Abstract
Immunotherapy represented by anti-PD-(L)1 and anti-CTLA-4 inhibitors has revolutionized cancer treatment, but challenges related to resistance and toxicity still remain. Due to the advancement of immuno-oncology, an increasing number of novel immunoregulatory targets and mechanisms are being revealed, with relevant therapies promising to improve clinical immunotherapy in the foreseeable future. Therefore, comprehending the larger picture is important. In this review, we analyze and summarize the current landscape of preclinical and translational mechanistic research, drug development, and clinical trials that brought about next-generation pharmacological immunoregulatory anti-cancer agents and drug candidates beyond classical immune checkpoint inhibitors. Along with further clarification of cancer immunobiology and advances in antibody engineering, agents targeting additional inhibitory immune checkpoints, including LAG-3, TIM-3, TIGIT, CD47, and B7 family members are becoming an important part of cancer immunotherapy research and discovery, as are structurally and functionally optimized novel anti-PD-(L)1 and anti-CTLA-4 agents and agonists of co-stimulatory molecules of T cells. Exemplified by bispecific T cell engagers, newly emerging bi-specific and multi-specific antibodies targeting immunoregulatory molecules can provide considerable clinical benefits. Next-generation agents also include immune epigenetic drugs and cytokine-based therapeutics. Cell therapies, cancer vaccines, and oncolytic viruses are not covered in this review. This comprehensive review might aid in further development and the fastest possible clinical adoption of effective immuno-oncology modalities for the benefit of patients.
Similar content being viewed by others
Introduction
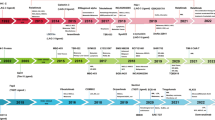
Immunotherapies attempt to harness the innate and adaptive immune system to attack cancer cells.1 Since early systematic clinical applications of immunotherapy in oncology, such as the use of Coley’s bacterial toxin for sarcoma more than 100 years ago and Bacillus Calmette-Guérin vaccine for bladder cancer in the 1970s,2 there has been an exponential evolution accelerated by the epochal FDA approvals of the first immune checkpoint inhibitors (ICIs), the antibody ipilimumab against anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) in 2011 and the first antibodies against anti-programmed cell death protein 1 (PD-1) pembrolizumab and nivolumab in 20143 (Fig. 1).
Timeline showing representative events of drug approval, clinical trials, and key biological discovery for immunoregulatory targets of anti-cancer therapeutics. Events involving immunoregulatory receptors and bispecific antibodies are in blue and dark gray boxes and those related to immuno-epigenetics and cytokines are in light gray and pink boxes, respectively. Boxes with solid lines indicate approvals, while other events are in dashed line boxes. Lines with rounded corners indicate the period of clinical trials. The approvals include the final approval, the accelerated approval by FDA, and the approval by EMA. Cancer stage descriptions such as advanced or metastatic, pathological subtypes, and details of combination therapies are omitted. ICI immune checkpoint inhibitors, mAb monoclonal antibody, BiTE bispecific T cell engager, ALL acute lymphoid leukemia, NSCLC non-small cell lung cancer, MSI-H microsatellite instability-high, dMMR deficient mismatch repair, TNBC triple-negative breast cancer, SCLC small cell lung cancer, bsAb bispecific antibody, HCC hepatocellular carcinoma, TCR T-cell receptor, TCE T cell engager, rhIL-2 recombinant human interleukin-2
Despite the remarkable success achieved by ICIs and ICI-based treatment combinations in some tumor entities,4,5,6,7,8,9,10,290,291 (Fig. 2). Therefore, the efficiency of agonists is affected by unique factors. First of all, agonists with very high affinity or at excessive dose can lose their agonistic function,292 suggesting a bell-shaped affinity-agonism and dose-response relationship and an optimal affinity and dose. Secondly, agonistic antibodies can bind both natural ligand binding sites and exclusive epitopes.290,293,294 For example, different domains of CD40 are associated with agonistic or antagonistic effects of anti-CD40 antibodies.295 Characterizing the antibody binding epitope is therefore very important for agonist development.
Moreover, the interaction between the antibody Fc domain and FcγRs can induce both agonist and ADCC/CDC effects. Except for the inhibitory FcγRIIB, other FcγRs are activating and FcγRI has the highest affinity for the Fc region. Binding FcγRIIB is proposed to promote target receptor crosslinking and to maintain immune synapses, thus providing true agonism290,291 (Fig. 2). Instead, binding activating FcγRs can elicit ADCC, which can be utilized to deplete Tregs, especially using the IgG1 isotype with the strongest binding to activating FcγRs290,291 (Fig. 2). Therefore, agonists can either activate anti-cancer immune cells or deplete immunosuppressive populations. However, issues might arise from indiscriminate ADCC triggered by activating FcγRs, depleting Tregs but also effector cells. Binding activating FcγRs also contributes to toxic side effects, e.g., in case of 4-1BB agonists.296,297 For these reasons, Fc engineering is crucial and has been shown to be highly useful for the development of pure agonists by removing the Fc segment,297,298 mutation methods abating Fc-FcγR interactions299 or selectively enhancing Fc-FcγRIIB binding.300
In particular, human IgG2 agonists can activate co-stimulatory molecules including CD40, 4-1BB, and CD28 independent of FcγRs.290,301 Later studies showed that agonists with rigid conformation constrained by “tight” hinge region promote clustering of co-stimulatory molecules301,302,303 and tend not to bind excess epitopes mediating antagonism as is the case for more flexible antibodies,301,304 thus providing sufficient agonism even without FcγRs,295 and this phenomenon exists on natural IgG2 isotype mAb.301,302
IgSF co-stimulatory receptor: ICOS
ICOS (CD278) is the receptor of ICOSL (B7-H2, CD275, B7h). Upon initial activation of TCR and CD28 signaling, ICOS is upregulated on T cells and this can non-redundantly enhance T cell immunity288,290,291 while ICOS is constitutively expressed on Tregs.291 ICOSL is constitutively expressed on APCs.288 After activation, ICOS induces phosphoinositide 3-kinase (PI3K)-Akt signaling,305 mammalian target of rapamycin (mTOR),306 and nuclear factor of activated T cells (NFAT)-responsive genes290 in T cells.
Anti-ICOS agonistic antibodies currently under development include vopratelimab and alomfilimab (Table 1 and Supplementary Table 3). The widely reported IgG4 pure agonist feladilimab has been removed from the GlaxoSmithKline pipeline due to its unsatisfactory clinical activity in phase II studies. The IgG1 mAbs vopratelimab and alomfilimab are designed to deplete intratumoral Tregs. Although vopratelimab plus nivolumab only elicited a total ORR of 2.3%, patients with ICOShigh CD4+ effector T cells had longer PFS and OS than patients without these cells (6.2 vs. 1.9 and 20.7 vs. 9.0, months).306 This finding guided the patient selection for the phase II SELECT study in NSCLC, where the combination of vopratelimab at 0.03 mg/kg with pimivalimab (a PD-1 inhibitor) yielded an ORR of 40% and a 6-month PFS rate of 80%. However, the study did not reach the primary endpoint of tumor shrinkage between vopratelimab plus pimivalimab and pimivalimab monotherapy groups.307 Alomfilimab depleted ICOShigh Tregs, had monotherapy anti-tumor efficacy, and improved anti-PD-L1 efficacy in a pre-clinical study.308 According to a preliminary report there were 5 OR cases out of 103 patients in a phase I/II trial testing alomfilimab ± atezolizumab.309 In summary, ICOS drug development is still challenging.
TNFRSF co-stimulatory receptor: CD40, OX40, and 4-1BB
Upon ligand trimer ligation, TNFRs on T cells trimerize to recruit TNFR-associated factor (TRAF)1-6 in different preferences and activate distinct downstream adapters but predominantly converge at nuclear factor-κB (NF-κB) signaling.289,290 According to the chronological impact on T cell activation as discussed above, we first discuss CD40, then focus on OX40 and 4-1BB that aroused most incentives of industries. Unlike OX40 and 4-1BB, the development of GITR agonists has been largely terminated due to limited responses.310,311,312,313,314,315 Similarly, agents targeting the CD27-CD70 pathway, such as the widely reported CD27 agonist varlilumab and CD70 agonist cusatuzumab, have also been removed from the pipelines of Celldex and Argenx, respectively, due to unfavorable developmental prospects. Likewise, the development of TNFR1/2 agonists remains immature, with almost all agents still under preclinical investigation.316,317 Therefore, other TNFRSF receptors, including GITR, CD27/CD70, and TNFR1/2, are not the focus of our discussion.
CD40
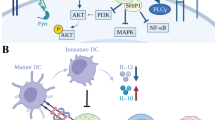
CD40 (TNFRSF5) expressed mainly by APCs plays an important role in initial activation of CD4+ T cells following the CD28 signal. CD40L (CD154) mainly expressed by CD4+ T cells ligates and activates CD40, triggering the maturation of DCs which is crucial for the efficient priming of T cells including CD4+ Th cells and cross-primed CD8+ T cells288,318 (Fig. 2). Activated CD40 stimulates expression of CD80 and CD86 on DCs thus stimulating the CD28 coreceptor on T cells which in turn leads to upregulation of CD40L on T cells coordinately driving T cell stimulation and DC maturation (Fig. 2).
The agents presently developed all entered phase II clinical trials (Table 1 and Supplementary Table 3), while only the development of selicrelumab has been discontinued. SEA-CD40, mitazalimab, sotigalimab, and giloralimab are IgG1 FcγR-dependent DC activators, whereas YH003 and CDX-1140 are IgG2 pure agonists. In the phase Ib/II PRINCE study of sotigalimab plus chemotherapy ± nivolumab in pancreatic adenocarcinoma, the total ORR was 58% in the phase Ib part,319 while in phase II part, the confirmed ORR of sotigalimab plus chemotherapy was 33%.319 Mitazalimab efficiently upregulated CD80/CD86 expression and IL-12 secretion by DCs, induced antigen-specific T cell proliferation and anti-tumor activity preclinically.320,321 Efficacy evaluation is ongoing in the phase II OPTIMIZE-1 study combining mitazalimab and chemotherapy in pancreatic ductal adenocarcinoma (PDAC) patients. More studies will be needed on combinations with other agents or regarding optimizing indication selection.
OX40
OX40 (CD134, TNFRSF4) is temporarily expressed by memory T cells and activated T cells following TCR/CD3 signaling and has important roles in their survival, yet it does not participate in T cell priming.288,291 It is also constitutively expressed by Tregs.288,291 Interestingly, OX40 agonism does not impair the immunosuppressive functions of Tregs but only confers them an inflammatory phenotype.322 Expression of OX40L (CD252) is upregulated on APCs after their activation and can be promoted by activated CD40.288 After binding of OX40L,323 trimerized OX40 recruits TRAF2-3 and TRAF5 to transmit canonical and non-canonical NF-κB and other signals288,289 (Fig. 2).
Several major companies have withdrawn from the development of OX40 agonists due to unfavorable clinical efficacies,324,325,326 indicating the necessity of strategy improvement for further development. OX40 agonists under development currently include revdofilimab, HFB301001, and BGB-A445 (Table 1 and Supplementary Table 3). The IgG1 agonist INCAGN1949 is proven to FcγR-dependently stimulate OX40 and deplete OX40high Tregs.327 However, in a phase I/II study, INCAGN1949 monotherapy only elicited an ORR of 1.15%,328 hence it has been removed from the pipeline of Agenus. Trials of other agonists are all still ongoing. The development of many OX40 agonists has been discontinued. Due to the transient expression of OX40, the timing of OX40 agonist administration may be important.291 Further development of OX40 agonists may need either combining with other agents in an appropriate order or develo** msAbs.
4-1BB
4-1BB (CD137, TNFRSF9) is also transiently upregulated following TCR/CD3-mediated signaling mainly on activated T cells289 but is also detected on NK cells and APCs.291 Upon ligation of 4-1BBL (TNFSF9), 4-1BB recruits TRAF1-2 to activate downstream signaling similar to OX40289 (Fig. 2). Considering the substantial liver toxicity at doses of ≥1 mg/kg290,293,294 and modest ORR of 3.8%329 observed in trials of the first generation 4-1BB agonistic antibodies urelumab and utomilumab respectively, Bristol-Myers Squibb and Pfizer deprioritized the development of these two drugs. However, subsequent analyses have guided further design of 4-1BB agonists. As many reports indicated, utomilumab showed insufficient clinical monotherapy activity while urelumab induces strong agonism but also severe toxicity in a fraction of the patients.291 Structural analysis indicated that utomilumab blocks natural ligands and binds 4-1BB at proximal domains while urelumab binds the distal one,330,331 which is consistent with antibodies against CD40.295 This reflects the importance of the binding epitopes in the design of agonists. The toxicity of urelumab mostly stems from Fc-FcγR interaction, thus Fc engineering is relevant for toxicity management of 4-1BB agonists. Based on such considerations, next-generation 4-1BB agonists including ADG106,332 LVGN6051,333 AGEN2373,334 and ATOR1017 have been developed and are being investigated in clinical trials (Table 1 and Supplementary Table 3). In a phase I trial of ADG106, treatment appeared to be safe with a DCR of 57%.332 LVGN6051 monotherapy elicited a DCR of 70% and induced preliminary ORR of 25% combined with pembrolizumab in a phase I study.335 AGEN2373 induced a DCR of 26.3% without liver toxicity.336 Dose escalation for ATOR-1017 is still ongoing with the best response of SD observed.337
In summary, agonists targeting costimulatory receptors appear powerful candidates for future immunotherapy and a wave of new agonistic molecules has been developed many of which have entered clinical trials. However, agonist development is more difficult than the development of antagonists because more parameters have to be taken into account. Clinical trials have shown that agonist monotherapies scarcely induce favorable responses hence combination with ICIs or other agents may become particularly important. Next-generation constructs including Fc-engineered mAbs, multi-valent mAbs, and bsAbs/msAbs seem promising.
Immunoregulatory bispecific and multi-specific antibodies
The concept of bsAb targeting two different molecules was proposed in the last century.338 At that time, shortly after gaining insights into immunoglobulin biology, Alfred Nisonoff envisioned combining two distinct antigen-binding sites within a single molecule. He connected rabbit Fab fragments with different specificities using chemical methods and demonstrated bispecificity of the resulting product.339 Subsequently, other researchers advanced the field of bsAbs by introducing hybridoma methods for mAbs, phage display techniques, and strategies to direct antibody effects towards various target cells.340,341,342 However, bsAbs/msAbs with promising efficacy and acceptable safety had not been developed until the last decade, when the CD3×CD19 bispecific T-cell engager (BiTE) blinatumomab was approved by the FDA.342 Along with the advances in antibody format design, and further comprehension of cancer immunology, anti-cancer bsAbs/msAbs targeting immunoregulatory and other cancer-related molecules are under intensive development. Here we present an update of the developmental landscape of these agents (Fig. 3a–d, Table 2 and Supplementary Table 4) compared with previous summaries342,343,344,345,346 according to data from the pipeline and clinical trials. We briefly introduce the characteristics of anti-cancer immunoregulatory bsAbs/msAbs, mainly discussing their categories according to mechanism-of-action, and clinical vista of widely reported agents.
Various formats and categories of bsAbs. a Examples of common bsAb formats. Short bars indicate the antibody Fab segment, long bars indicate the Fc segment, and for ImmTAC, the Fab segment is linked to an antigen-specific TCR. In the same antibody icon, different color combinations of the Fab segment indicate the binding to different target proteins. BsAb examples of these formats are in the gray box. b According to our statistics of bsAbs that have entered clinical trials, the TCEs comprising the anti-CD3 scFv account for about half of all bsAbs under development at present. The development of other bsAb categories presents a diversified landscape. The two ends of each arc indicate two targets of bsAbs. Only bsAbs are counted for this figure, with msAbs with higher valency excluded. The data of this figure are consistent with Table 2 and Supplementary Table 4. The statistic is up to October 2022 and bsAbs with terminated development are excluded. c Prism of developmental strategies of bsAbs. The strategy of bsAb development is mainly to combine four types of targets: immune cell targets, tumor cell targets, co-stimulatory molecules/immunostimulatory cytokines, and immune checkpoints or other immunosuppressive molecules. By these designs, immune cells and immunomodulatory signals can be introduced into the TAA-expressing environment. The black lines on the edge of the prism indicate that the corresponding bsAb category targets the target types directed by the arrows: (1) TCEs and NKEs; group I general bsAbs: (2) co-stimulatory molecule × TAA or TME protein and (3) co-stimulatory molecule × co-stimulatory molecule; group II general bsAbs: (4) inhibitory checkpoint × TAA and (5) inhibitory checkpoint × inhibitory checkpoint; group III general bsAbs: (6) inhibitory checkpoint × co-stimulatory molecule. d Mechanism-of-action of bispecific cell engagers and group I-III general immunoregulatory anti-cancer bsAbs. DART dual-affinity retargeting, scFv single-chain variable fragment, TAA tumor-associated antigen, HLA human leukocyte antigen, HSA human serum albumin, TriTAC Tri-specific T cell activating construct, CAF cancer-associated fibroblast, FAP fibroblast activation protein
BsAbs/msAbs have both similar and distinct mechanisms of action compared with mAbs. Fc-FcγR interactions are thought to be mainly responsible for the toxicity of early bsAbs, as in the case of the bispecific trifunctional antibody catumaxomab (anti-EpCAM×anti-×anti-CD3).342 Thus, now T cell engagers (TCEs) are mainly constructed without Fc segment or with a functionally silenced Fc segment. Complete removal of the Fc segment as in the cases of BiTEs and DARTs has not been the main trend and the development of many BiTEs and DARTs has been discontinued due to insufficient efficacy and safety issues. With the Fc segment silenced by mutation, the leading format of TCE development at present is the 1 + 1 asymmetric IgG-like form (Fig. 3a). The affinity toward different targets of a single bsAb can be fine-tuned by adjusting the two single-chain variable fragment (scFv) arms independently, thus ameliorating safety or pharmacokinetic/pharmacodynamic (PK/PD) properties. Moreover, in terms of PK/PD characteristics, the optimal dose for bsAbs is one that results in maximum target-bsAb-target trimer formation.347
Some bsAbs/msAbs can elicit biological effects that cannot be induced by the corresponding mAb mixture, therefore they are called obligate bsAbs/msAbs.342 For immunoregulatory anti-cancer bsAbs/msAbs, this has been demonstrated by redirecting CD3+ T cells, or immune cells expressing checkpoint receptors or co-stimulatory molecules to TAA-expressing cells or the TME. Moreover, bsAbs/msAbs binding different immunoregulatory targets can, at the same cellular spatial location, target multiple immunoreceptors or simultaneously enhance the co-stimulatory signal and inhibit immune checkpoints, hence potentially causing stronger anti-cancer immunity compared with the mAb mixture. These bsAbs/msAbs can be divided into cell engagers involving CD3, CD16a, or TAA-specific TCRs and general immunoregulatory anti-cancer bsAbs/msAbs combining all other immunoregulatory molecules or TAAs (Fig. 3c, d and Table 2).
