,12,13,14,15]. These effects can depend on the stimulation site and participant states of sensitivity and perception of pain. In general, however, single doses of selective serotonin reuptake inhibitors (SSRIs) tend to reduce the sensitivity and aversiveness of exogenous interoception of the digestive tract while depleting serotonin’s precursor tends to amplify it. Whether or not serotonin’s influence extends to interoception of ordinary endogenous gastric sensations is not yet clear.
Similarly, little is understood about serotonin’s role in the interoception of the heart. In a behavioural study of healthy volunteers, a single SSRI dose increased metacognitive insight into the interoception of heartbeats [16]. In major depression, patients tend to have blunted interoceptive processing of the heart and stomach [17]. Patients with depression receiving chronic SSRI treatment have demonstrated higher subjective interoceptive intensities than patients not receiving SSRI treatment [18]. However, the neural correlates of these SSRI effects have remained unclear.
An association between serotonin and ordinary interoception might be expected because both are commonly associated with anxiety. The serotonin-anxiety relationship has been established by considerable preclinical research [19,20,21] and SSRI effectiveness as a first-line pharmacological treatment of panic and generalised anxiety disorder [22, 23]. SSRIs can also increase anxiety in the short term [24, 25]. The interoception-anxiety relationship is characterised by a theoretical understanding of the role of interoception in the pathoaetiology and treatment of somatic anxiety symptoms and backed by empirical observations of interoceptive disturbance in the same anxiety disorders that are treated with SSRIs [7, 26,27,28]. Modern conceptualisations of irritable bowel syndrome also acknowledge neural, genetic, homoeostatic and pharmacological overlap between gastrointestinal sensation and anxious states [29]. In the lab, many of the same anxiety-linked responses that SSRIs acutely influence will vary with interoceptive sensation—including startle [30,31,32,33], fear [34, 35], and emotion recognition [36,37,38,39]. Conversely, SSRI treatment can also cause emotional blunting, which could be attributable to the general suppression of interoceptive processes, consistent with effects on aversive gastric interoception [15], or a change in interoception’s influence on subjective affective experience [40,41,42].
The neural substrates of interoception, anxiety, and serotoninergic influence on cognition also overlap. At serotonin terminals, this occurs within the insular cortex and amygdala [3, 17, 20, 29, 39, 43,44,45,46,47,48,49,50]. However, serotonin’s interoceptive effects could also occur at cell bodies within the raphe nuclei. The raphe nuclei contain cells that modulate appetitive, cardiac, respiratory, sensory, and thermal regulatory processes in response to interoceptive information [51, 52] and are thought to play a critical role in regulating anxiety [53]. SSRIs disproportionately increase extracellular serotonin at the raphe nuclei following acute doses [54].
Overall, our knowledge about the relationship of serotonin to ordinary interoceptive processing has been promising but limited by the testing of abnormal, painful, visceral sensations in small samples, the study of patient populations with disturbed interoception, absent exteroceptive control conditions, indirect inferences and lack of neural insight [55]. No direct link of serotonin to the neural processing of ordinary interoceptive sensation has been established.
Critically, some effects of serotonin on interoception are likely to be state-dependent. Serotonergic inhibitory effects on exteroceptive processing [9, 56, 57] and exogenous gastrointestinal interoception [10, 11, 14] are sensitive to the environment and state of the individual. This and other findings suggest that serotonin could constrain the impact of these states on emotion, cognition and behaviour through the regulation of sensory information processing [58]. One state of particular interest for interoception and serotonin is anxiety. The anterior insular cortex responds more to attended cardiac sensation in anxious individuals, and the cognition of anxious individuals can be more susceptible to interoceptive influence [44, 45, 59]. As such, we considered that some influences of serotonin on interoception might be particularly evident during anxious states and thereby modulate the interoception-anxiety relationship. Previously, the selectivity of serotonin effects for anxious states has been observed in the amygdala, wherein a single SSRI dose reduced the enhanced behavioural and neural response to fearful faces in relatively anxious individuals (also with past or present depression) but not in less-anxious, healthy controls [60, 61].
Neural responses to ordinary endogenous interoception can be probed by asking individuals to shift and maintain attention to a specific visceral sensation, which typically enhances the regional neural response underlying the corresponding interoceptive representation [17, 18, 62,63,64,65]. Neural responses to different interoceptive sensations have different implications for health and behaviour [2, 29, 59, 62]. Attended heart and stomach sensations have been shown to have overlap** but ultimately distinct representations in the human brain [66]. Correspondingly, researchers have examined the neural correlates of cardiac and gastric interoception separately within the VIA task in various clinical contexts after subtracting effects on visual attention to control for general effects on sensory processing [17, 18, 62,63,64,65]. We combined this approach with the established pharmacological technique of using a single SSRI dose in healthy volunteers [39, 50] to test the influence of increased extracellular serotonin on heart and stomach interoceptive processing. While long-term SSRI doses are essential for modelling the mechanisms of their delayed therapeutic effects, acute dose studies provide insight into the impacts of immediate serotonin changes on cognition without confounds of receptor desensitisation and neuroplasticity theoretically linked to long-term SSRI treatment [54, 67]. Single-dose studies can also provide needed mechanistic insight into early side effects of SSRIs, such as anxiety [25], and the immediate effects of SSRIs on affective bias that could theoretically contribute to and predict long-term clinical outcomes [39]. Studies of healthy volunteers avoid extraneous confounds associated with disorders and allow for a powerful within-subject analysis without requiring a placebo control in patients who would benefit from medication.