In summary, bsAbs/msAbs have several potential advantages, including (1) superior specificity, safety, and therapeutic efficacy compared with the corresponding mixture of mAbs, (2) the ability to bridge and recruit immune cells, and (3) dual or multiple signal regulation. Nevertheless, disadvantages of bsAbs/msAbs still exist including chain mispairing in production, risk of inducing cytokine release syndrome (CRS), and the potential for inducing anti-drug antibodies (ADAs). In bsAb/msAb production, diverse combinations of light and heavy chains could lead to the dilution of the target bsAb, posing challenges in its isolation and resulting in low yield.348,349 Innovative development platforms, such as CrossMab350,351 and orthogonal Fab interface,352 have emerged to mitigate the impact of this issue. CRS is a common and distinctive adverse effect in the clinical application of bsAbs,353,354,355 mainly associated with TCEs containing the anti-CD3 arm. It is a systemic inflammatory response with symptoms ranging from fever, fatigue, and headache to multiorgan failure, triggered by T cell activation, with myeloid cells and TNF-α being the main mediators of the systemic cytokine release.356,357 To advance the further application of TCEs, the management of their using and the handling of adverse events should be improved, for example, with stepwise dosing, properly using tocilizumab, corticosteroids, or TNF-α blockade, and supportive353,354,355,358 care. Regarding the induction of ADAs, increased engineering and artificial design may result in greater differences between bsAbs and endogenous immunoglobulins, and bsAbs could therefore potentially contain new epitopes that elevate antigenicity and subsequently increase the likelihood of ADA development. Therefore, early monitoring of immunogenicity is crucial for increasing clinical success rates in bsAb development.359,360
Bispecific T cell engagers
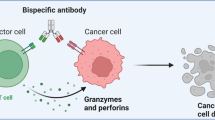
TCEs are representative obligate bsAbs combining anti-CD3 and anti-TAA scFvs to redirect any T cell to TAA-expressing tumor cells. TCEs make up nearly half of the immunoregulatory anti-cancer bsAbs/msAbs currently in clinical trials (Fig. 3b). Of note, the formats of TCEs comprise BiTE, dual-affinity re-targeting (DART), IgG-like full-length format, and others342,343 (Fig. 3a). Another type of TCE utilizing a TAA-specific TCR instead of an anti-TAA scFv is called ImmTAC. The development of TCEs surged after the approval of blinatumomab, which, as explained above, is an Fc-free BiTE. Blinatumomab yielded a CR rate of 43% in a phase II trial in Ph- relapsed or refractory (r/r) B-precursor acute lymphoblastic leukemia (ALL) patients361; it was thus approved by FDA in 2014. After blinatumomab, the CD3×CD20 IgG-like TCE mosunetuzumab was conditionally approved in the European Union,362 and also received accelerated approval by FDA in 2022 because it induced a CR rate of 60% for r/r follicular lymphoma (FL) in phase I and II trials.363,364 Likewise, teclistamab monotherapy was conditionally approved in the European Union365 and approved by FDA366 in 2022 for r/r multiple myeloma (MM) due to an ORR of 63.0%, a CR rate of 39.4% and mPFS of 11.3 months in the phase I/II MajesTEC-1 trial.367,368 Because of the reported mOS of 21.7 months in HLA-A*02:01+ uveal melanoma patients in a phase III trial,369 tebentafusp became the first approved ImmTAC in 2022.
The indications of TCEs depend on the TAA expression of the cancer type. For example, TCEs targeting CD20, CD19, and CD38 are all designed for hematological malignancies and are rivals of CAR-T cell therapies in hematology. The development of TCEs against solid tumors seems more challenging. Challenges include heterogeneity in TAA expression, on-target off-tumor toxicity for normal tissue, the immunosuppressive TME, disordered vasculature, and limited tumor penetration. These challenges might be overcome by further structure design exploration, antibody avidity fine-tuning, or therapy combinations.
At present, TCEs that have been approved or entered phase III clinical trials all target hematological TAAs. TCEs advanced into phase III trials before approval include epcoritamab (CD3×CD20), glofitamab (CD3×CD20), and elranatamab (CD3×B-cell maturation antigen) (Table 2 and Supplementary Table 4). For epcoritamab, the phase I/II EPCORE NHL-1 study showed an ORR of 68% and 90% for r/r B-cell non-Hodgkin lymphoma (B-NHL) and r/r FL patients with monotherapy,370 supporting the ongoing phase III EPCORE DLBCL-1 study. The majority of trials of glofitamab combine it with rituximab, obinutuzumab, or tocilizumab pretreatment to mitigate cytokine release.371 A phase I study combining glofitamab and obinutuzumab pretreatment in r/r B-NHL patients showed an ORR of 53.8% and a CR rate of 36.8%.372 For elranatamab, the phase I MagnetisMM-1 study has demonstrated an ORR of 75% at high doses,373 supporting two ongoing phase III trials.
Beyond conventional TCEs, other components are introduced in novel formats to refine immunostimulatory properties, PK/PD attributes, and toxicity (Fig. 3d and Table 2). By introducing a CD28 immunostimulatory arm, Sanofi designed Fc-silenced CD3 × CD38 × CD28 TCE with better stimulation of anti-tumoral T cells.374 Based on this design, SAR442257 has been developed and is being tested in a phase I trial (NCT04401020). Another category called Tri-specific T Cell-Activating Construct (TriTAC) introduced anti-human serum albumin scFv to improve PK/PD properties for solid tumors. Preclinical results showed superior T-cell killing compared with conventional BiTEs targeting EGFR or PSMA and favorable efficacy,375 supporting phase I/II trials (Table 2 and Supplementary Table 4). To improve safety, XTENylated protease-activated T cell engagers (XPATs) were created by introducing scFvs with TME-specific degradable masking, thus avoiding off-tumor T cell activation. Sanofi completed the acquisition of this technology in 2022, including the HER2 XPAT AMX-818. Moreover, as functions are being continuously discovered, innate immune cell populations are also evaluated for immuno-oncology agent development. BsAbs targeting CD16A/FcγRIIIa, an activating FcγR, to redirect NK cells to TAA-expressing cells are called NK cell engagers (NKEs) or innate cell engagers (ICEs) (Table 2 and Supplementary Table 4). Unfortunately, AFM13, a representative CD16A × CD30 NKE for r/r Hodgkin lymphoma, only induced an ORR below 25% in several trials as monotherapy.376,377 However, an ORR of 88% was induced by combining AFM13 and pembrolizumab,378 suggesting combination therapy for further development.
General immunoregulatory anti-cancer bsAbs/msAbs
Apart from CD3-engaging TCEs, many other anti-cancer bsAbs/msAbs target immunoregulatory proteins other than the CD3 complex. Based on the design, this category includes three subgroups: bsAbs/msAbs stimulating co-stimulatory molecules (group I), blocking immune checkpoints (group II), and the combination of these two tactics (group III) (Fig. 3d, Table 2 and Supplementary Table 4). These bsAbs/msAbs are currently mainly developed for the treatment of solid tumors.
Two designs are used for group I bsAbs/msAbs (Table 2). The first one is by binding co-stimulatory molecules on immune cells and TAA-expressing tumor cells or fibroblast activation protein on cancer-associated fibroblasts. The second one is to concurrently target distinct IgSF/TNFRSF co-stimulatory molecules on immune cells.
Group II bsAbs/msAbs include three subtypes (Table 2). The first one redirects PD-1/PD-L1 blockade toward TAAs or tyrosine kinase expression-enriched TME. The second one concurrently targets different immune checkpoint ligand-receptor axes. Due to thorough research on ICI combination therapies, the development of this subtype is the main trend for group II bsAbs/msAbs and is also most advanced in this category. The third one targets PD-1/PD-L1 and immunosuppressive molecules beyond IgSF checkpoints, such as CD47 and TGF-βRII. Group III includes designs mainly combining anti-PD-1/PD-L1 and co-stimulatory agonist arms, and fusion proteins combining ICI and immunostimulatory cytokines (immunocytokines) (Fig. 3d and Table 2). In a preclinical study, anti-PD1–IL-2v immunocytokine was proved to have superior ability to expand tumor-specific CD8+ effector-like T cells and therapeutic efficacy than the (agonistic) IL-2Rβγ-biased mutant IL-2 variant IL-2v in tandem with an anti-FAP scFv.379 These findings support the clinical development of RG6279, a bispecific anti-PD1–IL-2v fusion protein directing IL-2v to PD-1+ tumor-reactive T cells.
Encouraging preclinical results have been reported for various agents in these bsAb categories, including 4-1BB×HER2,380 4-1BB×CD40,381 4-1BB×PD-1/PD-L1,382,383 PD-1×GITRL,384 PD-L1×LAG-3,385 PD-1×CTLA-4,386 PD-L1×IL-15/IL-15RA387 bispecifics, and others. However, in general, except for several group II bsAbs, most others are still at early phases of development. BsAbs entered in phase III trials include cadonilimab, erfonrilimab, tebotelimab, retlirafusp alfa, and ivonescimab (Table 2 and Supplementary Table 4). Cadonilimab is an Fc-silenced symmetric IgG1 PD-1×CTLA-4 bsAb. Combined with chemotherapy, cadonilimab elicited an ORR of 65.9% in phase I/II trial for GC/GEJC.388 In the phase I/II trial for PD-L1 TPS ≥ 1% NSCLC, cadonilimab combined with anlotinib induced an ORR of 62.5%.389 Thus, cadonilimab combined with chemotherapy or targeted therapy elicited excellent ORRs (Table 2). Erfonrilimab is a symmetric full-length IgG1 PD-1×CTLA-4 bsAb. Combined with chemotherapy, erfonrilimab induced ORRs of 50.6%, 58.3%, and 55.6% in NSCLC,390 ESCC,391 and PDAC patients.392 A similar ORR of 57% was also observed combining erfonrilimab and lenvatinib in HCC patients.405 Notably, antagonizing m6A modifiers can sensitize tumors to PD-1 blockade in mice.406,407,408,409,410 However, most agents targeting m6A regulators are still in preclinical development and none has entered clinical evaluation.411 Thus, considering the volume and scope of this review, we mainly focus on DNA methylation by DNA methyltransferases (DNMTs), histone deacetylation by histone deacetylases (HDACs), recognition of acetylated histone by the mammalian bromodomain and extra-terminal (BET) proteins, and demethylation by histone methylase polycomb repressive complex 2 (PRC2) and lysine-specific histone demethylase 1 (LSD1) (Fig. 4).
Epigenetic targets and their impact on different immune cell types and tumor cells in the TME. Lines of the same color indicate the impacts on different cell types of the same epigenetic process. And the color of lines corresponds to the background color of the specific process those lines indicated. Epigenetic regulation mainly comprises transcriptional regulation via DNA methylation, histone modification, and post-transcriptional modification. Immune-related pharmacological development has mainly focused on DNA methylation by DNMTs, histone deacetylation by HDACs, recognition of acetylated histone by BET proteins, and histone demethylation by PRC2 and LSD1. DNA methylation, which is mainly mediated by DNMTs, represses gene transcription when located in a gene promoter and regulates anti-tumor immunity with the orchestration of different cell members. The aforementioned histone modifications are capable of remodeling chromatin structures and interactions with other regulating factors (e.g., recruitment of transcription factors) and affect gene transcription of various cell types in the TME. The post-transcriptional m6A methylation represents a new layer of epigenetic regulation that mainly affects the fate of RNAs via promoting or antagonizing their degradation or translation. Classification of drugs of each epigenetic target are indicated in the blue boxes. inh inhibitor, DNMT DNA methyltransferase, HDAC histone deacetylase, BET bromodomain and extraterminal domain, BRD bromodomain, BRDT bromodomain testis-specific protein, RNA pol RNA polymerase, PRC2 polycomb repressive complex 2, EZH enhancer of zeste homolog, EED embryonic ectoderm development, SUZ suppressor of zeste, LSD1 lysine-specific demethylase 1, m6A N6-methyladenosine, SASP senescence-associated secretory phenotype
DNA methyltransferases (DNMTs)
Targeting DNA methylation has become important for the treatment of certain hematological malignancies with the intention to reactivate tumor suppressors and promote differentiation of the malignant cells. Regarding anti-tumor immunity, therapeutic DNA demethylation can enhance tumor immunogenicity by inducing expression of endogeneous retroviral elements and of neoantigens normally silenced by DNA methylation. Expression of the former induces double-strand RNA which, in turn, can induce interferon-based innate immune activation essential for adaptive antitumor immunity, and is one reason why DNMT inhibitors can cause immunogenic cell death of malignant cells.412,413 Furthermore, therapeutic DNA demethylation can alter the composition and behavior of immune cells; it can increase the expression of MHC molecules, alleviate T cell exhaustion and enhance T cell effector and memory potential, increase secretion of Th1-type cytokines, and reduce immunosuppressive myeloid and Treg cells.412,413 DNA methylation status and demethylating agents can also directly affect the expression of multiple immune checkpoints, including PD-1,414,415 PD-L1,414,416,417, LAG3,414 TIM-3,414,418,419, CTLA-4,414,420 and TIGIT,421,422 by recruiting of proteins involved in gene repression or by inhibiting the binding of them.
Because of these interesting antitumor immune effects, combinations of hypomethylating agents (HMAs), currently mainly DNMT inhibitors, with immunotherapeutics are being investigated. Decitabine plus camrelizumab caused high response rates and long-term benefits in patients with Hodgkin’s lymphoma who failed PD-1 inhibitors.423,424 The combination of decitabine and pembrolizumab induced better response in patients with relapsed AML, with transcriptional signs of immune activation.425 Other combinations of HMAs and ICIs also show good safety and preliminary anti-tumor effects in patients with hematological malignancies in clinical trials426,427,428,429 (Supplementary Table 5). Regarding solid tumors, although the preclinical and some early clinical results using the combination of PD-1 blockade and HMAs are highly promising,430,431,432 most clinical data has been disappointing. No responses were observed after guadecitabine plus atezolizumab in metastatic urothelial carcinoma which had progressed on previous immune checkpoint blockade (ICB).433 The combination of guadecitabine or azacytidine and pembrolizumab or durvalumab produced only modest anti-tumor effects in a variety of solid tumors.434,435,436 The addition of azacytidine or CC-486 (oral azacytidine) to pembrolizumab437,438 or durvalumab439 was not more effective than standalone ICI treatment. Lack of robust tumor DNA demethylation and of viral mimicry was found to be associated with a missing clinical response in one study.439
Overall, the combination of HMAs and ICIs needs further studies, especially in solid tumors. Notably, investigations of how dosing and scheduling of these drug classes affect the immunomodulatory and anti-tumor effects in the clinical setting are expected. In mouse solid tumor models, low-dose HMAs plus ICIs outperform either HMAs or ICIs alone in restricting tumor growth and prolonging survival, with significant HMA-related immune modulation.430,431 Epigenetic priming using HMAs with sequential ICIs has the potential to produce durable clinical benefit associated with immune responses in patients with solid tumors.440,441 In addition, there is considerable interest in the development of compounds targeting a selective subtype of DNMTs, which may enhance the tolerability and efficacy.442,443,482 Considering the current evidence and the potential of BET proteins in cancer and immune-related diseases,483 the exploration of their impacts on anti-tumor immunity and the development of drugs targeting BETs worth more effort.
Histone methylase polycomb repressive complex 2 (PRC2)
PRC2, which is formed when zeste homolog 2 (EZH2) associates with embryonic ectoderm development (EED) protein and SUZ12, is responsible for histone methylation mainly at histone 3 lysine 27 (H3K27). It has a broad impact on cancer immunity484 (Fig. 4). It mediates long-term transcriptional silencing of the MHC-I antigen processing pathway485 and represses CXCL9 and CXCL10 production by tumors, two critical chemokines for effector T-cell trafficking.486,487 The orchestrated immune modulation also includes higher MDSC infiltration, less NK cell-mediated killing and more Treg-mediated immune suppression.484,488 EZH2 inhibition could enhance the efficacy and overcome resistance to current immunotherapies.488 Tazemetostat, an inhibitor of EZH2, the main catalytic unit, demonstrated clinical activity in epithelioid sarcoma in a phase II trial (ORR: 15%, duration of response: not reached).489 It was approved by FDA for locally advanced or metastatic epithelioid sarcoma in 2020. EZH1, a paralog of EZH2, can also form functional PRC2 complexes as a compensatory mechanism for tumor cells to escape EZH2 inhibition.490,491 Therefore, co-inhibition of EZH2 and EZH1492,493,494 or EED inhibition492,495 could more completely inhibit the activity of PRC2, especially in the presence of innate or acquired resistance mutations in EZH2 and by addressing the potential compensatory mechanism of EZH1-driven tumor growth. SETD2, an upstream regulator of EZH2, can also be targeted to combat EZH2-high tumors.496 Future preclinical and clinical investigations may identify novel drug targets and formats, and will provide more insight into the value of PRC2 inhibition in cancer immunotherapy.
Lysine-specific histone demethylase 1 (LSD1)
LSD1 inhibitors are widely applied in myeloid hematological malignancies as they promote the differentiation of myeloid cells via regulation of myeloid transcription factors GFI1 and PU.1.497,498 Regarding anti-tumor immunity, LSD1 undermines T cell-mediated cytotoxicity via promoting terminal differentiation of T cells499,500 (Fig. 4). Accordingly, LSD1 inhibition expands progenitor exhausted T cells with stem-like properties, thereby enhancing the efficacy of immunotherapy.499,500 LSD1 inhibition also increases antigen presentation mediated by MHC I complexes on cancer cells485,501 and decreases exosomal PD-L1.502 Although tranylcypromine-based flavin adenine dinucleotide (FAD) domain-binding irreversible inhibitors exert long-lasting inhibition on LSD1 and yield encouraging clinical results both in myeloid malignancies503 and solid tumors,504 they induce significant TRAEs due to their covalent binding to FAD domains contained in critical enzymes other than LSD1 and the ensuing off-target reactivity.505 This could be ameliorated using reversible LSD1 inhibitors. For example, minimal inhibition of the cytochrome P450 enzymes containing a FAD domain was reported using the reversible LSD1 inhibitor TACH101.506,507 Another two clinical stage reversible LSD1 inhibitors, seclidemstat508 and CC-90011,509 also show immune activation and efficacy in combination with ICIs. Selectively targeting nuclear LSD1 phosphorylated at serine 111 (nLSD1p) might also be a plausible therapeutic approach to tackle the safety issue.510 Similar to most other anti-cancer agents targeting immuno-epigenetic modifiers, LSD1 inhibitors are combined with PD-1/PD-L1 blockade in phase I/II trials with promising preliminary results.511
Targeting epigenetics faces problems related to broad specificity and pleiotropic activity. Discovered immunomodulatory effects of some existing epigenetic modulators might contrast their previously known antitumor functions. For example, although HDAC activity generally seems to impair anti-tumor immunity, the intrinsic HDAC activity of Tcf1 and Lef1 is crucial for maintaining CD8+ T cell identity.555,648,649 leads to an arduous efficacy-toxicity balance (Fig. 5a). Tissue-/cell-specific therapeutics and/or conditionally activated agents might help to overcome these problems. Both cytokine biology research and protein engineering and novel delivery platforms for cytokines have greatly advanced in recent years. They will hopefully help to design better drug structures and to expand the realm of targetable cytokines, continuously promoting the development of cytokine-based therapeutics.