We recognised that the influence of SSRIs could be expressed in different ways across the hierarchical network of interoceptive substrates, which change in function from supporting basic interoceptive sensory representations (e.g. mid-posterior insular cortex) to their integration into motivational and affective states (e.g. anterior insular cortex and amygdala) [3]. We, therefore, tested two hypotheses across these networks. Considering that serotonin tends to generally inhibit exteroceptive processing [8, 56,57,58], reduce visceral pain [10, 14, 15], increase emotional ‘blunting’ [40, 68], reduce responses to rewards and punishments [69], and reduce amygdala and insula responses to cues of threat [70]—the primary hypothesis was that an acute SSRI dose would attenuate the general neural response to ordinary internal sensations. The secondary hypothesis was that some SSRI effects on interoception would be selective for anxious states. We then undertook a post hoc exploration of associations between acute SSRI effects on interoception and changes in anxiety.
Methods and materials
Experimental design
We completed a placebo-controlled, double-blind, randomised cross-over, pharmacological neuroimaging experiment whereby the same participants were tested in two sessions, one following a single dose of an SSRI and one following a single placebo, in random order. The SSRI (20 mg oral citalopram (Cipramil, Lundbeck Ltd, Watford, UK) was chosen for its common use, its safety profile, and its exceptionally high selectivity for the serotonin transporter [71].
Participants
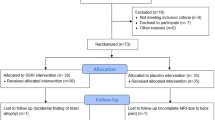
Ethical permission was granted by The Brighton and Sussex Medical School Research Governance and Ethics Committee (ER/JL332/9). Potential participants were screened with a health questionnaire and a standard psychiatric interview. Each participant provided informed consent. Exclusion criteria included: age under 18 years or over 35 years; the presence of significant ongoing medical condition; pregnancy or breastfeeding; currently taking any medication (excluding contraceptive pill); first-degree family history of bipolar disorder; an indication of current or historical mental health disorder, or scanner contraindications (e.g. metallic implants). Participants were instructed to abstain from alcohol or caffeine in the preceding 12 h before the start of test sessions.
Thirty-one healthy participants were recruited for the study and randomised to a treatment sequence. The sample size was determined by available funding, exceedance of sample sizes used to establish prior acute citalopram effects on neural responses to affective cognition [39], our within-subject design, and the probable 30% attrition rate. Seven participants withdrew from the study. Neuroimaging data from two participants were excluded due to excessive motion ( > 6% of volumes identified as motion outliers for either session scan). Neuroimaging data for one participant was excluded due to extreme nausea requiring exiting of the scanner. This left 21 participants measured in each condition, with 42 sessions in total across both treatments. This sample (mean age of 23.9 (SD = 3.3); and a mean body mass index of 21.9 (SD = 3.3)) included seven females. Trait anxiety scores (State-Trait Anxiety Inventory [72] (STAI)) averaged 34.9 (SD = 8.3). Eleven participants lived with low trait anxiety (score below 35), nine participants lived with moderate trait anxiety (score between 35 and 44), and one participant lived with high trait anxiety (score of 50). As measured by the Body Perception Questionnaire [73], the sample’s interoceptive tendencies fell within the normal range: their awareness subscale mean was 2.4 (SD = 1.0), and the stress subscale mean was 2.5 (SD = 0.88).
Study procedure
Participants were tested twice, with separate citalopram and placebo sessions, at least seven days apart (M = 10.3 days, SD = 6.87). The assignment of participants to treatment order was completed by a researcher with no contact with participants and no awareness of participant identities, using a computer-based algorithm for randomisation. Participants and researchers in contact with participants were blind to treatment conditions. Citalopram and placebo doses were contained in identical gelatine capsules filled with microcrystalline cellulose by a pharmacist.
After the dose, participants were given instructions and time to practice the VIA task before testing. Just before scanning, heart rate was recorded with the participant relaxed and sitting. Heart rate variability was measured as the standard deviation of the pulse interval. To allow citalopram levels to reach peak absorption, participants performed the VIA task approximately 3.75 h after treatment intake.
State anxiety (STAI-S) was measured immediately before and after the scan session. We used the average of the two measurements to estimate anxiety in the scanner. Similarly, to relate to prior studies and capture any overall change in mood, participant affect was measured with the Positive and Negative Affect Scale (PANAS) before and after scan sessions. The PANAS includes features of state anxiety in addition to other measures of affect. So, only the STAI-S was used to test hypotheses related to anxiety. Participants also completed eight visual analogue scales before and after the scan (the average of these taken for analysis, estimating scanner experiences). Three scales (from 0 to 100) were given to assess three somatic side effects (nausea, headache and dizziness). Five anxiety-related effects (pairs of antonyms: alert−drowsy, stimulated−sedated, restless−peaceful, irritable-good-humoured, anxious−calm) were used to confirm the STAI-S measures and alert the researchers to excessive side effects.
Visceral interoceptive awareness task
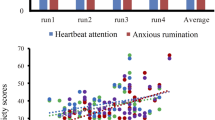
The VIA task (Fig. 1) has been used previously to identify changes in neural interoceptive processing in multiple clinical contexts [17, 18, 62,63,64,65]. The two interoceptive conditions consist of the Heart and Stomach cue trials, and the exteroceptive (visual) baseline condition consists of Target trials. In the Target trials, after a short interval, the target started changing from black to grey and cycled between these shades for a randomly varied time between 0.7 s and 1.1 s, designed to mimic the approximate frequency of a heartbeat. In approximately half the trials, participants were asked to rate the intensity of the sensations from the area of focus (interoceptive trials) or the intensity of the colour change (exteroceptive trials). These responses helped to ensure that participants focused but were too infrequent for statistical inference. The trials selected for intensity rating were the same for every participant in a randomised sequence. Sensory condition order (Heart, Stomach, Target) and shades of grey for the Target blocks were presented in the same randomised sequence for every participant. There were 15 trials in each condition for a total task duration of 15 min.
Participants were given the following instructions:
‘While the word ‘HEART’ or ‘STOMACH’ is shown on the screen, focus attention on the intensity of the sensations experienced from the area of your heart or stomach. When the word ‘TARGET’ is shown on the screen, it will sometimes change from black to different shades of grey. Focus attention on the amount that the colour changes. When a CROSS is shown on the screen, you can rest. Please keep your eyes open and try not to think of anything in particular. You will sometimes be asked to rate how intense the sensations or the colour changes were, using the buttons shown. Please make sure that you breathe evenly throughout the task. You must NOT hold your breath at any time.’
Neuroimaging procedures
Magnetic resonance imaging data were acquired using a Siemens Magnetom Prisma 3T scanner (Siemens Healthcare GmbH, Erlangen, Germany) and a 32-channel head coil. We acquired fMRI images during VIA task performance, field maps, perfusion images to test for treatment effects on cerebral blood flow (see Supplemental Material) and T1-weighted structural images for registration.
Image acquisition
fMRI images were acquired using T2*-weighted echo-planar imaging sensitive to blood oxygenation level-dependent signal changes (multiband factor 4, echo time 37 ms, repetition time 1500 ms, voxel size 2.2 × 2.2 × 2 mm, 104 × 104 voxels per slice, 72 slices, field of view (FOV) 205 × 205 mm2, flip angle 52°), with a varying number of volumes according to the speed of task completion (M = 580, SD = 36.8). Before the task fMRI sequence on both test sessions, pairs of phase-encode reversed images were acquired for distortion correction (FOV 228 × 228 mm2 104 × 104 voxels per slice, echo spacing 0.54 ms). In one session, a T1-weighted magnetisation-prepared rapid acquisition gradient echo (MPRAGE) structural image of the brain was acquired (echo time 2.2 ms, repetition time 2400 ms, 0.8 mm isotropic voxels, 300 × 320 voxels per slice, 208 slices, FOV 256 × 256 mm2, flip angle 8°).
Pre-processing
fMRI images were pre-processed with a standard FMRIB Software Library v. 6.02 (FSL) [74] pipeline including motion correction using the middle volume of the time series as a reference volume, identification of motion outlying volumes using FSL Motion Outliers (root mean squared intensity difference of adjacent volumes method), distortion correction using phase-encode reversed image pairs (using FSL tool ‘topup’), high-pass filtering at 80 s, brain extraction, and co-registration to participant structural images using linear registration. Registration from structural space to standard Montreal Neurological Institute space (MNI152 nonlinear 6th generation) was carried out using nonlinear registration. Independent Component Analysis (ICA) decomposition [75] was completed on unsmoothed data, and denoising was carried out manually, with noise components identified by an independent researcher using published criteria [76]. Spatial smoothing was completed with a 5 mm full-width at half maximum Gaussian kernel.
Analyses
Treatment differences in self-reported sensation intensities during the VIA task, heart rate, STAI-S and PANAS scores and VAS scales were analysed using paired t-tests.
Functional images were analysed using FSL’s FMRI Expert Analysis Tool (FEAT) v 6.0 with two-level generalised linear models (GLMs). The first-level models corresponded to individual scan sessions. These included events corresponding to trial conditions (Heart, Stomach and Target) along with regressors of no interest: intensity rating periods, button presses, outlying motion volumes and temporal derivatives of each event. Neural responses to exteroceptive focus (Target) served as high-level controls. They were subtracted from responses to interoceptive focus (Heart, Stomach) to generate contrast images of the relative interoceptive response to heart focus (heart-IR) and stomach focus (stomach-IR). The subtraction of exteroceptive effects controls for general treatment effects on sensory processing.
We completed three second-level analyses. Hypothesis 1 was tested by comparing heart-IR and stomach-IR between treatment conditions. This used FSL’s paired two-group difference design (i.e., a multilevel mixed effect model with a random intercept) with FMRIB’s Local Analysis of Mixed Effects (FLAME) 1 & 2 over the heart-IR and stomach-IR contrasts of all 42 scan sessions. Fixed factors were treatment (placebo, citalopram) and test session (1st or 2nd). Hypothesis 2 was tested by a second-level model designed to test for anxiety-dependent effects and how the relationship between anxiety and interoceptive response (heart-IR and stomach-IR) changed on citalopram. This was like the first model but added centred STAI-S score (which can vary between sessions within subjects) and anxiety-by-treatment interaction (STAI-S scores multiplied by +1 for citalopram and −1 for placebo) regressors. A drug-induced change in the relationship between anxiety and relative interoceptive response was tested by separate one-sample t-tests over the anxiety-by-treatment interaction for heart-IRs and stomach-IRs across all 42 sessions. To confirm that a significant anxiety-by-treatment interaction effect (observed for heart-IR) changed an existing baseline unmedicated relationship between anxiety and heart-IR (without citalopram), replicating prior studies, a third whole-brain search was conducted across all participants in the placebo condition, using regressors of the intercept, test session, and mean-centred STAI-S scores. A one-sample t-test was completed across the latter.
Unless otherwise specified, all reported findings at the second level were whole-brain searches, cluster-corrected using a conservative voxel-wise threshold of Z > 3.1 and a cluster significance level of p < 0.05. However, we applied small volume correction where a whole-brain search failed in the main heart-IR comparison. Since we had strong predictions that effects would be observed in or around the amygdala [50] and insular cortex [17], we generated masks for these regions with the Oxford-Harvard cortical and subcortical probabilistic atlases (using any probability of including the designated region). Only two masks were tested: bilateral amygdalae and bilateral insular cortices (including nearby planum temporale, Heschl’s Gyrus, and operculum).