It is worth noting that from a clinical perspective, differences exist in clinical practices for treatment of different cancer types. In fact, due to the varying immune backgrounds and intrinsic differences between cancer types, immunoregulatory anti-cancer therapies targeting different targets indeed have different optimal indications. Melanoma is well known for its robust immune responsiveness, which made it predestined for initial evaluation of therapeutic potential of LAG-3, TIM-3, CD40, and other immunoregulatory targets. Pembrolizumab induces CR in melanoma patients, and over 90% maintain CR for 5 years,650 highlighting potent efficacy of ICB. Relatlimab and tebentafusp were also approved for melanoma as their first indication. Activating the immune system against melanoma through cytokine-based therapies such as aldesleukin, darleukin, tavokinogene telseplasmid, has also proven to be effective.529,651,652
For other solid tumors, additional checkpoints like LAG-3 and TIGIT are likely to play significant roles. In the case of NSCLC, the relatively favorable immune environment in most NSCLC cases653 suggests that targeting these additional checkpoints could potentially be advantageous. LAG-3 agents have demonstrated efficacy in solid tumor entities such as NSCLC and HNSCC,52,53,54 and TIGIT agents are currently in several clinical trials in combination with PD-1/PD-L1 agents in NSCLC (Table 1). Exploration of B7 is ongoing across various solid tumors.654 The ADC enfortumab vedotin targeting nectin-4 has shown promising results particularly in combination with pembrolizumab in bladder cancer (NCT04223856). The situation is different for SCLC. While atezolizumab combined with carboplatin/etoposide is approved as first-line treatment for extensive-stage SCLC, many SCLC subtypes still respond weakly.655,656 These non-immunogenic tumor subtypes may rely on TAAs, such as DLL3 to be targeted, for example, with CD3×DLL3 TCE tarlatamab and HPN328. Neuroendocrine features of SCLC can also be managed with LSD1 inhibitors such as ladademstat to suppress neuroendocrine transcription factors.656,657,658
In gastrointestinal tumors, excellent efficacy for GC/GEJC is primarily observed with regimens based on anti-PD-1 agents and bsAbs containing anti-PD-1 scFv, such as cadonilimab388 and tebotelimab.659 For PDAC, CAFs are the main component of its TME, forming a strong physical barrier with the ECM that hampers T cell infiltration.660 CD40 agonists like sotigalimab can enhance T cell infiltration and show efficacy in combination with chemotherapy and nivolumab.319 Inhibiting TGF-β with NIS793 in combination with anti-PD-1 agents may help remodel the CAF-rich TME of PDAC.578,579 HCC is immune-privileged, with abundant MDSCs and an abnormal vascular system.661 Non-inflammatory HCC subtypes predominate,661 requiring anti-PD-1-based immunotherapy combined with anti-angiogenic therapy or dual immunotherapy to enhance immune response. Currently, atezolizumab plus bevacizumab is the first-line treatment for advanced HCC, with nivolumab plus ipilimumab and durvalumab plus tremelimumab also demonstrating efficacy.662,663 New bsAbs such as erfonrilimab and cadonilimab plus lenvatinib have achieved very high ORR.671,672 with no immunotherapy yet proven to improve prognosis for gliomas.672 Adenovirus-encoded IL-12 INXN 2001 (Table 3 and Supplementary Table 6) may help ameliorate the suppressive microenvironment. CD155 serves as both the ligand for the inhibitory receptor TIGIT and the poliovirus receptor. The polio-rhinovirus chimera lerapolturev (Table 1 and Supplementary Table 1) offers some hope for treatment of gliomas.673 Redirecting anti-tumor immunity relying on TAAs is also an important strategy for immunotherapy of pediatric and nervous system tumors, with the mAbs or TCEs targeting B7-H3 and GD2 showing promise.674,675
For gynecologic tumors, each of the main cancer types presents distinct characteristics. Immunotherapy for endometrial cancer is mainly limited to the MSI-H/dMMR subgroup, represented by PD-1 agents such as pembrolizumab and dostarlimab,676 with the potential of other immunoregulatory therapies yet to be explored. Ovarian cancer exhibits a highly immunosuppressive TME and relatively low immunogenicity,677 resulting in poor response to immunotherapy. Combination therapies blocking multiple checkpoints, bsAb like ubamatamab or non-α IL-2 variant nemvaleukin alfa modulating the TME, can possibly enhance immune responses.677,678,679 Cervical cancer shows better responses to immunotherapy,680 with promising outcomes observed with pembrolizumab plus chemotherapy (recently approved by FDA), anti-PD-1 plus anti-CTLA-4 agents, and bsAbs.680,681,682
Beyond the drug therapies discussed in detail, other therapies, such as cancer gene therapy and cancer vaccines, are also promising anti-cancer treatments with immunoregulatory effects. While they may not be totally classified as conventional drug therapies, their rapid development and effectiveness are noteworthy. Cancer gene therapy can alter genes in vivo or ex vivo. Ex vivo gene therapy, represented by CAR T cell therapy, has achieved great clinical successes.683,684,685 Additional genetic modifications hold promise to further improve cell therapy, as manifested by the good safety and feasibility of CRISPR-edited TCR T cells and CAR T cells in patients with solid tumors.686,687 In-vivo gene therapy introduces the target gene directly into patients using a vector. Stimulating intra-tumoral cytokine gene expression (elaborated in the section on cytokines) and co-stimulatory molecules as well as inhibiting immunosuppressive molecules/cell types with anti-sense oligonucleotides (ASOs) and small interfering RNAs (siRNAs) attract high research interest. The combined local delivery of OX40L, CD80, and CD86 mRNAs cause significant local and systemic immune activation and facilitate tumor regression at both local and abscopal sites.688
Cancer vaccines amplify the signal of tumor-specific antigens (TSAs) or TAAs via encapsulated antigen-encoding DNA and RNA, peptides, or antigen-loaded APCs.689,690,691 They actively stimulate patients’ own anti-tumor immune response at the very beginning of the cancer immunity cycle. With promising potential,692,693,694,695,696 current research focuses on identifying antigens with the best quality and optimizing the delivery platform.689,690,691 High-throughput sequencing and bioinformatics tools in recent years have greatly facilitated the screening of highly immunogenic neoantigens.692,697 Improved vectors698,699 and immune adjuvants700,701,702 have been reported to enhance the efficacy of vaccine delivery and the ensuing immune activation and tumor-killing effects in preclinical studies. Combining cancer vaccines with ICIs and TME-reprogramming may help tackle the problem of immunosuppressive TME observed in classical immunotherapies, and is now being extensively tested in clinical trials with some producing promising results and advancing into phase III evaluations (NCT05141721, NCT06077760), but the optimal combination, dosage, and sequence of combination therapy still require further exploration. In the broader landscape of immunoregulatory cancer therapeutics, it is essential to recognize the contributions of these diverse approaches.
In summary, we have reviewed highly promising avenues for the development of immunoregulatory anti-cancer therapeutics by analyzing a large volume of recent published research, also including conference reports, and clinical trials. We summarized recent advances in the understanding of the mechanisms of action of classes of immunotherapy drug targets and the progress of the corresponding drug development. Despite considerable success so far, further research is necessary to boost drug development to improve treatment responses and prolong cancer patient survival. Moreover, next-generation drug development in these immunotherapy fields will continue to rely on clarification of immunological target biology and progress in drug developmental platforms, whereas the final evaluation of drug efficacy depends on rigorous high-quality clinical trials. This needs effective cooperation of academia, pharmaceutical and biotech industry, and the clinical medical community. More and more promising pharmacological immunoregulatory anti-cancer therapeutics are likely to be developed in innovative forms to the benefit of patients. This will further expand and enrich the landscape of immunoregulatory anti-cancer therapies.
References
Topalian, S. L., Weiner, G. J. & Pardoll, D. M. Cancer immunotherapy comes of age. J. Clin. Oncol. 29, 4828–4836 (2011).
Rosenberg, S. A. Future prospects for immunotherapy. Cancer 36, 821–824 (1975).
Oiseth, S. J. & Aziz, M. S. Cancer immunotherapy: a brief review of the history, possibilities, and challenges ahead. J. Cancer Metastasis Treat. 3, 250 (2017).
Piulats, J. M. et al. Nivolumab plus ipilimumab for treatment-naïve metastatic uveal melanoma: an open-label, multicenter, phase II trial by the Spanish Multidisciplinary Melanoma Group (GEM-1402). J. Clin. Oncol. 39, 586–598 (2021).
Reck, M. et al. First-line nivolumab plus ipilimumab versus chemotherapy in advanced NSCLC With 1% or greater tumor PD-L1 expression: patient-reported outcomes from CheckMate 227 Part 1. J. Thorac. Oncol. 16, 665–676 (2021).
Hammers, H. J. et al. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: the CheckMate 016 Study. J. Clin. Oncol. 35, 3851–3858 (2017).
Larkin, J. et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 381, 1535–1546 (2019).
Cortes, J. et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 396, 1817–1828 (2020).
Janjigian, Y. Y. et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 398, 27–40 (2021).
Sun, J.-M. et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 398, 759–771 (2021).
**e, C. et al. Immune checkpoint blockade in combination with stereotactic body radiotherapy in patients with metastatic pancreatic ductal adenocarcinoma. Clin. Cancer Res. 26, 2318–2326 (2020).
Theelen, W. S. M. E. et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir. Med. 9, 467–475 (2021).
Gettinger, S. et al. Nivolumab plus erlotinib in patients with EGFR-mutant advanced NSCLC. J. Thorac. Oncol. 13, 1363–1372 (2018).
Gutzmer, R. et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 395, 1835–1844 (2020).
Motzer, R. et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N. Engl. J. Med. 384, 1289–1300 (2021).
Sugawara, S. et al. Nivolumab with carboplatin, paclitaxel, and bevacizumab for first-line treatment of advanced nonsquamous non-small-cell lung cancer. Ann. Oncol. 32, 1137–1147 (2021).
Powles, T. et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 21, 1563–1573 (2020).
Kelly, C. M. et al. Objective response rate among patients with locally advanced or metastatic sarcoma treated with talimogene laherparepvec in combination with pembrolizumab. JAMA Oncol. 6, 402 (2020).
Chesney, J. et al. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J. Clin. Oncol. 36, 1658–1667 (2018).
Rozeman, E. A. et al. Survival and biomarker analyses from the OpACIN-neo and OpACIN neoadjuvant immunotherapy trials in stage III melanoma. Nat. Med. 27, 256–263 (2021).
Miles, D. et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann. Oncol. 32, 994–1004 (2021).
Powles, T. et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 22, 931–945 (2021).
Pujade-Lauraine, E. et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): an open-label, three-arm, randomised, phase 3 study. Lancet Oncol. 22, 1034–1046 (2021).
Oxnard, G. R. et al. TATTON: a multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann. Oncol. 31, 507–516 (2020).
Yang, J. C.-H. et al. Osimertinib plus durvalumab versus osimertinib monotherapy in EGFR T790M–positive NSCLC following previous EGFR TKI therapy: CAURAL brief report. J. Thorac. Oncol. 14, 933–939 (2019).
Morad, G., Helmink, B. A., Sharma, P. & Wargo, J. A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 184, 5309–5337 (2021).
Hegde, P. S. & Chen, D. S. Top 10 challenges in cancer immunotherapy. Immunity 52, 17–35 (2020).
Voabil, P. et al. An ex vivo tumor fragment platform to dissect response to PD-1 blockade in cancer. Nat. Med. 27, 1250–1261 (2021).
Triebel, F. et al. LAG-3, a novel lymphocyte activation gene closely related to CD4. J. Exp. Med. 171, 1393–1405 (1990).
Okazaki, T. et al. PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J. Exp. Med. 208, 395–407 (2011).
Andrews, L. P., Marciscano, A. E., Drake, C. G. & Vignali, D. A. A. LAG3 (CD223) as a cancer immunotherapy target. Immunol. Rev. 276, 80–96 (2017).
Wherry, E. J. et al. Molecular signature of CD8 + T cell exhaustion during chronic viral infection. Immunity 27, 670–684 (2007).
Gandhi, M. K. et al. Expression of LAG-3 by tumor-infiltrating lymphocytes is coincident with the suppression of latent membrane antigen–specific CD8 + T-cell function in Hodgkin lymphoma patients. Blood 108, 2280–2289 (2006).
Huard, B. et al. Characterization of the major histocompatibility complex class II binding site on LAG-3 protein. Proc. Natl Acad. Sci. USA 94, 5744–5749 (1997).
Maruhashi, T. et al. LAG-3 inhibits the activation of CD4 + T cells that recognize stable pMHCII through its conformation-dependent recognition of pMHCII. Nat. Immunol. 19, 1415–1426 (2018).
Workman, C. J. & Vignali, D. A. A. The CD4-related molecule, LAG-3 (CD223), regulates the expansion of activated T cells. Eur. J. Immunol. 33, 970–979 (2003).
Huard, B., Tournier, M., Hercend, T., Triebel, F. & Faure, F. Lymphocyte-activation gene 3/major histocompatibility complex class II interaction modulates the antigenic response of CD4 + T lymphocytes. Eur. J. Immunol. 24, 3216–3221 (1994).
Workman, C. J. & Vignali, D. A. A. Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223). J. Immunol. 174, 688–695 (2005).
Grosso, J. F. et al. LAG-3 regulates CD8 + T cell accumulation and effector function in murine self- and tumor-tolerance systems. J. Clin. Invest. 117, 3383–3392 (2007).
Workman, C. J. et al. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J. Immunol. 172, 5450–5455 (2004).
Matsuzaki, J. et al. Tumor-infiltrating NY-ESO-1–specific CD8 + T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc. Natl Acad. Sci. USA 107, 7875–7880 (2010).
Woo, S.-R. et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 72, 917–927 (2012).
Kouo, T. et al. Galectin-3 shapes antitumor immune responses by suppressing CD8 T Cells via LAG-3 and inhibiting expansion of plasmacytoid dendritic cells. Cancer Immunol. Res. 3, 412–423 (2015).
Ming, Q. et al. LAG3 ectodomain structure reveals functional interfaces for ligand and antibody recognition. Nat. Immunol. 23, 1031–1041 (2022).
Maruhashi, T. et al. Binding of LAG-3 to stable peptide-MHC class II limits T cell function and suppresses autoimmunity and anti-cancer immunity. Immunity 55, 912–924.e8 (2022).
Guy, C. et al. LAG3 associates with TCR–CD3 complexes and suppresses signaling by driving co-receptor–Lck dissociation. Nat. Immunol. 23, 757–767 (2022).
Andreae, S., Buisson, S. & Triebel, F. MHC class II signal transduction in human dendritic cells induced by a natural ligand, the LAG-3 protein (CD223). Blood 102, 2130–2137 (2003).
Casati, C. et al. Soluble human LAG-3 molecule amplifies the in vitro generation of type 1 tumor-specific immunity. Cancer Res. 66, 4450–4460 (2006).
Shen, R. et al. LAG-3 expression on peripheral blood cells identifies patients with poorer outcomes after immune checkpoint blockade. Sci. Transl. Med. 5107, 1–13 (2021).
He, Y. et al. LAG-3 protein expression in non–small cell lung cancer and its relationship with PD-1/PD-L1 and tumor-infiltrating lymphocytes. J. Thorac. Oncol. 12, 814–823 (2017).
Datar, I. et al. Expression analysis and significance of PD-1, LAG-3, and TIM-3 in human non–small cell lung cancer using spatially resolved and multiparametric single-cell analysis. Clin. Cancer Res. 25, 4663–4673 (2019).
Tawbi, H. A. et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N. Engl. J. Med. 386, 24–34 (2022).
Atkinson, V. et al. Eftilagimod alpha, a soluble lymphocyte activation gene-3 (LAG-3) protein plus pembrolizumab in patients with metastatic melanoma. J. Immunother. Cancer 8, e001681 (2020).
Brana, I. et al. Results from a phase II study of eftilagimod alpha (soluble LAG-3 protein) and pembrolizumab in patients with PD-L1 unselected metastatic second-line squamous head and neck carcinoma. J. Clin. Oncol. 39, 6028–6028 (2021).
Wolf, Y., Anderson, A. C. & Kuchroo, V. K. TIM3 comes of age as an inhibitory receptor. Nat. Rev. Immunol. 20, 173–185 (2020).
Sánchez-Fueyo, A. et al. Tim-3 inhibits T helper type 1–mediated auto- and alloimmune responses and promotes immunological tolerance. Nat. Immunol. 4, 1093–1101 (2003).
Monney, L. et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 415, 536–541 (2002).
Hastings, W. D. et al. TIM-3 is expressed on activated human CD4 + T cells and regulates Th1 and Th17 cytokines. Eur. J. Immunol. 39, 2492–2501 (2009).
Sakuishi, K. et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 207, 2187–2194 (2010).
Zhu, C. et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 6, 1245–1252 (2005).
Huang, Y. H. et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature 517, 386–390 (2015).
Rangachari, M. et al. Bat3 promotes T cell responses and autoimmunity by repressing Tim-3-mediated cell death and exhaustion. Nat. Med. 18, 1394–1400 (2012).
Zhu, C. et al. Tim-3 adaptor protein Bat3 is a molecular checkpoint of T cell terminal differentiation and exhaustion. Sci. Adv. 7, eabd2710 (2021).
Kataoka, S. et al. The costimulatory activity of Tim-3 requires Akt and MAPK signaling and its recruitment to the immune synapse. Sci. Signal. 14, eaba0717 (2021).
Avery, L., Filderman, J., Szymczak-Workman, A. L. & Kane, L. P. Tim-3 co-stimulation promotes short-lived effector T cells, restricts memory precursors, and is dispensable for T cell exhaustion. Proc. Natl Acad. Sci. USA 115, 2455–2460 (2018).