Order effects were tested independently to establish no confounding effects of the session or session × treatment interaction.
Post-hoc, the six significant clusters from the treatment effects of the first second-level model (Hypothesis 1) were tested for association with increases and decreases of anxiety between sessions after controlling for anxiety-associated side effects and treatment order. Criteria for reporting was either a significance level beyond a Bonferroni corrected threshold (p < 0.0083) or a similar effect in the same region on the other side of the brain, which would be unlikely to occur by chance.
Supplemental tests examined the robustness of effects against side effects of the treatments, treatment order, changes in cerebral blood flow, and guess of treatment condition. A separate supplemental regression was also conducted between treatment effects on cardiac interoceptive awareness reported elsewhere [16] and treatment effects on heart-IR. Correlations between clusters were explored. A separate regression between trait anxiety and citalopram’s effect on heart-RIR was also conducted. For all of the above, please see Supplemental Material.
Data associated with this project can be freely downloaded at https://doi.org/10.6084/m9.figshare.22786274
Results
Heart rate and subjective experience
Heart rate was lower on citalopram (M = 63.1 bpm, SD = 8.44) than on placebo (M = 66.53 bpm, SD = 10.6), consistent with the effective absorption of citalopram (t(20) = −2.45, p = 0.02) [77]. There was no change in a specific recorded subjective experience. However, participants rated the likelihood of having received citalopram higher (t(20) = 2.42, p = 0.03) on a scale of 1–100 when they had received citalopram (M = 56.9, SD = 22) compared to placebo (M = 32.0, SD = 22), indicating a potential summative influence of subjective effects on conscious awareness of the drug condition. See Supplemental Material for a thorough investigation into the robustness of results relating to this effect. See Table S1 for all measures of heart rate and subjective experience.
Cerebral blood flow
Analysis of ASL image pairs (Supplemental Material) showed no significant clusters at the familywise error rate, suggesting that citalopram did not affect cerebral blood flow. Therefore, any effects on BOLD responses were unlikely to be mediated by general effects on blood flow in the brain.
Interoceptive Attention: fMRI
Relative to placebo, citalopram reduced the relative interoceptive response to stomach sensation (stomach-IR) within the amygdalae, bilateral posterior insular cortex, and neighbouring regions (Table 1, Fig. 2) using a whole brain search. These are referred to henceforth as (left and right) amygdalar and posterior insular clusters, respectively. During heart focus, treatment differences did not reach the same threshold as the stomach focus when correcting for multiple comparisons across the whole brain. Using small volume correction, citalopram was shown to reduce the heart-IR within the right and left amygdalae (Table 1, Fig. 3). However, there was no evidence for a similar reduction in the insular cortices. A separate analysis demonstrated no order effects within suprathreshold clusters of BOLD activity affected by citalopram.
A Reduced activity (blue) of the stomach-IR with citalopram (vs. placebo). Maps were developed with a voxel threshold of Z > 3.1 and a cluster significance threshold of p < 0.05. Maps are overlayed onto the standard MNI 152 brain (x, y, z coord: top 43,−15, 12; bottom −22, 2, −18). R and L indicate right and left. B Plots of the average contrast parameter estimates (PE, arbitrary units) for each significant cluster in A. Green = citalopram. Orange = placebo.
A Reduced amygdala activity (blue) of the heart-IR with citalopram (vs. placebo). Maps were developed with a voxel threshold of Z > 3.1, a cluster significance threshold of p < 0.05, and small volume correction within an anatomical mask (light green) of amygdalae from the Harvard-Oxford Subcortical Structural Atlas. Maps are overlayed onto the standard MNI 152 brain (x = 16, z = −18). B Plots of the average contrast parameter estimates for heart-IR (PE, arbitrary units) in each treatment condition for each significant cluster in A. Green = citalopram. Orange = placebo.
Anxiety
State anxiety decreased for some participants and increased for others following an acute dose of citalopram. This resulted in no average anxiety change (p > 0.72) (Table S1).
In a whole-brain search, we discovered an effect of citalopram that increased with state anxiety within symmetrical bilateral clusters that spanned anterior insular and posterior orbitofrontal cortices (Table 1, Fig. 4A). This was the effect of reduced association between anxiety and heart-IR in these clusters on citalopram. A visual appraisal of the data indicated that the heart-IR within these regions increased with state anxiety when participants were on a placebo, but this relationship was reversed on citalopram (Fig. 4B). This was confirmed in a follow-up independent whole brain cluster-corrected analysis of the placebo condition. State anxiety increased with heart-IR in the right anterior insular/orbitofrontal cortex (316 voxels, Peak Z = 5.13, MNI coord: 44, 30, −12). On the left, this effect was also present but did not survive cluster correction (58 voxels, p < 0.001 uncorrected, peak MNI coord −44, 20, 8). No such effect was present on citalopram (Fig. 4A, Table S2). Further associations between state anxiety and heart-IR were observed on a placebo, but only the anterior insular/orbitofrontal clusters demonstrated a reduced association on citalopram. A similar anxiety-dependent effect was not observed for stomach-IR.
A Positive association between heart-IR and state anxiety on placebo (red/green) and the significant reduction of this relationship on citalopram (blue). Red and blue maps result from whole-brain searches using a voxel threshold of Z > 3.1 and a cluster significance threshold of p < 0.05. Green maps result from a whole brain search, uncorrected at p < 0.001. Maps are overlayed onto the standard MNI 152 brain (z = −10). B Plots of state anxiety (STAI-S) associations with average heart-IR parameter estimates (PE, arbitrary units) within the frontal orbital cortex / insular clusters of the citalopram < placebo contrast of A. Refer to Table 1 for statistical inference.
The state anxiety measure captured anxiety during the scan, which medication side effects could influence. The state anxiety-by-treatment interaction was robust to covariates of nausea, dizziness, headache, guess of drug condition, and the interactions of these measures with the treatment condition (see Supplemental Material). However, to further rule out the influence of side effects, we tested and found that trait anxiety (STAI-T), measured before any treatment and therefore unrelated to side effects, also predicted the effect of citalopram on heart-IR within the same bilateral orbitofrontal/insular cortex clusters (Table S3, Figure S2). This supported the conclusion that this treatment effect on heart-IR relates to levels of anxiety rather than drug-induced correlates.
Changes in state anxiety were associated with changes in nausea (r = 0.68, p < 0.001), headache (r = 0.54, p = 0.014), and dizziness (r = 0.45, p = 0.047) after controlling for treatment order. In the post-hoc examination of the main effects of heart-IR and stomach-IR clusters, the variance in state anxiety change after controlling for these associations was associated with treatment differences within the average extracted parameter estimates of the left amygdala cluster (b = 2.2, t(15) = 3.1, p = 0.008, r = 0.62) and right amygdala cluster (b = 0.5, t(15) = 2.2, p = 0.044, r = 0.49) of the stomach-IR (Fig. 5). A Bonferroni corrected alpha level for this post hoc analysis (6 tests) is p = 0.0083. The right amygdala effect is reported here due to the same effect on the left.
Metacognitive Interoceptive Insight
In a previous study involving contributions from the same participants, a single citalopram dose increased conscious awareness of the reliability of inferences made from heartbeat interoception [16]. We explored whether this effect correlated with the SSRI-induced reduction of heart-IR, which it did within the left (but not right) amygdala (see Supplemental Material, Figure S1).
Robustness tests
For a thorough examination of the robustness of effects to treatment side effects, treatment order, and guess of treatment condition, see Supplemental Material.
Discussion
A single dose of the SSRI citalopram reduced the relative neural response to attended normal internal sensation in viscerosensory (e.g. posterior insular cortex) and integrative/emotion-processing regions (e.g. amygdala) of interoceptive processing pathways [3, citalopram in proportion to anxiety levels, with a significant flattening of the anxiety-interoception relationship observed on placebo. Critically, this effect represents a reduction in the link between anxiety and interoception, not an anxiety reduction. Reduced links between anxiety and behavioural measures of interoception have been previously associated with increased anhedonia [42]. One might, therefore, wish to explore whether SSRI-induced changes in the neural anxiety-interoception relationship in the anterior insula relate to emotional blunting associated with SSRI treatment in some individuals [40]. Moreover, anxious states have been proposed to relate to accumulating, reciprocal relationships between levels of anxiety, heightened interoceptive responses, and further anxiety about those heightened interoceptive responses [27, 39]. So, our finding also sets the stage for investigations of whether serotonergic perturbation of the anxiety-interoception relationship could have a role in long-term SSRI treatment outcomes. More generally, however, this result confirms that some modulatory effects of serotonin on ordinary interoceptive processing are state-dependent, as previously suggested for serotonin influence on exteroceptive processes [9, 56,57,58].
As expected from prior research, anxiety responses increased for some participants and decreased for others, resulting in no net change [25, 39, 50]. We have provided preliminary insight into the individual differences in anxiety response, suggesting that the amygdala’s response to stomach sensation relates to increases and decreases in anxiety following an SSRI dose. The bilaterality of the effect provides confidence in this result. If replicated in a larger sample, this effect would underscore the importance of considering links between anxiety and gastrointestinal sensation in psychiatric and internal medicine research [29, 85, 86].
Like similar experimental medicine studies, we employed a single-dose SSRI protocol to understand the cognitive impact of acutely increased endogenous serotonin [39, 50]. Acute effects of SSRIs can differ from effects after seven or more days due to desensitisation of autoreceptors and other adaptive effects [67, 87]. Additional research would therefore be needed to extrapolate to the longer-term effects of SSRI treatment. Secondly, this was a study of young, healthy volunteers. This provides controlled inferences about normal function unencumbered by interactions with symptoms or other medications and avoids ethical challenges of within-subject pharmacological study in a patient group. However, only with further research should one extrapolate these findings to clinical contexts and experiences across the lifespan. There is also much more to learn about the precise mechanisms of these effects. Citalopram has exceptional selectivity for the serotonin transporter. However, the role of knock-on effects on other neurotransmitter systems and the roles of specific serotonin receptors remains unknown.
Overall, we found that an acute increase in extracellular serotonin reduces central neural responses to interoceptive information in healthy individuals in both general and anxiety-dependent fashion. This opens new avenues of research in other populations and contexts for a better understanding of interoception, its regulation, and its relationship to affective states.
References
Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–66.
Tsakiris M, Preester HD (eds.). The Interoceptive Mind: From Homeostasis to Awareness. Oxford University Press: Oxford, New York, 2018.
Craig A. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70.
Barrett LF. The theory of constructed emotion: an active inference account of interoception and categorization. Soc Cogn Affect Neurosci. 2017;12:1–23.
Hsueh B, Chen R, Jo Y, Tang D, Raffiee M, Kim YS, et al. Cardiogenic control of affective behavioural state. Nature. 2023;615:292–9.
Garfinkel SN, Critchley HD. Threat and the body: how the heart supports fear processing. Trends Cogn Sci. 2016;20:34–46.