Gorman, J. V. et al. Tim-3 directly enhances CD8 T cell responses to acute listeria monocytogenes infection. J. Immunol. 192, 3133–3142 (2014).
Sims, G. P., Rowe, D. C., Rietdijk, S. T., Herbst, R. & Coyle, A. J. HMGB1 and RAGE in inflammation and cancer. Annu. Rev. Immunol. 28, 367–388 (2010).
Chiba, S. et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat. Immunol. 13, 832–842 (2012).
de Mingo Pulido, Á. et al. The inhibitory receptor TIM-3 limits activation of the cGAS-STING pathway in intra-tumoral dendritic cells by suppressing extracellular DNA uptake. Immunity 54, 1154–1167.e7 (2021).
Nakayama, M. et al. Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood 113, 3821–3830 (2009).
Dixon, K. O. et al. TIM-3 restrains anti-tumour immunity by regulating inflammasome activation. Nature 595, 101–106 (2021).
Ghiringhelli, F. et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1Β-dependent adaptive immunity against tumors. Nat. Med. 15, 1170–1178 (2009).
Liu, Z. et al. Novel effector phenotype of TIM-3+ Regulatory T cells leads to enhanced suppressive function in head and neck cancer patients. Clin. Cancer Res. 24, 4529–4538 (2018).
Granier, C. et al. Tim-3 expression on tumor-infiltrating PD-1 + CD8 + T cells correlates with poor clinical outcome in renal cell carcinoma. Cancer Res 77, 1075–1082 (2017).
Fucikova, J. et al. TIM-3 dictates functional orientation of the immune infiltrate in ovarian cancer. Clin. Cancer Res. 25, 4820–4831 (2019).
Severson, J. J. et al. PD-1+Tim-3 + CD8 + T lymphocytes display varied degrees of functional exhaustion in patients with regionally metastatic differentiated thyroid cancer. Cancer Immunol. Res. 3, 620–630 (2015).
Fourcade, J. et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8 + T cell dysfunction in melanoma patients. J. Exp. Med. 207, 2175–2186 (2010).
Fourcade, J. et al. PD-1 and Tim-3 regulate the expansion of tumor antigen-specific CD8 + T cells induced by melanoma vaccines. Cancer Res 74, 1045–1055 (2014).
Li, H. et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology 56, 1342–1351 (2012).
Kurtulus, S. et al. Checkpoint blockade immunotherapy induces dynamic changes in PD-1 − CD8 + tumor-infiltrating T cells. Immunity 50, 181–194.e6 (2019).
Kim, J. E. et al. Combination therapy with anti-PD-1, anti-TIM-3, and focal radiation results in regression of murine gliomas. Clin. Cancer Res. 23, 124–136 (2017).
Curigliano, G. et al. Phase I/Ib clinical trial of sabatolimab, an anti–TIM-3 antibody, alone and in combination with spartalizumab, an Anti–PD-1 antibody, in advanced solid tumors. Clin. Cancer Res. 27, 3620–3629 (2021).
Harding, J. J. et al. Blocking TIM-3 in treatment-refractory advanced solid tumors: a phase Ia/b study of LY3321367 with or without an Anti-PD-L1 antibody. Clin. Cancer Res. 27, 2168–2178 (2021).
Hollebecque, A. et al. Safety and antitumor activity of α-PD-L1 antibody as monotherapy or in combination with α-TIM-3 antibody in patients with microsatellite instability–high/mismatch repair–deficient tumors. Clin. Cancer Res. 27, 6393–6404 (2021).
Brunner, A. M. et al. Efficacy and safety of sabatolimab (MBG453) in combination with hypomethylating agents (HMAs) in patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (HR-MDS): updated results from a phase 1b study. Blood 136, 1–2 (2020).
Novartis. Novartis receives FDA fast track designation for sabatolimab (MBG453) in myelodysplastic syndromes | Novartis. https://www.novartis.com/news/novartis-receives-fda-fast-track-designation-sabatolimab-mbg453-myelodysplastic-syndromes (2021).
Yu, X. et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 10, 48–57 (2009).
Stanietsky, N. et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc. Natl Acad. Sci. USA 106, 17858–17863 (2009).
Johnston, R. J. et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8 + T cell effector function. Cancer Cell 26, 923–937 (2014).
Zhang, Q. et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 19, 723–732 (2018).
Hasan, M. M. et al. Implication of TIGIT+ human memory B cells in immune regulation. Nat. Commun. 12, 1534 (2021).
Riquelme, P. et al. TIGIT+ iTregs elicited by human regulatory macrophages control T cell immunity. Nat. Commun. 9, 2858 (2018).
Kurtulus, S. et al. TIGIT predominantly regulates the immune response via regulatory T cells. J. Clin. Invest. 125, 4053–4062 (2015).
Zhang, H. et al. Direct interaction between CD155 and CD96 promotes immunosuppression in lung adenocarcinoma. Cell Mol. Immunol. 18, 1575–1577 (2021).
Chan, C. J. et al. The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions. Nat. Immunol. 15, 431–438 (2014).
Dougall, W. C., Kurtulus, S., Smyth, M. J. & Anderson, A. C. TIGIT and CD96: new checkpoint receptor targets for cancer immunotherapy. Immunol. Rev. 276, 112–120 (2017).
Mittal, D. et al. CD96 is an immune checkpoint that regulates CD8 + T-cell antitumor function. Cancer Immunol. Res. 7, 559–571 (2019).
Blake, S. J. et al. Suppression of metastases using a new lymphocyte checkpoint target for cancer immunotherapy. Cancer Discov. 6, 446–459 (2016).
Zhu, Y. et al. Identification of CD112R as a novel checkpoint for human T cells. J. Exp. Med. 213, 167–176 (2016).
Whelan, S. et al. PVRIG and PVRL2 are induced in cancer and inhibit CD8 + T-cell function. Cancer Immunol. Res. 7, 257–268 (2019).
Murter, B. et al. Mouse PVRIG has CD8 + T cell–specific coinhibitory functions and dampens antitumor immunity. Cancer Immunol. Res. 7, 244–256 (2019).
Li, Y. et al. Blockade of checkpoint receptor PVRIG unleashes anti-tumor immunity of NK cells in murine and human solid tumors. J. Hematol. Oncol. 14, 100 (2021).
Wojtowicz, W. M. et al. A human IgSF cell-surface interactome reveals a complex network of protein-protein interactions. Cell 182, 1027–1043.e17 (2020).
Cao, S. et al. A membrane protein display platform for receptor interactome discovery. Proc. Natl Acad. Sci. USA 118, e2025451118 (2021).
Cisneros, E., Moraru, M., Gómez-Lozano, N., López-Botet, M. & Vilches, C. KIR2DL5: an orphan inhibitory receptor displaying complex patterns of polymorphism and expression. Front. Immunol. 3, 289 (2012).
Shibuya, A. et al. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity 4, 573–581 (1996).
Nabekura, T. et al. Critical role of DNAX accessory molecule-1 (DNAM-1) in the development of acute graft-versus-host disease in mice. Proc. Natl Acad. Sci. USA 107, 18593–18598 (2010).
Shibuya, K. et al. CD226 (DNAM-1) is involved in lymphocyte function–associated antigen 1 costimulatory signal for naive T cell differentiation and proliferation. J. Exp. Med. 198, 1829–1839 (2003).
Weulersse, M. et al. Eomes-dependent loss of the co-activating receptor CD226 restrains CD8 + T cell anti-tumor functions and limits the efficacy of cancer immunotherapy. Immunity 53, 824–839.e10 (2020).
Du, X. et al. CD226 regulates natural killer cell antitumor responses via phosphorylation-mediated inactivation of transcription factor FOXO1. Proc. Natl Acad. Sci. USA 115, E11731–E11740 (2018).
Deng, Y. et al. Transcription factor Foxo1 is a negative regulator of natural killer cell maturation and function. Immunity 42, 457–470 (2015).
Anderson, A. C., Joller, N. & Kuchroo, V. K. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity 44, 989–1004 (2016).
Chiu, D. K.-C. et al. Hepatocellular carcinoma cells up-regulate PVRL1, stabilizing PVR and inhibiting the cytotoxic T-cell response via TIGIT to mediate tumor resistance to PD1 inhibitors in mice. Gastroenterology 159, 609–623 (2020).
Reches, A. et al. Nectin4 is a novel TIGIT ligand which combines checkpoint inhibition and tumor specificity. J. Immunother. Cancer 8, e000266 (2020).
Lozano, E., Dominguez-Villar, M., Kuchroo, V. & Hafler, D. A. The TIGIT/CD226 axis regulates human T cell function. J. Immunol. 188, 3869–3875 (2012).
Banta, K. L. et al. Mechanistic convergence of the TIGIT and PD-1 inhibitory pathways necessitates co-blockade to optimize anti-tumor CD8 + T cell responses. Immunity 55, 512–526.e9 (2022).
Lepletier, A. et al. Tumor CD155 expression is associated with resistance to anti-PD1 immunotherapy in metastatic melanoma. Clin. Cancer Res. 26, 3671–3681 (2020).
He, W. et al. CD155T/TIGIT signaling regulates CD8 + T-cell metabolism and promotes tumor progression in human gastric cancer. Cancer Res. 77, 6375–6388 (2017).
Masson, D. et al. Overexpression of the CD155 gene in human colorectal carcinoma. Gut 49, 236–240 (2001).
Wu, L. et al. Blockade of TIGIT/CD155 signaling reverses T-cell exhaustion and enhances antitumor capability in head and neck squamous cell carcinoma. Cancer Immunol. Res. 7, 1700–1713 (2019).
Carlsten, M. et al. Primary human tumor cells expressing CD155 impair tumor targeting by down-regulating DNAM-1 on NK cells. J. Immunol. 183, 4921–4930 (2009).
Li, X.-Y. et al. CD155 loss enhances tumor suppression via combined host and tumor-intrinsic mechanisms. J. Clin. Invest. 128, 2613–2625 (2018).
Chauvin, J.-M. et al. IL15 stimulation with TIGIT blockade reverses CD155-mediated NK-cell dysfunction in melanoma. Clin. Cancer Res. 26, 5520–5533 (2020).
Freed-Pastor, W. A. et al. The CD155/TIGIT axis promotes and maintains immune evasion in neoantigen-expressing pancreatic cancer. Cancer Cell 39, 1342–1360.e14 (2021).
Josefsson, S. E. et al. T cells expressing checkpoint receptor TIGIT are enriched in follicular lymphoma tumors and characterized by reversible suppression of T-cell receptor signaling. Clin. Cancer Res. 24, 870–881 (2018).
Sun, H. et al. Human CD96 correlates to natural killer cell exhaustion and predicts the prognosis of human hepatocellular carcinoma. Hepatology 70, 168–183 (2019).
Lozano, E. et al. Nectin-2 expression on malignant plasma cells is associated with better response to TIGIT blockade in multiple myeloma. Clin. Cancer Res. 26, 4688–4698 (2020).
Yang, Z.-Z. et al. TIGIT expression is associated with T-cell suppression and exhaustion and predicts clinical outcome and anti–PD-1 response in follicular lymphoma. Clin. Cancer Res. 26, 5217–5231 (2020).
Josefsson, S. E. et al. TIGIT and PD-1 mark intratumoral T cells with reduced effector function in B-cell non-Hodgkin lymphoma. Cancer Immunol. Res. 7, 355–362 (2019).
Chauvin, J.-M. et al. TIGIT and PD-1 impair tumor antigen–specific CD8 + T cells in melanoma patients. J. Clin. Invest. 125, 2046–2058 (2015).
Kong, Y. et al. T-cell immunoglobulin and ITIM domain (TIGIT) associates with CD8 + T-cell exhaustion and poor clinical outcome in AML patients. Clin. Cancer Res. 22, 3057–3066 (2016).
Cho, B. C. et al. Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol. 23, 781–792 (2022).
Rudin, C. M. et al. SKYSCRAPER-02: primary results of a phase III, randomized, double-blind, placebo-controlled study of atezolizumab (atezo) + carboplatin + etoposide (CE) with or without tiragolumab (tira) in patients (pts) with untreated extensive-stage small cell lung cancer (ES-SCLC). J. Clin. Oncol. 40, LBA8507–LBA8507 (2022).
Niu, J. et al. First-in-human phase 1 study of the anti-TIGIT antibody vibostolimab as monotherapy or with pembrolizumab for advanced solid tumors, including non-small-cell lung cancer. Ann. Oncol. 33, 169–180 (2022).
Reinhold, M. I. et al. In vivo expression of alternatively spliced forms of integrin-associated protein (CD47). J. Cell Sci. 108, 3419–3425 (1995).
Van Duijn, A., Van Der Burg, S. H. & Scheeren, F. A. CD47/SIRPα axis: bridging innate and adaptive immunity. J. Immunother. Cancer 10, e004589 (2022).
Chen, S. et al. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 8, 207 (2023).
Demaria, O. et al. Harnessing innate immunity in cancer therapy. Nature 574, 45–56 (2019).
Jiang, Z., Sun, H., Yu, J., Tian, W. & Song, Y. Targeting CD47 for cancer immunotherapy. J. Hematol. Oncol. 14, 1–18 (2021).
Liu, Y. et al. Emerging phagocytosis checkpoints in cancer immunotherapy. Signal. Transduct. Target. Ther. 8, 104 (2023).
Logtenberg, M. E. W., Scheeren, F. A. & Schumacher, T. N. The CD47-SIRPα immune checkpoint. Immunity 52, 742–752 (2020).
Brooke, G., Holbrook, J. D., Brown, M. H. & Barclay, A. N. Human lymphocytes interact directly with CD47 through a novel member of the signal regulatory protein (SIRP) family. J. Immunol. 173, 2562–2570 (2004).
Liu, H. et al. A comprehensive immunoreceptor phosphotyrosine-based signaling network revealed by reciprocal protein-peptide array screening. Mol. Cell. Proteom. 14, 1846–1858 (2015).
Barclay, A. N. & Van Den Berg, T. K. The interaction between signal regulatory protein alpha (SIRPα) and CD47: structure, function, and therapeutic target. Annu. Rev. Immunol. 32, 25–50 (2014).
Fujioka, Y. et al. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol. Cell. Biol. 16, 6887–6899 (1996).
Timms, J. F. et al. SHPS-1 is a scaffold for assembling distinct adhesion-regulated multi-protein complexes in macrophages. Curr. Biol. 9, 927–930 (1999).
Adams, S. et al. Signal-regulatory protein is selectively expressed by myeloid and neuronal cells. J. Immunol. 161, 1853–1859 (1998).
Tsai, R. K. & Discher, D. E. Inhibition of ‘self’ engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J. Cell Biol. 180, 989–1003 (2008).
Kharitonenkov, A. et al. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature 386, 181–186 (1997).
Veillette, A., Thibaudeaut, E. & Latour, S. High expression of inhibitory receptor SHPS-1 and its association with protein-tyrosine phosphatase SHP-1 in macrophages. J. Biol. Chem. 273, 22719–22728 (1998).
Tang, Z. et al. CD47 masks pro-phagocytic ligands in cis on tumor cells to suppress antitumor immunity. Nat. Immunol. 24, 2032–2041 (2023).
Brown, E. J. & Frazier, W. A. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 11, 130–135 (2001).
Gao, A. G. & Frazier, W. A. Identification of a receptor candidate for the carboxyl-terminal cell binding domain of thrombospondins. J. Biol. Chem. 269, 29650–29657 (1994).
Lindberg, F. P., Gresham, H. D., Reinhold, M. I. & Brown, E. J. Integrin-associated protein immunoglobulin domain is necessary for efficient vitronectin bead binding. J. Cell Biol. 134, 1313–1322 (1996).
T, Y. et al. Negative regulation of platelet clearance and of the macrophage phagocytic response by the transmembrane glycoprotein SHPS-1. J. Biol. Chem. 277, 39833–39839 (2002).
Ding, X. et al. Loss of microglial SIRPα promotes synaptic pruning in preclinical models of neurodegeneration. Nat. Commun. 12, 2030 (2021).
Jiang, D. et al. Neuronal signal-regulatory protein alpha drives microglial phagocytosis by limiting microglial interaction with CD47 in the retina. Immunity 55, 2318–2335.e7 (2022).
Lehrman, E. K. et al. CD47 protects synapses from excess microglia-mediated pruning during development. Neuron 100, 120–134.e6 (2018).
Lysenko, V. et al. Blocking the CD47-SIRPα interaction reverses the disease phenotype in a polycythemia vera mouse model. Leukemia 37, 1277–1286 (2023).
Dulgeroff, L. B. T. et al. CD47 blockade reduces the pathologic features of experimental cerebral malaria and promotes survival of hosts with Plasmodium infection. Proc. Natl Acad. Sci. USA 118, e1907653118 (2021).
Singla, B. et al. Loss of myeloid cell-specific SIRPα, but not CD47, attenuates inflammation and suppresses atherosclerosis. Cardiovasc. Res. 118, 3097–3111 (2022).
Roquilly, A. et al. Alveolar macrophages are epigenetically altered after inflammation, leading to long-term lung immunoparalysis. Nat. Immunol. 21, 636–648 (2020).
Li, J. et al. Overexpression of CD47 is associated with brain overgrowth and 16p11.2 deletion syndrome. Proc. Natl Acad. Sci. USA 118, e2005483118 (2021).
Huang, W. et al. MIR-708 promotes phagocytosis to eradicate T-ALL cells by targeting CD47. Mol. Cancer 17, 12 (2018).
Chao, M. P. et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 142, 699–713 (2010).
Majeti, R. et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 138, 286–299 (2009).
Willingham, S. B. et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl Acad. Sci. USA 109, 6662–6667 (2012).
Dooling, L. J. et al. Cooperative phagocytosis of solid tumours by macrophages triggers durable anti-tumour responses. Nat. Biomed. Eng. 7, 1081–1096 (2023).
Van, V. Q. et al. CD47(high) expression on CD4 effectors identifies functional long-lived memory T cell progenitors. J. Immunol. 188, 4249–4255 (2012).
Myers, L. M. et al. A functional subset of CD8 + T cells during chronic exhaustion is defined by SIRPα expression. Nat. Commun. 10, 794 (2019).
Zhou, Z. et al. Tumor-intrinsic SIRPA promotes sensitivity to checkpoint inhibition immunotherapy in melanoma. Cancer Cell 40, 1324–1340.e8 (2022).
Komori, S. et al. CD47 promotes peripheral T cell survival by preventing dendritic cell-mediated T cell necroptosis. Proc. Natl Acad. Sci. USA 120, e2304943120 (2023).
Fisher, G. A. et al. A phase Ib/II study of the anti-CD47 antibody magrolimab with cetuximab in solid tumor and colorectal cancer patients. J. Clin. Oncol. 38, 114–114 (2020).