Khalsa SS, Adolphs R, Cameron OG, Critchley HD, Davenport PW, Feinstein JS, et al. Interoception and mental health: a roadmap. Biol Psychiatry: Cogn Neurosci Neuroimaging. 2018;3:501–13.
Jacob SN, Nienborg H. Monoaminergic neuromodulation of sensory processing. Front Neural Circuits. 2018;12:51 https://www.frontiersin.org/articles/10.3389/fncir.2018.00051
Sizemore TR, Hurley LM, Dacks AM. Serotonergic modulation across sensory modalities. J Neurophysiol. 2020;123:2406–25.
Berman SM, Chang L, Suyenobu B, Derbyshire SW, Stains J, FitzGerald L, et al. Condition-specific deactivation of brain regions by 5-HT3 receptor antagonist Alosetron. Gastroenterology. 2002;123:969–77.
Labus JS, Mayer EA, Jarcho J, Kilpatrick LA, Kilkens TOC, Evers EAT et al. Acute tryptophan depletion alters the effective connectivity of emotional arousal circuitry during visceral stimuli in healthy women. Gut 2011; 60: https://doi.org/10.1136/gut.2010.213447.
Kilkens TOC, Honig A, Fekkes D, Brummer R-JM. The effects of an acute serotonergic challenge on brain–gut responses in irritable bowel syndrome patients and controls. Alimentary Pharmacol Therapeutics. 2005;22:865–74.
Kilkens TOC, Honig A, van Nieuwenhoven MA, Riedel WJ, Brummer R-JM. Acute tryptophan depletion affects brain-gut responses in irritable bowel syndrome patients and controls. Gut. 2004;53:1794–1800.
Broers C, Geeraerts A, Boecxstaens V, Van Houtte B, Geysen H, Peersman N, et al. The role of serotonin in the control of esophageal sensitivity assessed by multimodal stimulation in health. Neurogastroenterol Motil. 2021;33:e14057.
Broekaert D, Fischler B, Sifrim D, Janssens J, Tack J. Influence of citalopram, a selective serotonin reuptake inhibitor, on oesophageal hypersensitivity: a double-blind, placebo-controlled study. Alimentary Pharmacol Therapeutics. 2006;23:365–70.
Livermore JJA, Holmes CL, Moga G, Adamatzky K, Critchley HD, Garfinkel SN, et al. A single oral dose of citalopram increases interoceptive insight in healthy volunteers. Psychopharmacology. 2022. https://doi.org/10.1007/s00213-022-06115-7
Avery JA, Drevets WC, Moseman SE, Bodurka J, Barcalow JC, Simmons WK. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol Psychiatry. 2014;76:258–66.
Burrows K, DeVille DC, Cosgrove KT, Kuplicki RT, Bodurka J, Guinjoan S, et al. Impact of serotonergic medication on interoception in major depressive disorder. Biol Psychol. 2022;169:108286.
Żmudzka E, Sałaciak K, Sapa J, Pytka K. Serotonin receptors in depression and anxiety: Insights from animal studies. Life Sci. 2018;210:106–24.
Nash JR, Sargent PA, Rabiner EA, Hood SD, Argyropoulos SV, Potokar JP, et al. Serotonin 5-HT1A receptor binding in people with panic disorder: positron emission tomography study. Br J Psychiatry. 2008;193:229–34.
Zangrossi H, Del Ben CM, Graeff FG, Guimarães FS. Serotonin in panic and anxiety disorders. In: Müller CP, Cunningham KA (eds). Handbook of Behavioral Neuroscience. Elsevier, 2020, 611–33.
National Institute for Health and Care Excellence. Generalised anxiety disorder and panic disorder in adults: management. 2011. https://www.nice.org.uk/guidance/cg113.
Jakubovski E, Johnson JA, Nasir M, Müller-Vahl K, Bloch MH. Systematic review and meta-analysis: dose–response curve of SSRIs and SNRIs in anxiety disorders. Depression Anxiety. 2019;36:198–212.
Gollan JK, Fava M, Kurian B, Wisniewski SR, Rush AJ, Daly E et al. What are the clinical implications of new onset or worsening anxiety during the first two weeks of SSRI treatment for depression? Depress Anxiety 2012; 29: https://doi.org/10.1002/da.20917.
Sinclair LI, Christmas DM, Hood SD, Potokar JP, Robertson A, Isaac A, et al. Antidepressant-induced jitteriness/anxiety syndrome: systematic review. Br J Psychiatry. 2009;194:483–90.
Paulus MP, Feinstein JS, Khalsa SS. An active inference approach to interoceptive psychopathology. Annu Rev Clin Psychol. 2019;15:97–122.
Domschke K, Stevens S, Pfleiderer B, Gerlach AL. Interoceptive sensitivity in anxiety and anxiety disorders: An overview and integration of neurobiological findings. Clin Psychol Rev. 2010;30:1–11.
Khoury NM, Lutz J, Schuman-Olivier Z. Interoception in psychiatric disorders: a review of randomized, controlled trials with interoception-based interventions. Harv Rev Psychiatry. 2018;26:250–63.
Mayer EA, Ryu HJ, Bhatt RR. The neurobiology of irritable bowel syndrome. Mol Psychiatry. 2023;28:1451–65.
Grillon C, Levenson J, Pine DS. A single dose of the selective serotonin reuptake inhibitor citalopram exacerbates anxiety in humans: a fear-potentiated startle study. Neuropsychopharmacology. 2007;32:225–31.
Schulz A, van Dyck Z, Lutz APC, Rost S, Vögele C. Gastric modulation of startle eye blink. Biol Psychol. 2017;127:25–33.