Zeidan, A. M. et al. A phase I study of CC-90002, a monoclonal antibody targeting CD47, in patients with relapsed and/or refractory (R/R) acute myeloid leukemia (AML) and high-risk myelodysplastic syndromes (MDS): final results. Blood 134, 1320–1320 (2019).
Daver, N. et al. Phase I/II study of azacitidine (AZA) with venetoclax (VEN) and magrolimab (Magro) in patients (pts) with newly diagnosed older/unfit or high-risk acute myeloid leukemia (AML) and relapsed/refractory (R/R) AML. Blood 138, 371–371 (2021).
Brierley, C. K. et al. The effects of monoclonal anti‐CD47 on RBCs, compatibility testing, and transfusion requirements in refractory acute myeloid leukemia. Transfusion 59, 2248–2254 (2019).
Ingram, J. R. et al. Localized CD47 blockade enhances immunotherapy for murine melanoma. Proc. Natl Acad. Sci. USA 114, 10184–10189 (2017).
Kojima, Y. et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 536, 86–90 (2016).
Sallman, D. A. et al. The first-in-class anti-CD47 antibody Hu5F9-G4 is active and well tolerated alone or with azacitidine in AML and MDS patients: Initial phase 1b results. J. Clin. Oncol. 37, 7009–7009 (2019).
Advani, R. et al. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin’s lymphoma. N. Engl. J. Med. 379, 1711–1721 (2018).
Osorio, J. C., Smith, P., Knorr, D. A. & Ravetch, J. V. The antitumor activities of anti-CD47 antibodies require Fc-FcγR interactions. Cancer Cell 41, 2051–2065.e6 (2023).
Khalaji, A. et al. Don’t eat me/eat me signals as a novel strategy in cancer immunotherapy. Heliyon 9, e20507 (2023).
Li, Y. et al. A pH-dependent anti-CD47 antibody that selectively targets solid tumors and improves therapeutic efficacy and safety. J. Hematol. Oncol. 16, 2 (2023).
Ma, L. et al. Preclinical development of a novel CD47 nanobody with less toxicity and enhanced anti-cancer therapeutic potential. J. Nanobiotechnol. 18, 12 (2020).
Cao, A. et al. CD47-blocking antibody ZL-1201 promotes tumor-associated macrophage phagocytic activity and enhances the efficacy of the therapeutic antibodies and chemotherapy. Cancer Res. Commun. 2, 1404–1417 (2022).
Puro, R. J. et al. Development of AO-176, a next-generation humanized anti-CD47 antibody with novel anticancer properties and negligible red blood cell binding. Mol. Cancer Ther. 19, 835–846 (2020).
Peluso, M. O. et al. The fully human anti-CD47 antibody SRF231 exerts dual-mechanism antitumor activity via engagement of the activating receptor CD32a. J. Immunother. Cancer 8, e000413 (2020).
Meng, Z., Wang, Z., Guo, B., Cao, W. & Shen, H. TJC4, a differentiated anti-CD47 antibody with novel epitope and RBC sparing properties. Blood 134, 4063–4063 (2019).
Gan, H. K. et al. Safety of AK117, an anti-CD47 monoclonal antibody, in patients with advanced or metastatic solid tumors in a phase I study. J. Clin. Oncol. 39, 2630–2630 (2021).
Burris, H. A. III et al. A first-in-human study of AO-176, a highly differentiated anti-CD47 antibody, in patients with advanced solid tumors. J. Clin. Oncol. 39, 2516–2516 (2021).
Patnaik, A. et al. Results of a first-in-human phase I study of SRF231, a fully human, high-affinity anti-CD47 antibody. J. Clin. Oncol. 38, 3064–3064 (2020).
Daver, N. G. et al. Lemzoparlimab (lemzo) with venetoclax (ven) and/or azacitidine (aza) in patients (pts) with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS): A phase 1b dose escalation study. J. Clin. Oncol. 40, TPS7067–TPS7067 (2022).
Mehta, A. et al. Lemzoparlimab, a differentiated anti-CD47 antibody in combination with rituximab in relapsed and refractory non-Hodgkin’s lymphoma: initial clinical results. Blood 138, 3542–3542 (2021).
Qi, J. et al. A phase I/IIa study of lemzoparlimab, a monoclonal antibody targeting CD47, in patients with relapsed and/or refractory acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS): initial phase I results. Blood 136, 30–31 (2020).
Petrova, P. S. et al. TTI-621 (SIRPαFc): a CD47-blocking innate immune checkpoint inhibitor with broad antitumor activity and minimal erythrocyte binding. Clin. Cancer Res. 23, 1068–1079 (2017).
Patel, K. et al. CD47-blocker TTI-622 shows single-agent activity in patients with advanced relapsed or refractory lymphoma: update from the ongoing first-in-human dose escalation study. Blood 138, 3560–3560 (2021).
Movva, S. et al. Safety and clinical activity of TTI-621 in combination with doxorubicin in patients with unresectable or metastatic high-grade leiomyosarcoma: results from the low-dose expansion cohort. J. Clin. Oncol. 41, 11508–11508 (2023).
Daver, N. G. et al. Tolerability and efficacy of the anticluster of differentiation 47 antibody magrolimab combined with azacitidine in patients with previously untreated AML: phase Ib results. J. Clin. Oncol. 41, 4893–4904 (2023).
Tong, H. et al. Preliminary results of a phase 2 study of IMM01 combined with azacitidine (AZA) as the first-line treatment in adult patients with chronic myelomonocytic leukemia (CMML). Blood 142, 1859–1859 (2023).
Lakhani, N. J. et al. Evorpacept alone and in combination with pembrolizumab or trastuzumab in patients with advanced solid tumours (ASPEN-01): a first-in-human, open-label, multicentre, phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 22, 1740–1751 (2021).
Lakhani, N. J. et al. A phase Ib study of the anti-CD47 antibody magrolimab with the PD-L1 inhibitor avelumab (A) in solid tumor (ST) and ovarian cancer (OC) patients. J. Clin. Oncol. 38, 18–18 (2020).
Reid, T. et al. Phase 1 pilot study of RRx-001 + nivolumab in patients with advanced metastatic cancer (PRIMETIME). Front. Immunol. 14, 1104753 (2023).
Morgensztern, D. et al. RRx-001 followed by platinum plus etoposide in patients with previously treated small-cell lung cancer. Br. J. Cancer 121, 211–217 (2019).
Reid, T. et al. Safety and activity of RRx-001 in patients with advanced cancer: a first-in-human, open-label, dose-escalation phase 1 study. Lancet Oncol. 16, 1133–1142 (2015).
**a, Q. et al. The safety and efficacy of cadonilimab in combination with AK117 (anti-CD47 antibody) plus chemotherapy as first-line treatment for advanced gastric (G) or gastroesophageal junction (GEJ) cancer. J. Clin. Oncol. 41, e16050–e16050 (2023).
Drakaki, A. et al. Atezolizumab plus magrolimab, niraparib, or tocilizumab versus atezolizumab monotherapy in platinum-refractory metastatic urothelial carcinoma: a phase Ib/II open-label, multicenter, randomized umbrella study (MORPHEUS Urothelial Carcinoma). Clin. Cancer Res. 29, 4373–4384 (2023).
Zhang, P. et al. STING agonist-loaded, CD47/PD-L1-targeting nanoparticles potentiate antitumor immunity and radiotherapy for glioblastoma. Nat. Commun. 14, 1610 (2023).
Luo, J.-Q. et al. Nanoparticle-mediated CD47-SIRPα blockade and calreticulin exposure for improved cancer chemo-immunotherapy. ACS Nano 17, 8966–8979 (2023).
Guo, Y., Bao, Q., Hu, P. & Shi, J. Nanomedicine-based co-delivery of a calcium channel inhibitor and a small molecule targeting CD47 for lung cancer immunotherapy. Nat. Commun. 14, 7306 (2023).
Tang, Y. et al. Precise delivery of nanomedicines to M2 macrophages by combining “Eat Me/Don’t Eat Me” signals and its anticancer application. ACS Nano 15, 18100–18112 (2021).
Zhao, C. et al. Vesicular antibodies: shedding light on antibody therapeutics with cell membrane nanotechnology. Adv. Mater. 35, 2207875 (2023).
Rao, L. et al. Hybrid cellular membrane nanovesicles amplify macrophage immune responses against cancer recurrence and metastasis. Nat. Commun. 11, 4909 (2020).
Meng, Q. et al. Genetically programmable fusion cellular vesicles for cancer immunotherapy. Angew. Chem. Int. Ed. Engl. 60, 26320–26326 (2021).
**a, Y. et al. Engineering macrophages for cancer immunotherapy and drug delivery. Adv. Mater. 32, 2002054 (2020).
Andrews, L. P., Yano, H. & Vignali, D. A. A. Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4: breakthroughs or backups. Nat. Immunol. 20, 1425–1434 (2019).
Zhang, G., Dong, Q., Xu, Y., Yu, G. & Zhang, X. B7-H3: another molecule marker for Mo-DCs? Cell Mol. Immunol. 2, 307–311 (2005).
Chapoval, A. I. et al. B7-H3: a costimulatory molecule for T cell activation and IFN-γ production. Nat. Immunol. 2, 269–274 (2001).
Suh, W. K. et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat. Immunol. 4, 899–906 (2003).
Vigdorovich, V. et al. Structure and T cell inhibition properties of B7 family member, B7-H3. Structure 21, 707–717 (2013).
Xu, J. et al. Soluble mouse B7-H3 down-regulates dendritic cell stimulatory capacity to allogenic T cell proliferation and production of IL-2 and IFN-gamma. Cell Mol. Immunol. 3, 235–240 (2006).
Hashiguchi, M. et al. Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7-H3 and enhances T cell responses. Proc. Natl Acad. Sci. USA 105, 10495–10500 (2008).
Kreymborg, K. et al. Ablation of B7-H3 but not B7-H4 results in highly increased tumor burden in a murine model of spontaneous prostate cancer. Cancer Immunol. Res. 3, 849–854 (2015).
Husain, B. et al. A platform for extracellular interactome discovery identifies novel functional binding partners for the immune receptors B7-H3/CD276 and PVR/CD155. Mol. Cell. Proteom. 18, 2310–2323 (2019).
Rutz, S., Wang, X. & Ouyang, W. The IL-20 subfamily of cytokines—from host defence to tissue homeostasis. Nat. Rev. Immunol. 14, 783–795 (2014).
Gao, W. et al. IL20RA signaling enhances stemness and promotes the formation of an immunosuppressive microenvironment in breast cancer. Theranostics 11, 2564–2580 (2021).
Miyamoto, T. et al. B7-H3 suppresses antitumor immunity via the CCL2–CCR2–M2 macrophage axis and contributes to ovarian cancer progression. Cancer Immunol. Res. 10, 56–69 (2022).
Yonesaka, K. et al. B7-H3 negatively modulates CTL-mediated cancer immunity. Clin. Cancer Res. 24, 2653–2664 (2018).
Carvajal-Hausdorf, D. et al. Expression and clinical significance of PD-L1, B7-H3, B7-H4 and TILs in human small cell lung Cancer (SCLC). J. Immunother. Cancer 7, 65 (2019).
Akcakanat, A. et al. Genomic, transcriptomic, and proteomic profiling of metastatic breast cancer. Clin. Cancer Res. 27, 3243–3252 (2021).
Arigami, T. et al. B7-H3 ligand expression by primary breast cancer and associated with regional nodal metastasis. Ann. Surg. 252, 1044–1051 (2010).
Roth, T. J. et al. B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res. 67, 7893–7900 (2007).
Zang, X. et al. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc. Natl Acad. Sci. USA 104, 19458–19463 (2007).
Boorjian, S. A. et al. T-Cell coregulatory molecule expression in urothelial cell carcinoma: clinicopathologic correlations and association with survival. Clin. Cancer Res. 14, 4800–4808 (2008).
Crispen, P. L. et al. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin. Cancer Res. 14, 5150–5157 (2008).
Chen, J.-T. et al. Glycoprotein B7-H3 overexpression and aberrant glycosylation in oral cancer and immune response. Proc. Natl Acad. Sci. USA 112, 13057–13062 (2015).
Aung, P. P. et al. B7-H3 expression in merkel cell carcinoma–associated endothelial cells correlates with locally aggressive primary tumor features and increased vascular density. Clin. Cancer Res. 25, 3455–3467 (2019).
Parra, E. R. et al. Immunohistochemical and image analysis-based study shows that several immune checkpoints are co-expressed in non–small cell lung carcinoma tumors. J. Thorac. Oncol. 13, 779–791 (2018).
Schneider, T. et al. Non-small cell lung cancer induces an immunosuppressive phenotype of dendritic cells in tumor microenvironment by upregulating B7-H3. J. Thorac. Oncol. 6, 1162–1168 (2011).
Lee, Y. et al. Inhibition of the B7-H3 immune checkpoint limits tumor growth by enhancing cytotoxic lymphocyte function. Cell Res. 27, 1034–1045 (2017).
Wang, C. et al. CD276 expression enables squamous cell carcinoma stem cells to evade immune surveillance. Cell Stem Cell 28, 1597–1613.e7 (2021).
Seaman, S. et al. Eradication of tumors through simultaneous ablation of CD276/B7-H3-positive tumor cells and tumor vasculature. Cancer Cell 31, 501–515.e8 (2017).
Kendsersky, N. M. et al. The B7-H3–targeting antibody–drug conjugate m276-SL-PBD is potently effective against pediatric cancer preclinical solid tumor models. Clin. Cancer Res. 27, 2938–2946 (2021).
Loo, D. et al. Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activity. Clin. Cancer Res. 18, 3834–3845 (2012).
Sica, G. L. et al. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity 18, 849–861 (2003).
Wei, J., Loke, P., Zang, X. & Allison, J. P. Tissue-specific expression of B7x protects from CD4 T cell-mediated autoimmunity. J. Exp. Med. 208, 1683–1694 (2011).
Zhu, G. et al. B7-H4-deficient mice display augmented neutrophil-mediated innate immunity. Blood 113, 1759–1767 (2009).
Nurieva, R. et al. T-cell tolerance or function is determined by combinatorial costimulatory signals. EMBO J. 25, 2623–2633 (2006).
Quandt, D., Fiedler, E., Boettcher, D., Marsch, W. C. & Seliger, B. B7-H4 expression in human melanoma: Its association with patients’ survival and antitumor immune response. Clin. Cancer Res. 17, 3100–3111 (2011).
Tringler, B. et al. B7-H4 is highly expressed in ductal and lobular breast cancer. Clin. Cancer Res. 11, 1842–1848 (2005).
Simon, I. et al. B7-H4 Is a novel membrane-bound protein and a candidate serum and tissue biomarker for ovarian cancer. Cancer Res. 66, 1570–1575 (2006).
Krambeck, A. E. et al. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc. Natl Acad. Sci. USA 103, 10391–10396 (2006).
Guo, M. et al. Gene signature driving invasive mucinous adenocarcinoma of the lung. EMBO Mol. Med. 9, 462–481 (2017).
Cheng, H. et al. Wide expression and significance of alternative immune checkpoint molecules, B7x and HHLA2, in PD-L1–negative human lung cancers. Clin. Cancer Res. 24, 1954–1964 (2018).
Kryczek, I. et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J. Exp. Med. 203, 871–881 (2006).
Kryczek, I. et al. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. 67, 8900–8905 (2007).
Yao, Y. et al. B7-H4(B7x)-mediated cross-talk between glioma-initiating cells and macrophages via the IL6/JAK/STAT3 pathway lead to poor prognosis in glioma patients. Clin. Cancer Res. 22, 2778–2790 (2016).
Li, J. et al. Co-inhibitory molecule B7 superfamily member 1 expressed by tumor-infiltrating myeloid cells induces dysfunction of anti-tumor CD8 + T Cells. Immunity 48, 773–786.e5 (2018).
Rahbar, R. et al. B7-H4 expression by nonhematopoietic cells in the tumor microenvironment promotes antitumor immunity. Cancer Immunol. Res. 3, 184–195 (2015).
Chen, Y. et al. Expression of the novel co-stimulatory molecule B7-H4 by renal tubular epithelial cells. Kidney Int. 70, 2092–2099 (2006).
Dangaj, D. et al. Novel recombinant human B7-H4 antibodies overcome tumoral immune escape to potentiate T-cell antitumor responses. Cancer Res. 73, 4820–4829 (2013).
Yuan, X. et al. Blockade of immune-checkpoint B7-H4 and lysine demethylase 5B in esophageal squamous cell carcinoma confers protective immunity against P. gingivalis infection. Cancer Immunol. Res. 7, 1440–1456 (2019).
Wang, L. et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J. Exp. Med. 208, 577–592 (2011).
Lines, J. L. et al. VISTA is an immune checkpoint molecule for human T cells. Cancer Res. 74, 1924–1932 (2014).
Yuan, L., Tatineni, J., Mahoney, K. M. & Freeman, G. J. VISTA: a mediator of quiescence and a promising target in cancer immunotherapy. Trends Immunol. 42, 209–227 (2021).
Tinoco, R. et al. PSGL-1 is an immune checkpoint regulator that promotes T cell exhaustion. Immunity 44, 1190–1203 (2016).
Johnston, R. J. et al. VISTA is an acidic pH-selective ligand for PSGL-1. Nature 574, 565–570 (2019).
Flies, D. B. et al. Coinhibitory receptor PD-1H preferentially suppresses CD4 + T cell–mediated immunity. J. Clin. Invest. 124, 1966–1975 (2014).
ElTanbouly, M. A. et al. VISTA is a checkpoint regulator for naïve T cell quiescence and peripheral tolerance. Science 367, eaay0524 (2020).
Liu, J. et al. Immune-checkpoint proteins VISTA and PD-1 nonredundantly regulate murine T-cell responses. Proc. Natl Acad. Sci. USA 112, 6682–6687 (2015).
Han, X. et al. PD-1H (VISTA)–mediated suppression of autoimmunity in systemic and cutaneous lupus erythematosus. Sci. Transl. Med. 11, eaax1159 (2019).
Wang, L. et al. Disruption of the immune-checkpoint VISTA gene imparts a proinflammatory phenotype with predisposition to the development of autoimmunity. Proc. Natl Acad. Sci. USA 111, 14846–14851 (2014).
Ceeraz, S. et al. VISTA deficiency accelerates the development of fatal murine lupus nephritis. Arthritis Rheumatol. 69, 814–825 (2017).
Le Mercier, I. et al. VISTA regulates the development of protective antitumor immunity. Cancer Res 74, 1933–1944 (2014).
Hong, S. et al. Analysis of VISTA expression and function in renal cell carcinoma highlights VISTA as a potential target for immunotherapy. Protein Cell 10, 840–845 (2019).
Blando, J. et al. Comparison of immune infiltrates in melanoma and pancreatic cancer highlights VISTA as a potential target in pancreatic cancer. Proc. Natl Acad. Sci. USA 116, 1692–1697 (2019).
Liu, H. et al. A crucial role of the PD-1H coinhibitory receptor in suppressing experimental asthma. Cell Mol. Immunol. 15, 838–845 (2018).
Li, H. et al. The allergy mediator histamine confers resistance to immunotherapy in cancer patients via activation of the macrophage histamine receptor H1. Cancer Cell 40, 36–52.e9 (2022).
Deng, J. et al. Hypoxia-induced VISTA promotes the suppressive function of myeloid-derived suppressor cells in the tumor microenvironment. Cancer Immunol. Res. 7, 1079–1090 (2019).
Thakkar, D. et al. Rationally targeted anti-VISTA antibody that blockades the C-C’ loop region can reverse VISTA immune suppression and remodel the immune microenvironment to potently inhibit tumor growth in an Fc independent manner. J. Immunother. Cancer 10, e003382 (2022).
Pan, J. et al. Inhibition of lung tumorigenesis by a small molecule CA170 targeting the immune checkpoint protein VISTA. Commun. Biol. 4, 906 (2021).
Radhakrishnan, V. et al. Excellent CBR and prolonged PFS in non-squamous NSCLC with oral CA-170, an inhibitor of VISTA and PD-L1. Ann. Oncol. 30, v494 (2019).
Radhakrishnan, V. S. et al. Phase 2 trial of CA-170, a novel oral small molecule dual inhibitor of immune checkpoints VISTA and PD-1, in patients (pts) with advanced solid tumor and Hodgkin lymphoma. J. Immunother. Cancer 6, P714 (2018).
Mai, H.-Q. et al. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial. Nat. Med. 27, 1536–1543 (2021).
Zhou, C. et al. Sintilimab plus platinum and gemcitabine as first-line treatment for advanced or metastatic squamous NSCLC: results from a randomized, double-blind, phase 3 trial (ORIENT-12). J. Thorac. Oncol. 16, 1501–1511 (2021).
Arce Vargas, F. et al. Fc effector function contributes to the activity of human anti-CTLA-4 antibodies. Cancer Cell 33, 649–663.e4 (2018).
Zhang, F. et al. Structural basis of a novel PD-L1 nanobody for immune checkpoint blockade. Cell Discov. 3, 17004 (2017).
Roopenian, D. C. & Akilesh, S. FcRn: the neonatal Fc receptor comes of age. Nat. Rev. Immunol. 7, 715–725 (2007).
Edner, N. M., Carlesso, G., Rush, J. S. & Walker, L. S. K. Targeting co-stimulatory molecules in autoimmune disease. Nat. Rev. Drug Discov. 19, 860–883 (2020).
Ward-Kavanagh, L. K., Lin, W. W., Šedý, J. R. & Ware, C. F. The TNF receptor superfamily in co-stimulating and co-inhibitory responses. Immunity 44, 1005–1019 (2016).
Mayes, P. A., Hance, K. W. & Hoos, A. The promise and challenges of immune agonist antibody development in cancer. Nat. Rev. Drug Discov. 17, 509–527 (2018).
Kraehenbuehl, L., Weng, C.-H., Eghbali, S., Wolchok, J. D. & Merghoub, T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat. Rev. Clin. Oncol. 19, 37–50 (2022).
Chodorge, M. et al. A series of Fas receptor agonist antibodies that demonstrate an inverse correlation between affinity and potency. Cell Death Differ. 19, 1187–1195 (2012).
Al-Shamkhani, A. et al. OX40 is differentially expressed on activated rat and mouse T cells and is the sole receptor for the OX40 ligand. Eur. J. Immunol. 26, 1695–1699 (1996).
Segal, N. H. et al. Results from an integrated safety analysis of urelumab, an agonist anti-CD137 monoclonal antibody. Clin. Cancer Res. 23, 1929–1936 (2017).
Yu, X. et al. Complex interplay between epitope specificity and isotype dictates the biological activity of anti-human CD40 antibodies. Cancer Cell 33, 664–675.e4 (2018).
Chester, C., Sanmamed, M. F., Wang, J. & Melero, I. Immunotherapy targeting 4-1BB: mechanistic rationale, clinical results, and future strategies. Blood 131, 49–57 (2018).
Compte, M. et al. A tumor-targeted trimeric 4-1BB-agonistic antibody induces potent anti-tumor immunity without systemic toxicity. Nat. Commun. 9, 4809 (2018).
Compte, M. et al. An Fc-free EGFR-specific 4-1BB-agonistic trimerbody displays broad antitumor activity in humanized murine cancer models without toxicity. Clin. Cancer Res. 27, 3167–3177 (2021).
Wang, X., Mathieu, M. & Brezski, R. J. IgG Fc engineering to modulate antibody effector functions. Protein Cell 9, 63–73 (2018).
Dahan, R. et al. Therapeutic activity of agonistic, human anti-cd40 monoclonal antibodies requires selective FcγR engagement. Cancer Cell 29, 820–831 (2016).
White, A. L. et al. Conformation of the human immunoglobulin G2 hinge imparts superagonistic properties to immunostimulatory anticancer antibodies. Cancer Cell 27, 138–148 (2015).
Liu, X. et al. Human immunoglobulin G hinge regulates agonistic anti-CD40 immunostimulatory and antitumour activities through biophysical flexibility. Nat. Commun. 10, 4206 (2019).
Orr, C. M. et al. Hinge disulfides in human IgG2 CD40 antibodies modulate receptor signaling by regulation of conformation and flexibility. Sci. Immunol. 7, eabm3723 (2022).
Yu, X. et al. Isotype switching converts anti-CD40 antagonism to agonism to elicit potent antitumor activity. Cancer Cell 37, 850–866.e7 (2020).
Fos, C. et al. ICOS ligation recruits the p50α PI3K regulatory subunit to the immunological synapse. J. Immunol. 181, 1969–1977 (2008).
Yap, T. A. et al. First-in-human phase I/II ICONIC trial of the ICOS agonist vopratelimab alone and with nivolumab: ICOS-high CD4 T-cell populations and predictors of response. Clin. Cancer Res. 28, 3695–3708 (2022).
Gumus, M. et al. 181 P SELECT: a phase II randomized trial evaluating 2 doses of vopratelimab (V) + pimivalimab (P) vs P in TISvopra selected patients (pts). ImmunoOncol. Technol. 16, 100293 (2022).
Sainson, R. C. A. et al. An antibody targeting ICOS increases intratumoral cytotoxic to regulatory T-cell ratio and induces tumor regression. Cancer Immunol. Res. 8, 1568–1582 (2020).
Patel, M. R. et al. A phase 1/2 open-label study of KY1044, an anti-ICOS antibody with dual mechanism of action, as single agent and in combination with atezolizumab, in adult patients with advanced malignancies. J. Clin. Oncol. 39, 2624–2624 (2021).
Papadopoulos, K. P. et al. Phase I study of MK-4166, an anti-human glucocorticoid-induced TNF receptor antibody, alone or with pembrolizumab in advanced solid tumors. Clin. Cancer Res. 27, 1904–1911 (2021).
Heinhuis, K. M. et al. Safety, tolerability, and potential clinical activity of a glucocorticoid-induced TNF receptor–related protein agonist alone or in combination with nivolumab for patients with advanced solid tumors. JAMA Oncol. 6, 100 (2020).
Geva, R. et al. First‐in‐human phase 1 study of MK‐1248, an anti–glucocorticoid‐induced tumor necrosis factor receptor agonist monoclonal antibody, as monotherapy or with pembrolizumab in patients with advanced solid tumors. Cancer 126, 4926–4935 (2020).
Tran, B. et al. Dose escalation results from a first-in-human, phase 1 study of glucocorticoid-induced TNF receptor–related protein agonist AMG 228 in patients with advanced solid tumors. J. Immunother. Cancer 6, 93 (2018).
Balmanoukian, A. S. et al. Safety and clinical activity of MEDI1873, a novel GITR agonist, in advanced solid tumors. Clin. Cancer Res. 26, 6196–6203 (2020).
Davar, D. et al. Phase IB study of GITR agonist antibody TRX518 singly and in combination with gemcitabine, pembrolizumab, or nivolumab in patients with advanced solid tumors. Clin. Cancer Res. 28, 3990–4002 (2022).
Fischer, R., Kontermann, R. E. & Pfizenmaier, K. Selective targeting of TNF receptors as a novel therapeutic approach. Front. Cell Dev. Biol. 8, 401 (2020).
Bai, J., Ding, B. & Li, H. Targeting TNFR2 in cancer: all roads lead to rome. Front. Immunol. 13, 844931 (2022).
Caux, C. et al. Activation of human dendritic cells through CD40 Cross-linking. J. Exp. Med. 180, 1263–1272 (1994).
O’Hara, M. H. et al. CD40 agonistic monoclonal antibody APX005M (sotigalimab) and chemotherapy, with or without nivolumab, for the treatment of metastatic pancreatic adenocarcinoma: an open-label, multicentre, phase 1b study. Lancet Oncol. 22, 118–131 (2021).
Mangsbo, S. M. et al. The human agonistic CD40 antibody ADC-1013 eradicates bladder tumors and generates T-cell–dependent tumor immunity. Clin. Cancer Res. 21, 1115–1126 (2015).
Deronic, A. et al. The human anti-CD40 agonist antibody mitazalimab (ADC-1013; JNJ-64457107) activates antigen-presenting cells, improves expansion of antigen-specific T cells, and enhances anti-tumor efficacy of a model cancer vaccine in vivo. Cancer Immunol. Immunother. 70, 3629–3642 (2021).
Polesso, F., Sarker, M., Weinberg, A. D., Murray, S. E. & Moran, A. E. OX40 agonist tumor immunotherapy does not impact regulatory T cell suppressive function. J. Immunol. 203, 2011–2019 (2019).
Compaan, D. M. & Hymowitz, S. G. The crystal structure of the costimulatory OX40-OX40L complex. Structure 14, 1321–1330 (2006).
Diab, A. et al. A phase I, open-label, dose-escalation study of the OX40 agonist ivuxolimab in patients with locally advanced or metastatic cancers. Clin. Cancer Res. 28, 71–83 (2022).
Kim, T. W. et al. First-in-human phase I study of the OX40 agonist MOXR0916 in patients with advanced solid tumors. Clin. Cancer Res. 28, 3452–3463 (2022).
Goldman, J. W. et al. Safety and tolerability of MEDI0562, an OX40 agonist mAb, in combination with durvalumab or tremelimumab in adult patients with advanced solid tumors. Clin. Cancer Res. 28, 3709–3719 (2022).
Davis, E. J. et al. First-in-human phase I/II, open-label study of the anti-OX40 agonist INCAGN01949 in patients with advanced solid tumors. J. Immunother. Cancer 10, e004235 (2022).
Incyte Corporation. A phase 1/2, open-label, dose-escalation, safety study of INCAGN01949 in subjects with advanced or metastatic solid tumors—study results—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/results/NCT02923349 (2019).
Segal, N. H. et al. Phase I study of single-agent utomilumab (PF-05082566), a 4-1bb/cd137 agonist, in patients with advanced cancer. Clin. Cancer Res. 24, 1816–1823 (2018).
Chin, S. M. et al. Structure of the 4-1BB/4-1BBL complex and distinct binding and functional properties of utomilumab and urelumab. Nat. Commun. 9, 4679 (2018).
Li, Y. et al. Limited cross-linking of 4-1BB by 4-1BB ligand and the agonist monoclonal antibody utomilumab. Cell Rep. 25, 909–920.e4 (2018).
Zhang, L. et al. A phase I, dose-escalation study of ADG106, a fully human anti-CD137 agonistic antibody, in subjects with advanced solid tumors or relapsed/refractory non-Hodgkin lymphoma. J. Clin. Oncol. 38, 3105–3105 (2020).
Qi, X. et al. Optimization of 4-1BB antibody for cancer immunotherapy by balancing agonistic strength with FcγR affinity. Nat. Commun. 10, 2141 (2019).
Galand, C. et al. 377 AGEN2373 is a CD137 agonist antibody designed to leverage optimal CD137 and FcγR co-targeting to promote antitumor immunologic effects. J. Immunother. Cancer 8, A229.2–A230 (2020).
Fu, S. et al. Early safety and efficacy from a phase I open-label clinical trial of CD137(4-1BB) agonistic antibody LVGN6051 as monotherapy and in combination with pembrolizumab. J. Clin. Oncol. 39, 2521–2521 (2021).
Tolcher, A. W. et al. Initial findings of the first-in-human phase I study of AGEN2373, a conditionally active CD137 agonist antibody, in patients (pts) with advanced solid tumors. J. Clin. Oncol. 39, 2634–2634 (2021).
Ullenhag, G. J. et al. A first-in-human, multicenter, open-label, phase 1 study of ATOR-1017, a 4-1BB antibody, in patients with advanced solid malignancies. J. Clin. Oncol. 39, 2646–2646 (2021).
Nisonoff, A., Wissler, F. C. & Lipman, L. N. Properties of the major component of a peptic digest of rabbit antibody. Science 132, 1770–1771 (1960).
Fudenberg, H. H., Drews, G. & Nisonoff, A. Serologic demonstration of dual specificity of rabbit bivalent hybrid antibody. J. Exp. Med. 119, 151–166 (1964).
Hudson, P. J. & Souriau, C. Engineered antibodies. Nat. Med. 9, 129–134 (2003).
Riethmüller, G. Symmetry breaking: bispecific antibodies, the beginnings, and 50 years on. Cancer Immun. 12, 12 (2012).
Labrijn, A. F., Janmaat, M. L., Reichert, J. M. & Parren, P. W. H. I. Bispecific antibodies: a mechanistic review of the pipeline. Nat. Rev. Drug Discov. 18, 585–608 (2019).
Goebeler, M. E. & Bargou, R. C. T cell-engaging therapies—BiTEs and beyond. Nat. Rev. Clin. Oncol. 17, 418–434 (2020).
de Miguel, M., Umana, P., de Morais, A. L. G., Moreno, V. & Calvo, E. T-cell-engaging therapy for solid tumors. Clin. Cancer Res. 27, 1595–1603 (2021).
Blanco, B., Domínguez-Alonso, C. & Alvarez-Vallina, L. Bispecific immunomodulatory antibodies for cancer immunotherapy. Clin. Cancer Res. 27, 5457–5464 (2021).
Esfandiari, A., Cassidy, S. & Webster, R. M. Bispecific antibodies in oncology. Nat. Rev. Drug Discov. 21, 411–412 (2022).
Li, T. et al. Novel semi-mechanistic model leveraging preclinical and clinical data to inform the recommended phase 2 dose (RP2D) selection for epcoritamab (DuoBody CD3xCD20). Blood 136, 35–36 (2020).
Suurs, F. V., Lub-de Hooge, M. N., de Vries, E. G. E. & de Groot, D. J. A. A review of bispecific antibodies and antibody constructs in oncology and clinical challenges. Pharmacol. Ther. 201, 103–119 (2019).
Li, H., Er Saw, P. & Song, E. Challenges and strategies for next-generation bispecific antibody-based antitumor therapeutics. Cell Mol. Immunol. 17, 451–461 (2020).
Schaefer, W. et al. Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies. Proc. Natl Acad. Sci. USA 108, 11187–11192 (2011).
Surowka, M., Schaefer, W. & Klein, C. Ten years in the making: application of CrossMab technology for the development of therapeutic bispecific antibodies and antibody fusion proteins. MAbs 13, 1967714 (2021).
Lewis, S. M. et al. Generation of bispecific IgG antibodies by structure-based design of an orthogonal Fab interface. Nat. Biotechnol. 32, 191–198 (2014).
Lee, D. W. et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124, 188–195 (2014).
Ludwig, H. et al. Prevention and management of adverse events during treatment with bispecific antibodies and CAR T cells in multiple myeloma: a consensus report of the European Myeloma Network. Lancet Oncol. 24, e255–e269 (2023).
Kroschinsky, F. et al. New drugs, new toxicities: Severe side effects of modern targeted and immunotherapy of cancer and their management. Crit. Care 21, 1–11 (2017).
Li, J. et al. CD3 bispecific antibody-induced cytokine release is dispensable for cytotoxic T cell activity. Sci. Transl. Med. 11, eaax8861 (2019).
Leclercq-Cohen, G. et al. Dissecting the mechanisms underlying the cytokine release syndrome (CRS) mediated by T-cell bispecific antibodies. Clin. Cancer Res. 29, 4449–4463 (2023).
Meng, Q. F. et al. Inhalation delivery of dexamethasone with iSEND nanoparticles attenuates the COVID-19 cytokine storm in mice and nonhuman primates. Sci. Adv. 9, eadg3277 (2023).
Zhou, Y. et al. Immunogenicity assessment of bispecific antibody-based immunotherapy in oncology. J. Immunother. Cancer 10, e004225 (2022).
Cohen, S. et al. An integrated approach for characterizing immunogenic responses toward a bispecific antibody. MAbs 13, 1944017 (2021).
Topp, M. S. et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 16, 57–66 (2015).
Roche. European Commission approves Roche’s first-in-class bispecific antibody Lunsumio for people with relapsed or refractory follicular lymphoma. https://www.roche.com/investors/updates/inv-update-2022-06-08 (2022).
Budde, L. E. et al. Single-agent mosunetuzumab Shows durable complete responses in patients with relapsed or refractory B-cell lymphomas: phase I dose-escalation study. J. Clin. Oncol. 40, 481–491 (2022).
Budde, L. E. et al. Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: a single-arm, multicentre, phase 2 study. Lancet Oncol. 23, 1055–1065 (2022).
Johnson & Johnson. Janssen Marks First Approval Worldwide for TECVAYLI® (teclistamab) with EC Authorisation of First-in-Class Bispecific Antibody for the Treatment of Patients with Multiple Myeloma | Johnson & Johnson. https://www.jnj.com/janssen-marks-first-approval-worldwide-for-tecvayli-teclistamab-with-ec-authorisation-of-first-in-class-bispecific-antibody-for-the-treatment-of-patients-with-multiple-myeloma (2022).
FDA. FDA approves teclistamab-cqyv for relapsed or refractory multiple myeloma | FDA. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-teclistamab-cqyv-relapsed-or-refractory-multiple-myeloma (2022).
Usmani, S. Z. et al. Teclistamab, a B-cell maturation antigen × CD3 bispecific antibody, in patients with relapsed or refractory multiple myeloma (MajesTEC-1): a multicentre, open-label, single-arm, phase 1 study. Lancet 398, 665–674 (2021).
Moreau, P. et al. Teclistamab in relapsed or refractory multiple myeloma. N. Engl. J. Med. 387, 495–505 (2022).