Schulz A, Matthey JH, Vögele C, Schaan V, Schächinger H, Adler J, et al. Cardiac modulation of startle is altered in depersonalization-/derealization disorder: Evidence for impaired brainstem representation of baro-afferent neural traffic. Psychiatry Res. 2016;240:4–10.
Harmer CJ, Reid CB, Ray MK, Goodwin GM, Cowen PJ. 5HT3 antagonism abolishes the emotion potentiated startle effect in humans. Psychopharmacology. 2006;186:18–24.
Garfinkel SN, Minati L, Gray MA, Seth AK, Dolan RJ, Critchley HD. Fear from the heart: sensitivity to fear stimuli depends on individual heartbeats. J Neurosci. 2014;34:6573–82.
Burghardt NS, Sullivan GM, McEwen BS, Gorman JM, LeDoux JE. The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol Psychiatry. 2004;55:1171–8.
Terasawa Y, Moriguchi Y, Tochizawa S, Umeda S. Interoceptive sensitivity predicts sensitivity to the emotions of others. Cogn Emot. 2014;28:1435–48.
Hübner AM, Trempler I, Gietmann C, Schubotz RI. Interoceptive sensibility predicts the ability to infer others’ emotional states. PLoS ONE. 2021;16:e0258089.
Jung W-M, Lee Y-S, Lee I-S, Wallraven C, Ryu Y, Chae Y. Enhanced bodily states of fear facilitates bias perception of fearful faces. Mol Brain. 2020;13:157.
Godlewska BR, Harmer CJ. Cognitive neuropsychological theory of antidepressant action: a modern-day approach to depression and its treatment. Psychopharmacology. 2021;238:1265–78.
Masdrakis VG, Markianos M, Baldwin DS. Apathy associated with antidepressant drugs: a systematic review. Acta Neuropsychiatr. 2023;35:189–204. https://doi.org/10.1017/neu.2023.6.
Furman DJ, Waugh CE, Bhattacharjee K, Thompson RJ, Gotlib IH. Interoceptive awareness, positive affect, and decision making in major depressive disorder. J Affect Disord. 2013;151:780–5.
Dunn BD, Stefanovitch I, Evans D, Oliver C, Hawkins A, Dalgleish T. Can you feel the beat? Interoceptive awareness is an interactive function of anxiety- and depression-specific symptom dimensions. Behav Res Ther. 2010;48:1133–8.
Akimova E, Lanzenberger R, Kasper S. The Serotonin-1A receptor in anxiety disorders. Biol Psychiatry. 2009;66:627–35.
Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189.
Tan Y, Wei D, Zhang M, Yang J, Jelinčić V, Qiu J. The role of mid-insula in the relationship between cardiac interoceptive attention and anxiety: evidence from an fMRI study. Sci Rep. 2018;8:17280.
Stern ER. Neural circuitry of interoception: new insights into anxiety and obsessive-compulsive disorders. Curr Treat Options Psych. 2014;1:235–47.
Javanmard M, Shlik J, Kennedy SH, Vaccarino FJ, Houle S, Bradwejn J. Neuroanatomic correlates of CCK-4-induced panic attacks in healthy humans: a comparison of two time points. Biol Psychiatry. 1999;45:872–82.
Cameron OG, Huang GC, Nichols T, Koeppe RA, Minoshima S, Rose D, et al. Reduced γ-Aminobutyric Acida–benzodiazepine binding sites in insular cortex of individuals with panic disorder. Arch Gen Psychiatry. 2007;64:793–800.
Critchley HD, Harrison NA. Visceral influences on brain and behavior. Neuron. 2013;77:624–38.
Ma Y. Neuropsychological mechanism underlying antidepressant effect: a systematic meta-analysis. Mol Psychiatry. 2015;20:311–9.
Lovick TA. The medullary raphe nuclei: a system for integration and gain control in autonomic and somatomotor responsiveness? Exp Physiol. 1997;82:31–41.
Bhave VM, Nectow AR. The dorsal raphe nucleus in the control of energy balance. Trends Neurosci. 2021;44:946–60.
Hale MW, Shekhar A, Lowry CA. Stress-related serotonergic systems: implications for symptomatology of anxiety and affective disorders. Cell Mol Neurobiol. 2012;32:695–708.
Stahl SM, Muntner N. Stahl’s essential psychopharmacology: neuroscientific basis and practical application. Fourth edition. Cambridge University Press: Cambridge; 2013.
Nord CL, Lawson RP, Dalgleish T. Disrupted dorsal mid-insula activation during interoception across psychiatric disorders. AJP. 2021;178:761–70.
Hurley LM, Hall IC. Context-dependent modulation of auditory processing by serotonin. Hear Res. 2011;279:74–84.
Hurley L, Devilbiss D, Waterhouse B. A matter of focus: monoaminergic modulation of stimulus coding in mammalian sensory networks. Curr Opin Neurobiol. 2004;14:488–95.
Spoont MR. Modulatory role of serotonin in neural information processing: implications for human psychopathology. Psychological Bull. 1992;112:330–50.
Garfinkel SN, Gould van Praag CD, Engels M, Watson D, Silva M, Evans SL, et al. Interoceptive cardiac signals selectively enhance fear memories. J Exp Psychol: Gen. 2021;150:1165–76.
Godlewska BR, Norbury R, Selvaraj S, Cowen PJ, Harmer CJ. Short-term SSRI treatment normalises amygdala hyperactivity in depressed patients. Psychological Med. 2012;42:2609–17.
Bhagwagar Z, Cowen PJ, Goodwin GM, Harmer CJ. Normalization of enhanced fear recognition by acute SSRI treatment in subjects with a previous history of depression. AJP. 2004;161:166–8.