Nathan, P. et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N. Engl. J. Med. 385, 1196–1206 (2021).
Hutchings, M. et al. Dose escalation of subcutaneous epcoritamab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an open-label, phase 1/2 study. Lancet 398, 1157–1169 (2021).
Bacac, M. et al. CD20-TCB with obinutuzumab pretreatment as next-generation treatment of hematologic malignancies. Clin. Cancer Res. 24, 4785–4797 (2018).
Hutchings, M. et al. Glofitamab, a novel, bivalent CD20-targeting T-cell–engaging bispecific antibody, induces durable complete remissions in relapsed or refractory B-cell lymphoma: a phase I trial. J. Clin. Oncol. 39, 1959–1970 (2021).
Bahlis, N. J. et al. Efficacy and safety of elranatamab (PF-06863135), a B-cell maturation antigen (BCMA)-CD3 bispecific antibody, in patients with relapsed or refractory multiple myeloma (MM). J. Clin. Oncol. 39, 8006–8006 (2021).
Wu, L. et al. Trispecific antibodies enhance the therapeutic efficacy of tumor-directed T cells through T cell receptor co-stimulation. Nat. Cancer 1, 86–98 (2020).
Austin, R. J. et al. TriTACs, a novel class of T-cell–engaging protein constructs designed for the treatment of solid tumors. Mol. Cancer Ther. 20, 109–120 (2021).
Rothe, A. et al. A phase 1 study of the bispecific anti-CD30/CD16A antibody construct AFM13 in patients with relapsed or refractory Hodgkin lymphoma. Blood 125, 4024–4031 (2015).
Sasse, S. et al. AFM13 in patients with relapsed or refractory classical Hodgkin lymphoma: final results of an open-label, randomized, multicenter phase II trial. Leuk. Lymphoma 63, 1871–1878 (2022).
Bartlett, N. L. et al. A phase 1b study of AFM13 in combination with pembrolizumab in patients with relapsed or refractory Hodgkin lymphoma. Blood 136, 2401–2409 (2020).
Codarri Deak, L. et al. PD-1-cis IL-2R agonism yields better effectors from stem-like CD8 + T cells. Nature 610, 161–172 (2022).
Hinner, M. J. et al. Tumor-localized costimulatory T-cell engagement by the 4-1BB/HER2 bispecific antibody-anticalin fusion PRS-343. Clin. Cancer Res. 25, 5878–5889 (2019).
Muik, A. et al. DuoBody-CD40x4-1BB induces dendritic-cell maturation and enhances T-cell activation through conditional CD40 and 4-1BB agonist activity. J. Immunother. Cancer 10, e004322 (2022).
Qiao, Y. et al. Cancer immune therapy with PD-1-dependent CD137 co-stimulation provides localized tumour killing without systemic toxicity. Nat. Commun. 12, 6360 (2021).
Geuijen, C. et al. A human CD137×PD-L1 bispecific antibody promotes anti-tumor immunity via context-dependent T cell costimulation and checkpoint blockade. Nat. Commun. 12, 4445 (2021).
Chan, S. et al. An anti-PD-1–GITR-L bispecific agonist induces GITR clustering-mediated T cell activation for cancer immunotherapy. Nat. Cancer 3, 337–354 (2022).
Kraman, M. et al. FS118, a bispecific antibody targeting lag-3 and PD-L1, enhances T-cell activation resulting in potent antitumor activity. Clin. Cancer Res. 26, 3333–3344 (2020).
Dovedi, S. J. et al. Design and efficacy of a monovalent bispecific PD-1/CTLA4 antibody that enhances CTLA4 blockade on PD-1+ activated T cells. Cancer Discov. 11, 1100–1117 (2021).
Martomo, S. A. et al. Single-dose anti–PD-L1/IL-15 fusion protein KD033 generates synergistic antitumor immunity with robust tumor-immune gene signatures and memory responses. Mol. Cancer Ther. 20, 347–356 (2021).
Ji, J. et al. A phase Ib/II, multicenter, open-label study of AK104, a PD-1/CTLA-4 bispecific antibody, combined with chemotherapy (chemo) as first-line therapy for advanced gastric (G) or gastroesophageal junction (GEJ) cancer. J. Clin. Oncol. 40, 308–308 (2022).
Wu, L. et al. 1300 P A phase Ib/II trial of AK104 (PD-1/CTLA-4 bispecific antibody) in combination with anlotinib in advanced NSCLC. Ann. Oncol. 32, S1006 (2021).
Yang, Y. et al. A phase 2, open-label, multicenter study to evaluate the efficacy, safety, and tolerability of KN046 in combination with chemotherapy in subjects with advanced non-small cell lung cancer. J. Clin. Oncol. 39, 9060–9060 (2021).
Xu, J. et al. Efficacy and safety of KN046 plus paclitaxel/cisplatin as first-line treatment for unresectable locally advanced, recurrent or metastatic esophageal squamous cell carcinoma (ESCC). J. Clin. Oncol. 39, 4062–4062 (2021).
**, G. et al. Efficacy and safety of KN046 plus nab-paclitaxel/gemcitabine as first-line treatment for unresectable locally advanced or metastatic pancreatic ductal adenocarcinoma (PDAC). J. Clin. Oncol. 39, 4138–4138 (2021).
**ng, B. 938 P KN046 (an anti-PD-L1/CTLA-4 bispecific antibody) in combination with lenvatinib in the treatment for advanced unresectable or metastatic hepatocellular carcinoma (HCC): preliminary efficacy and safety results of a prospective phase II trial. Ann. Oncol. 32, S822 (2021).
Liu, D. et al. Phase 1 study of SHR-1701, a bifunctional fusion protein targeting PD-L1 and TGF-β, in patients with advanced solid tumors. J. Clin. Oncol. 39, 2503–2503 (2021).
Shi, M. et al. SHR-1701, a bifunctional fusion protein targeting PD-L1 and TGF-β, for advanced NSCLC with EGFR mutations: Data from a multicenter phase 1 study. J. Clin. Oncol. 39, 9055–9055 (2021).
Liu, D. et al. 1375 P SHR-1701, a novel bifunctional anti-PD-L1/TGF-βRII agent, for pretreated recurrent/refractory (r/r) gastric cancer (GC): Data from a first-in-human phase I study. Ann. Oncol. 32, S1042 (2021).
Feng, J. et al. 1278 P SHR-1701, a bifunctional fusion protein targeting PD-L1 and TGF-β, as first-line therapy for PD-L1+ advanced/metastatic NSCLC: Data from a clinical expansion cohort of a phase I study. Ann. Oncol. 32, S995 (2021).
Merck. Merck announces update on the INTR@PID clinical program including Lung 037 study. https://www.merckgroup.com/en/news/bintrafusp-alfa-037-update-20-01-2021.html (2021).
Zhao, Y. et al. A phase II study of AK112 (PD-1/VEGF bispecific) in combination with chemotherapy in patients with advanced non-small cell lung cancer. J. Clin. Oncol. 40, 9019–9019 (2022).
Bates, S. E. Epigenetic therapies for cancer. N. Engl. J. Med. 383, 650–663 (2020).
Cheng, Y. et al. Targeting epigenetic regulators for cancer therapy: mechanisms and advances in clinical trials. Signal Transduct. Target. Ther. 4, 62 (2019).
Dao, F.-Y., Lv, H., Fullwood, M. J. & Lin, H. Accurate identification of DNA replication origin by fusing epigenomics and chromatin interaction information. Research 2022, 9780293 (2022).
Li, X., Ma, S., Deng, Y., Yi, P. & Yu, J. Targeting the RNA m6A modification for cancer immunotherapy. Mol. Cancer 21, 76 (2022).
Shulman, Z. & Stern-Ginossar, N. The RNA modification N6-methyladenosine as a novel regulator of the immune system. Nat. Immunol. 21, 501–512 (2020).
Cao, X. et al. m6A methylation: a process resha** the tumour immune microenvironment and regulating immune evasion. Mol. Cancer 22, 42 (2023).
Li, T. et al. Methionine deficiency facilitates antitumour immunity by altering m6A methylation of immune checkpoint transcripts. Gut 72, 501–511 (2023).
Han, D. et al. Anti-tumour immunity controlled through mRNA m6A methylation and YTHDF1 in dendritic cells. Nature 566, 270–274 (2019).
Yang, S. et al. m6A mRNA demethylase FTO regulates melanoma tumorigenicity and response to anti-PD-1 blockade. Nat. Commun. 10, 2782 (2019).
Chen, H. et al. METTL3 inhibits antitumor immunity by targeting m6A-BHLHE41-CXCL1/CXCR2 axis to promote colorectal cancer. Gastroenterology 163, 891–907 (2022).
Li, N. et al. ALKBH5 regulates anti–PD-1 therapy response by modulating lactate and suppressive immune cell accumulation in tumor microenvironment. Proc. Natl Acad. Sci. USA 117, 20159–20170 (2020).
Deng, L. J. et al. m6A modification: recent advances, anticancer targeted drug discovery and beyond. Mol. Cancer 21, 52 (2022).
Saleh, M. H., Wang, L. & Goldberg, M. S. Improving cancer immunotherapy with DNA methyltransferase inhibitors. Cancer Immunol. Immunother. 65, 787–796 (2016).
Jones, P. A., Ohtani, H., Chakravarthy, A. & De Carvalho, D. D. Epigenetic therapy in immune-oncology. Nat. Rev. Cancer 19, 151–161 (2019).
Sasidharan Nair, V. et al. DNA methylation and repressive H3K9 and H3K27 trimethylation in the promoter regions of PD-1, CTLA-4, TIM-3, LAG-3, TIGIT, and PD-L1 genes in human primary breast cancer. Clin. Epigenetics 10, 78 (2018).
Chi, Z., Lu, Y., Yang, Y., Li, B. & Lu, P. Transcriptional and epigenetic regulation of PD-1 expression. Cell. Mol. Life Sci. 78, 3239–3246 (2021).
Micevic, G., Thakral, D., McGeary, M. & Bosenberg, M. W. PD‐L1 methylation regulates PD‐L1 expression and is associated with melanoma survival. Pigment Cell Melanoma Res. 32, 435–440 (2019).
Wathikthinnakon, M. et al. Combination gemcitabine and PD-L1xCD3 bispecific T cell engager (BiTE) enhances T lymphocyte cytotoxicity against cholangiocarcinoma cells. Sci. Rep. 12, 6154 (2022).
Chou, F.-C., Kuo, C.-C., Chen, H.-Y., Chen, H.-H. & Sytwu, H.-K. DNA demethylation of the TIM-3 promoter is critical for its stable expression on T cells. Genes Immun. 17, 179–186 (2016).
Zhang, L. et al. SUV39H1-DNMT3A-mediated epigenetic regulation of Tim-3 and galectin-9 in the cervical cancer. Cancer Cell Int. 20, 325 (2020).
Hoffmann, F. et al. CTLA4 DNA methylation is associated with CTLA-4 expression and predicts response to immunotherapy in head and neck squamous cell carcinoma. Clin. Epigenetics 15, 112 (2023).
Niebel, D. et al. DNA methylation regulates TIGIT expression within the melanoma microenvironment, is prognostic for overall survival, and predicts progression-free survival in patients treated with anti-PD-1 immunotherapy. Clin. Epigenetics 14, 50 (2022).
Corley, M. J., Chew, G., Pang, A. P. & Ndhlovu, L. DNA methylation tightly regulates TIGIT expression in CD8 + T cells during chronic HIV infection. J. Immunol. 204, 95.19–95.19 (2020).
Wang, C. et al. Efficacy of decitabine plus anti-PD-1 camrelizumab in patients with Hodgkin lymphoma who progressed or relapsed after PD-1 blockade monotherapy. Clin. Cancer Res. 27, 2782–2791 (2021).
Nie, J. et al. Addition of low-dose decitabine to anti–PD-1 antibody camrelizumab in relapsed/refractory classical Hodgkin lymphoma. J. Clin. Oncol. 37, 1479–1489 (2019).
Goswami, M. et al. Pembrolizumab and decitabine for refractory or relapsed acute myeloid leukemia. J. Immunother. Cancer 10, e003392 (2022).
Brunner, A. M. et al. Efficacy and safety of sabatolimab (MBG453) in combination with hypomethylating agents (HMAs) in patients (Pts) with very high/high-risk myelodysplastic syndrome (vHR/HR-MDS) and acute myeloid leukemia (AML): final analysis from a phase Ib study. Blood 138, 244–244 (2021).
Sallman, D. A. et al. Magrolimab in combination with azacitidine in patients with higher-risk myelodysplastic syndromes: final results of a phase Ib study. J. Clin. Oncol. 41, 2815–2826 (2023).
Miao, M. et al. A phase 1b study to evaluate safety and efficacy of IBI188 in combination with azacitidine (AZA) as a first-line treatment in subjects with newly diagnosed higher risk myelodysplastic syndrome. Blood 140, 4045–4046 (2022).
Wong, K. K., Hassan, R. & Yaacob, N. S. Hypomethylating agents and immunotherapy: therapeutic synergism in acute myeloid leukemia and myelodysplastic syndromes. Front. Oncol. 11, 624742 (2021).
Wang, Y. et al. Low-dose decitabine priming endows CAR T cells with enhanced and persistent antitumour potential via epigenetic reprogramming. Nat. Commun. 12, 409 (2021).
Amaro, A. et al. Guadecitabine increases response to combined anti-CTLA-4 and anti-PD-1 treatment in mouse melanoma in vivo by controlling T-cells, myeloid derived suppressor and NK cells. J. Exp. Clin. Cancer Res. 42, 67 (2023).
Papadatos-Pastos, D. et al. Phase 1, dose-escalation study of guadecitabine (SGI-110) in combination with pembrolizumab in patients with solid tumors. J. Immunother. Cancer 10, e004495 (2022).
Jang, H. J. et al. A phase II trial of guadecitabine plus atezolizumab in metastatic urothelial carcinoma progressing after initial immune checkpoint inhibitor therapy. Clin. Cancer Res. 29, 2052–2065 (2023).
Matei, D. et al. Phase II trial of guadecitabine priming and pembrolizumab in platinum resistant recurrent ovarian cancer. J. Clin. Oncol. 38, 6025–6025 (2020).
Algaze, S. et al. A phase Ib study of guadecitabine and durvalumab in patients with advanced hepatocellular carcinoma, pancreatic adenocarcinoma, and biliary cancers. J. Clin. Oncol. 40, 574–574 (2022).
Kuang, C. et al. Pembrolizumab plus azacitidine in patients with chemotherapy refractory metastatic colorectal cancer: a single-arm phase 2 trial and correlative biomarker analysis. Clin. Epigenetics 14, 3 (2022).
Safyan, R. A. et al. Phase 2 study of azacitidine (AZA) plus pembrolizumab (pembro) as second-line treatment in patients with advanced pancreatic ductal adenocarcinoma. J. Clin. Oncol. 40, 4158–4158 (2022).
Levy, B. P. et al. Randomised phase 2 study of pembrolizumab plus CC-486 versus pembrolizumab plus placebo in patients with previously treated advanced non-small cell lung cancer. Eur. J. Cancer 108, 120–128 (2019).
Taylor, K. et al. An open-label, phase II multicohort study of an oral hypomethylating agent CC-486 and durvalumab in advanced solid tumors. J. Immunother. Cancer 8, e000883 (2020).
Di Giacomo, A. M. et al. Safety and immunobiological activity of guadecitabine sequenced with ipilimumab in metastatic melanoma patients: the phase Ib NIBIT-M4 study. J. Clin. Oncol. 37, 2549–2549 (2019).
Chen, S. et al. Epigenetic priming enhances antitumor immunity in platinum-resistant ovarian cancer. J. Clin. Invest. 132, e158800 (2022).
Pappalardi, M. B. et al. Discovery of a first-in-class reversible DNMT1-selective inhibitor with improved tolerability and efficacy in acute myeloid leukemia. Nat. Cancer 2, 1002–1017 (2021).
Mehdipour, P., Chen, R. & De Carvalho, D. D. The next generation of DNMT inhibitors. Nat. Cancer 2, 1000–1001 (2021).
**e, T. et al. Insight into the selective binding mechanism of DNMT1 and DNMT3A inhibitors: a molecular simulation study. Phys. Chem. Chem. Phys. 21, 12931–12947 (2019).
Khan, A. N. H., Gregorie, C. J. & Tomasi, T. B. Histone deacetylase inhibitors induce TAP, LMP, Tapasin genes and MHC class I antigen presentation by melanoma cells. Cancer Immunol. Immunother. 57, 647–654 (2008).
Nguyen, A. et al. HDACi delivery reprograms tumor-infiltrating myeloid cells to eliminate antigen-loss variants. Cell Rep. 24, 642–654 (2018).
Li, X. et al. HDAC inhibition potentiates anti-tumor activity of macrophages and enhances anti-PD-L1-mediated tumor suppression. Oncogene 40, 1836–1850 (2021).
Wang, H.-F. et al. Histone deacetylase inhibitors deplete myeloid-derived suppressor cells induced by 4T1 mammary tumors in vivo and in vitro. Cancer Immunol. Immunother. 66, 355–366 (2017).
Mantovani, A., Marchesi, F., Malesci, A., Laghi, L. & Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 14, 399–416 (2017).
Ny, L. et al. The PEMDAC phase 2 study of pembrolizumab and entinostat in patients with metastatic uveal melanoma. Nat. Commun. 12, 5155 (2021).
O’Shaughnessy, J. et al. Results of ENCORE 602 (TRIO025), a phase II, randomized, placebo-controlled, double-blinded, multicenter study of atezolizumab with or without entinostat in patients with advanced triple-negative breast cancer (aTNBC). J. Clin. Oncol. 38, 1014 (2020).
Cadoo, K. A. et al. A phase II randomized study of avelumab plus entinostat versus avelumab plus placebo in patients (pts) with advanced epithelial ovarian cancer (EOC). J. Clin. Oncol. 37, 5511 (2019).
Tsimberidou, A. M. et al. Preclinical development and first-in-human study of KA2507, a selective and potent inhibitor of histone deacetylase 6, for patients with refractory solid tumors. Clin. Cancer Res. 27, 3584–3594 (2021).
Awad, M. M. et al. Selective histone deacetylase inhibitor ACY-241 (Citarinostat) plus nivolumab in advanced non-small cell lung cancer: results from a phase Ib study. Front. Oncol. 11, 3156 (2021).
Hashimoto, A., Fukumoto, T., Zhang, R. & Gabrilovich, D. Selective targeting of different populations of myeloid-derived suppressor cells by histone deacetylase inhibitors. Cancer Immunol. Immunother. 69, 1929–1936 (2020).