Kerr KL, Moseman SE, Avery JA, Bodurka J, Zucker NL, Simmons WK. Altered insula activity during visceral interoception in weight-restored patients with anorexia nervosa. Neuropsychopharmacol. 2016;41:521–8.
Avery JA, Burrows K, Kerr KL, Bodurka J, Khalsa SS, Paulus MP, et al. How the brain wants what the body needs: the neural basis of positive alliesthesia. Neuropsychopharmacol. 2017;42:822–30.
DeVille DC, Kuplicki R, Stewart JL, Tulsa 1000 Investigators, Paulus MP, Khalsa SS. Diminished responses to bodily threat and blunted interoception in suicide attempters. eLife. 2020;9:e51593.
Simmons WK, Avery JA, Barcalow JC, Bodurka J, Drevets WC, Bellgowan P. Kee** the body in mind: Insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Hum Brain Mapp. 2013;34:2944–58.
Haruki Y, Ogawa K. Cardiac and gastric interoceptive awareness have distinct neural substrates. eNeuro. 2023;10:ENEURO.0157–22.2023.
Harmer CJ, Cowen PJ. ‘It’s the way that you look at it’—a cognitive neuropsychological account of SSRI action in depression. Philos Trans R Soc B: Biol Sci. 2013;368:20120407.
Langley C, Armand S, Luo Q, Savulich G, Segerberg T, Søndergaard A, et al. Chronic escitalopram in healthy volunteers has specific effects on reinforcement sensitivity: a double-blind, placebo-controlled semi-randomised study. Neuropsychopharmacol. 2023;48:664–70.
McCabe C, Mishor Z, Cowen PJ, Harmer CJ. Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biol Psychiatry. 2010;67:439–45.
Murphy SE, Norbury R, O’Sullivan U, Cowen PJ, Harmer CJ. Effect of a single dose of citalopram on amygdala response to emotional faces. Br J Psychiatry. 2009;194:535–40.
Owens MJ, Knight DL, Nemeroff CB. Second-generation SSRIs: human monoamine transporter binding profile of escitalopram and R-fluoxetine. Biol Psychiatry. 2001;50:345–50.
Spielberger CD Manual for the State-Trait Anxiety Inventory (STAI). Consulting Psychologists Press: PaloAlto, CA, 1983.
Porges S. Body perception questionnaire. Laboratory of Developmental Assessment, University of Maryland, 1993.
Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62:782–90.
Beckmann C, Smith S. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–52.
Griffanti L, Douaud G, Bijsterbosch J, Evangelisti S, Alfaro-Almagro F, Glasser MF, et al. Hand classification of fMRI ICA noise components. NeuroImage. 2017;154:188–205.
Rasmussen SL, Overø KF, Tanghøj P. Cardiac safety of citalopram: prospective trials and retrospective analyses. J Clin Psychopharmacol. 1999;19:407–15.
Kleckner IR, Zhang J, Touroutoglou A, Chanes L, **a C, Simmons WK, et al. Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nat Hum Behav. 2017;1:0069.
Evrard HC. The organization of the primate insular cortex. Front Neuroanat. 2019;13:43 https://www.frontiersin.org/articles/10.3389/fnana.2019.00043
Wang G-J, Tomasi D, Backus W, Wang R, Telang F, Geliebter A, et al. Gastric distention activates satiety circuitry in the human brain. NeuroImage. 2008;39:1824–31.
Fermin ASR, Friston K, Yamawaki S. An insula hierarchical network architecture for active interoceptive inference. R Soc Open Sci. 2022;9:220226.
Ghaziri J, Tucholka A, Girard G, Boucher O, Houde J-C, Descoteaux M, et al. Subcortical structural connectivity of insular subregions. Sci Rep. 2018;8:8596.
Maniscalco JW, Rinaman L. Vagal interoceptive modulation of motivated behavior. Physiology. 2018;33:151–67.
Gray MA, Harrison NA, Wiens S, Critchley HD. Modulation of emotional appraisal by false physiological feedback during fMRI. PLoS ONE. 2007;2:e546.
Joo M-K, Kim D-H. Vagus nerve-dependent effects of fluoxetine on anxiety- and depression-like behaviors in mice. Eur J Pharmacol. 2023;953:175862.
McVey Neufeld K-A, Bienenstock J, Bharwani A, Champagne-Jorgensen K, Mao Y, West C, et al. Oral selective serotonin reuptake inhibitors activate vagus nerve dependent gut-brain signalling. Sci Rep. 2019;9:14290.
Adell A. Revisiting the role of raphe and serotonin in neuropsychiatric disorders. J Gen Physiol. 2015;145:257–9.
Acknowledgements
We would like to thank Dr. Jason Avery for his advice on the VIA task at the early stages of this project. We also thank Dr. Andrew Barritt and Dr. Iulia Bogdan for providing medical cover. The University of Sussex sponsored and funded this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: DCM, JJAL. Design: DCM, JJAL, HC, SG. Investigation: JJAL, KA. Data preparation: DCM, JJAL, LS. Statistical Analysis: DCM (primary), JJAL (perfusion). Project administration: JJAL, DCM. Writing—original draft: DCM. Writing—review & editing: DCM, JJAL, LS, HC, SG, LS.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
All work was completed at the University of Sussex.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Livermore, J.J.A., Skora, L.I., Adamatzky, K. et al. General and anxiety-linked influences of acute serotonin reuptake inhibition on neural responses associated with attended visceral sensation. Transl Psychiatry 14, 241 (2024). https://doi.org/10.1038/s41398-024-02971-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-024-02971-3
- Springer Nature Limited