Hassel, J. C. et al. 1370 P—Phase Ib/II study (SENSITIZE) assessing safety, pharmacokinetics (PK), pharmacodynamics (PD), and clinical outcome of domatinostat in combination with pembrolizumab in patients with advanced melanoma refractory/non-responding to prior checkpoint inhibitor therapy. Ann. Oncol. 30, v559 (2019).
Reijers, I. L. M. et al. P01.15 Personalized combination of neoadjuvant domatinostat, nivolumab (NIVO) and ipilimumab (IPI) in macroscopic stage III melanoma patients stratified according to interferon-gamma (IFN-gamma) signature—the DONIMI study. J. Immunother. Cancer 8, A15–A16 (2020).
Bretz, A. C. et al. Domatinostat favors the immunotherapy response by modulating the tumor immune microenvironment (TIME). J. Immunother. Cancer 7, 294 (2019).
Rodriguez, C. P. et al. A phase II trial of pembrolizumab and vorinostat in recurrent metastatic head and neck squamous cell carcinomas and salivary gland cancer. Clin. Cancer Res. 26, 837–845 (2020).
Saltos, A. N. et al. Phase II randomized trial of first-line pembrolizumab and vorinostat in patients with metastatic NSCLC (mNSCLC). J. Clin. Oncol. 38, 9567 (2020).
Peterson, L. M. et al. (18)F-Fluoroestradiol PET imaging in a phase II trial of vorinostat to restore endocrine sensitivity in ER + /HER2- metastatic breast cancer. J. Nucl. Med. 62, 184–190 (2021).
Kilgour, J. M. et al. Phase II open-label, single-arm trial to investigate the efficacy and safety of topical remetinostat gel in patients with basal cell carcinoma. Clin. Cancer Res. 27, 4717–4725 (2021).
Ueno, M. et al. A randomized, double-blind, phase II study of oral histone deacetylase inhibitor resminostat plus S-1 versus placebo plus S-1 in biliary tract cancers previously treated with gemcitabine plus platinum-based chemotherapy. Cancer Med. 10, 2088–2099 (2021).
Tak, W. Y. et al. Phase I/II study of first-line combination therapy with sorafenib plus resminostat, an oral HDAC inhibitor, versus sorafenib monotherapy for advanced hepatocellular carcinoma in east Asian patients. Invest. N. Drugs 36, 1072–1084 (2018).
San-Miguel, J. F. et al. Overall survival of patients with relapsed multiple myeloma treated with panobinostat or placebo plus bortezomib and dexamethasone (the PANORAMA 1 trial): a randomised, placebo-controlled, phase 3 trial. Lancet Haematol. 3, e506–e515 (2016).
San-Miguel, J. F. et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 15, 1195–1206 (2014).
San-Miguel, J. F. et al. Panobinostat plus bortezomib and dexamethasone: impact of dose intensity and administration frequency on safety in the PANORAMA 1 trial. Br. J. Haematol. 179, 66–74 (2017).
Laubach, J. P. et al. Efficacy and safety of oral panobinostat plus subcutaneous bortezomib and oral dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma (PANORAMA 3): an open-label, randomised, phase 2 study. Lancet Oncol. 22, 142–154 (2021).
Aggarwal, R. R. et al. Exceptional responders to abexinostat (ABX) plus pazopanib (PAZ) in pretreated renal cell carcinoma (RCC) and other solid tumors: Long-term follow-up of a phase 1b study. J. Clin. Oncol. 37, 3022 (2019).
Tjulandin, S. et al. A multicenter phase II study of the efficacy and safety of quisinostat (an HDAC inhibitor) in combination with paclitaxel and carboplatin chemotherapy (CT) in patients (pts) with recurrent platinum resistant high grade serous epithelial ovarian, primarily peritoneal or fallopian tube carcinoma cancer (OC). J. Clin. Oncol. 35, 5541 (2017).
Hajmirza, A. et al. BET family protein BRD4: an emerging actor in NFκB signaling in inflammation and cancer. Biomedicines 6, 16 (2018).
Ozer, H. G. et al. BRD4 profiling identifies critical chronic lymphocytic leukemia oncogenic circuits and reveals sensitivity to PLX51107, a novel structurally distinct BET inhibitor. Cancer Discov. 8, 458–477 (2018).
Mao, W. et al. Immunogenicity of prostate cancer is augmented by BET bromodomain inhibition. J. Immunother. Cancer 7, 277 (2019).
Li, X. et al. BRD4 inhibition by AZD5153 promotes antitumor immunity via depolarizing M2 macrophages. Front. Immunol. 11, 89 (2020).
Zhu, H. et al. BET bromodomain inhibition promotes anti-tumor immunity by suppressing PD-L1 expression. Cell Rep. 16, 2829–2837 (2016).
Li, W. et al. Targeting MYC activity in double-hit lymphoma with MYC and BCL2 and/or BCL6 rearrangements with epigenetic bromodomain inhibitors. J. Hematol. Oncol. 12, 73 (2019).
Tasdemir, N. et al. BRD4 connects enhancer remodeling to senescence immune surveillance. Cancer Discov. 6, 612–629 (2016).
Lheureux, S. et al. Abstract LB104: dose-finding/expansion phase Ib study to evaluate the safety and activity of BET inhibitor RO6870810 (RO) and atezolizumab (A) in patients (pts) with advanced ovarian cancer (OC) or triple negative breast cancer (TNBC). Cancer Res. 81, LB104–LB104 (2021).
Shapiro, G. I. et al. A phase 1 study of RO6870810, a novel bromodomain and extra-terminal protein inhibitor, in patients with NUT carcinoma, other solid tumours, or diffuse large B-cell lymphoma. Br. J. Cancer 124, 744–753 (2020).
Hilton, J. et al. BMS-986158, a small molecule inhibitor of the bromodomain and extraterminal domain proteins, in patients with selected advanced solid tumors: results from a phase 1/2a trial. Cancers 14, 4079 (2022).
Kuang, C., Cai, M., Risnik, D., Giles, F. & Zhang, L. Abstract 1763: NEO2734, a novel dual bromodomain and histone acetyltransferase inhibitor, in the treatment of colorectal cancer. Cancer Res. 80, 1763 (2020).
Giles, F., Witcher, M. & Brown, B. NEO2734: a novel potent oral dual BET and P300/CBP inhibitor. Ann. Oncol. 29, viii140–viii141 (2018).
Wang, N., Wu, R., Tang, D. & Kang, R. The BET family in immunity and disease. Signal Transduct. Target. Ther. 6, 23 (2021).
Eich, M.-L., Athar, M., Ferguson, J. E. & Varambally, S. EZH2-targeted therapies in cancer: hype or a reality. Cancer Res. 80, 5449–5458 (2020).
Zhou, Z. et al. An organoid-based screen for epigenetic inhibitors that stimulate antigen presentation and potentiate T-cell-mediated cytotoxicity. Nat. Biomed. Eng. 5, 1320–1335 (2021).
Peng, D. et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 527, 249–253 (2015).
Nagarsheth, N. et al. PRC2 epigenetically silences Th1-type chemokines to suppress effector T-cell trafficking in colon cancer. Cancer Res. 76, 275–282 (2016).
Kim, H.-J., Cantor, H. & Cosmopoulos, K. Overcoming immune checkpoint blockade resistance via EZH2 inhibition. Trends Immunol. 41, 948–963 (2020).
Gounder, M. et al. Tazemetostat in advanced epithelioid sarcoma with loss of INI1/SMARCB1: an international, open-label, phase 2 basket study. Lancet Oncol. 21, 1423–1432 (2020).
Chammas, P., Mocavini, I. & di Croce, L. Engaging chromatin: PRC2 structure meets function. Br. J. Cancer 122, 315–328 (2020).
Kouznetsova, V. L., Tchekanov, A., Li, X., Yan, X. & Tsigelny, I. F. Polycomb repressive 2 complex—molecular mechanisms of function. Protein Sci. 28, 1387–1399 (2019).
Burr, M. L. et al. An evolutionarily conserved function of polycomb silences the MHC class I antigen presentation pathway and enables immune evasion in cancer. Cancer Cell 36, 385–401.e8 (2019).
Honma, D. et al. DS-3201, a potent EZH1/2 dual inhibitor, demonstrates antitumor activity against non-Hodgkin lymphoma (NHL) regardless of EZH2 mutation. Blood 132, 2217–2217 (2018).
Jung, S. H. et al. Abstract 1142: A novel and potent EZH1/2 dual inhibitor, HM97662, demonstrates antitumor activity in malignant tumors. Cancer Res. 81, 1142–1142 (2021).
Daemen, A. et al. Abstract 1131: ORIC-944, a potent and selective allosteric PRC2 inhibitor, demonstrates robust in vivo activity in prostate cancer models. Cancer Res. 81, 1131–1131 (2021).
Yuan, H. et al. SETD2 restricts prostate cancer metastasis by integrating EZH2 and AMPK signaling pathways. Cancer Cell 38, 350–365.e7 (2020).
Condamine, T. et al. Abstract 4635: The LSD1 specific inhibitor INCB059872 enhances the activity of immune checkpoint blockade by resha** the myeloid compartment in the syngeneic 4T1 mouse mammary tumor model. Cancer Res. 77, 4635 (2017).
Barth, J. et al. Correction: LSD1 inhibition by tranylcypromine derivatives interferes with GFI1-mediated repression of PU.1 target genes and induces differentiation in AML. Leukemia 33, 1541–1541 (2019).
Liu, Y. et al. LSD1 inhibition sustains T cell invigoration with a durable response to PD-1 blockade. Nat. Commun. 12, 6831 (2021).
Blank, C. U. et al. Defining ‘T cell exhaustion’. Nat. Rev. Immunol. 19, 665–674 (2019).
Sheng, W. et al. LSD1 ablation stimulates anti-tumor immunity and enables checkpoint blockade. Cell 174, 549–563.e19 (2018).
Shen, D.-D. et al. LSD1 deletion decreases exosomal PD-L1 and restores T-cell response in gastric cancer. Mol. Cancer 21, 75 (2022).
Watts, J. M. et al. The lysine-specific demethylase 1 (LSD1) inhibitor tranylcypromine (TCP) in combination with ATRA is tolerable and has anti-leukemic activity in adult patients with relapsed/refractory AML and MDS. Blood 132, 2721 (2018).
Ropacki, M. et al. P2.12-04 CLEPSIDRA: a phase II trial combining iadademstat with platinum-etoposide in platinum-sensitive relapsed SCLC patients. J. Thorac. Oncol. 14, S813 (2019).
Dai, X. J. et al. Reversible lysine specific demethylase 1 (LSD1) Inhibitors: a promising wrench to impair LSD1. J. Med. Chem. 64, 2466–2488 (2021).
Yoo, S. et al. Abstract 2128: TACH101, a first-in-class pan inhibitor of KDM4 histone lysine demethylases. Cancer Res. 81, 2128 (2021).
Chandhasin, C. et al. Inhibition of histone lysine demethylases with TACH101, a first-in-class pan-inhibitor of KDM4. J. Clin. Oncol. 39, 3105 (2021).
Soldi, R. et al. The novel reversible LSD1 inhibitor SP-2577 promotes anti-tumor immunity in SWItch/Sucrose-NonFermentable (SWI/SNF) complex mutated ovarian cancer. PLoS ONE 15, e0235705 (2020).
Aix, S. P. et al. 50 P A phase Ib study of CC-90011, a potent, reversible, oral LSD1 inhibitor, plus etoposide and cisplatin (EP) or carboplatin (EC) in patients (Pts) with first-line (1 L) extensive-stage (ES) small cell lung cancer (SCLC): Updated results. J. Thorac. Oncol. 16, S722–S723 (2021).
Tu, W. J. et al. Targeting nuclear LSD1 to reprogram cancer cells and reinvigorate exhausted T cells via a novel LSD1-EOMES switch. Front. Immunol. 11, 1228 (2020).
Chawla, S. P. et al. Preliminary efficacy from an ongoing phase 1 dose escalation study of seclidemstat (SP-2577) in patients (pts) with advanced solid tumors (AST). J. Clin. Oncol. 39, 3073 (2021).
**ng, S. et al. Tcf1 and Lef1 transcription factors establish CD8 + T cell identity through intrinsic HDAC activity. Nat. Immunol. 17, 695–703 (2016).
Siddiqui, I. et al. Intratumoral Tcf1+PD-1 + CD8 + T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity 50, 195–211.e10 (2019).
Siu, L. L. et al. 438 O—METEOR-1: a phase I study of GSK3326595, a first-in-class protein arginine methyltransferase 5 (PRMT5) inhibitor, in advanced solid tumours. Ann. Oncol. 30, v159 (2019).
Kim, H. et al. PRMT5 control of cGAS/STING and NLRC5 pathways defines melanoma response to antitumor immunity. Sci. Transl. Med. 12, eaaz5683 (2020).
Webb, L. M. et al. Protein arginine methyltransferase 5 promotes cholesterol biosynthesis-mediated Th17 responses and autoimmunity. J. Clin. Invest. 130, 1683–1698 (2020).
Strobl, C. D. et al. Selective PRMT5 inhibitors suppress human CD8 + T Cells by Upregulation of p53 and impairment of the AKT pathway similar to the tumor metabolite MTA. Mol. Cancer Ther. 19, 409–419 (2020).
Jiang, Y. et al. PRMT5 disruption drives antitumor immunity in cervical cancer by reprogramming T cell-mediated response and regulating PD-L1 expression. Theranostics 11, 9162–9176 (2021).
Hu, R. et al. PRMT5 inhibition promotes PD-L1 expression and immuno-resistance in lung cancer. Front. Immunol. 12, 722188 (2022).
Cao, J. & Yan, Q. Cancer epigenetics, tumor immunity, and immunotherapy. Trends Cancer 6, 580–592 (2020).
Yang, J. et al. Epigenetic regulation in the tumor microenvironment: molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 8, 210 (2023).
Mo, F. et al. An engineered IL-2 partial agonist promotes CD8( + ) T cell stemness. Nature 597, 544–548 (2021).
Pipkin, M. E. et al. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity 32, 79–90 (2010).
Janas, M. L., Groves, P., Kienzle, N. & Kelso, A. IL-2 regulates perforin and granzyme gene expression in CD8 + T cells independently of its effects on survival and proliferation. J. Immunol. 175, 8003–8010 (2005).
Fyfe, G. et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J. Clin. Oncol. 13, 688–696 (1995).
Fontenot, J. D., Rasmussen, J. P., Gavin, M. A. & Rudensky, A. Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 6, 1142–1151 (2005).
Krieg, C., Létourneau, S., Pantaleo, G. & Boyman, O. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc. Natl Acad. Sci. USA 107, 11906–11911 (2010).
Diab, A. et al. Bempegaldesleukin (NKTR-214) plus Nivolumab in Patients with Advanced Solid Tumors: Phase I Dose-Escalation Study of Safety, Efficacy, and Immune Activation (PIVOT-02). Cancer Discov. 10, 1158–1173 (2020).
Diab, A. et al. PIVOT IO 001: First disclosure of efficacy and safety of bempegaldesleukin (BEMPEG) plus nivolumab (NIVO) vs NIVO monotherapy in advanced melanoma… | OncologyPRO. https://oncologypro.esmo.org/meeting-resources/esmo-congress/pivot-io-001-first-disclosure-of-efficacy-and-safety-of-bempegaldesleukin-bempeg-plus-nivolumab-nivo-vs-nivo-monotherapy-in-advanced-melanoma (2022).
Tannir, N. et al. LBA68 Bempegaldesleukin (BEMPEG) plus nivolumab (NIVO) compared to the investigator’s choice of sunitinib or cabozantinib in previously untreated advanced renal cell carcinoma (RCC): results from a phase III randomized study (PIVOT-09). Ann. Oncol. 33, S1433 (2022).
Bristol Myers Squibb. Bristol Myers Squibb—Nektar and Bristol Myers Squibb announce update on clinical development program for Bempegaldesleukin (BEMPEG) in combination with Opdivo (nivolumab). https://news.bms.com/news/corporate-financial/2022/Nektar-and-Bristol-Myers-Squibb-Announce-Update-on-Clinical-Development-Program-for-Bempegaldesleukin-BEMPEG-in-Combination-with-Opdivo-nivolumab/default.aspx (2022).
Liu, Y. et al. IL-2 regulates tumor-reactive CD8( + ) T cell exhaustion by activating the aryl hydrocarbon receptor. Nat. Immunol. 22, 358–369 (2021).
Onyshchenko, K. et al. Expansion of circulating stem-like CD8 + T cells by adding CD122-directed IL-2 complexes to radiation and anti-PD1 therapies in mice. Nat. Commun. 14, 2087 (2023).
Hashimoto, M. et al. PD-1 combination therapy with IL-2 modifies CD8 + T cell exhaustion program. Nature 610, 173–181 (2022).
Emmerich, J. et al. Abstract 1744: STK-012, an alpha/beta selective IL-2 mutein for the activation of the antigen-activated T cells in solid tumor. Cancer Res. 81, 1744 (2021).
Boni, V. et al. ARTISTRY-1: Nemvaleukin alfa monotherapy and in combination with pembrolizumab in patients (pts) with advanced solid tumors. J. Clin. Oncol. 39, 2513 (2021).
Hamid, O. et al. Selection of the recommended phase 2 dose (RP2D) for subcutaneous nemvaleukin alfa: ARTISTRY-2. J. Clin. Oncol. 39, 2552 (2021).
Bempeg failure unlikely to affect other IL2 Drugs. Cancer Discov. 12, 1604–1605 (2022).
Kohli, K. et al. Abstract 607: IL-15 is the most potent of tested gamma chain cytokines at inducing in situ proliferation of T cells in human pancreatic cancer. Cancer Res. 81, 607 (2021).
Wrangle, J. M. et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. Lancet Oncol. 19, 694–704 (2018).
Wrangle, J. M. et al. Preliminary data from QUILT 3.055: a phase 2 multi-cohort study of N803 (IL-15 superagonist) in combination with checkpoint inhibitors (CPI). J. Clin. Oncol. 39, 2596 (2021).
Waldhauer, I. et al. Simlukafusp alfa (FAP-IL2v) immunocytokine is a versatile combination partner for cancer immunotherapy. MAbs 13, 1913791 (2021).
Klein, C. et al. Cergutuzumab amunaleukin (CEA-IL2v), a CEA-targeted IL-2 variant-based immunocytokine for combination cancer immunotherapy: overcoming limitations of aldesleukin and conventional IL-2-based immunocytokines. Oncoimmunology 6, e1277306 (2017).
Bishop, J. L. et al. Abstract 1788: Increasing the therapeutic index of IL12 by engineering for tumor specific protease activation. Cancer Res. 81, 1788 (2021).
Steiner, P. et al. Abstract 1716: WTX-330, a conditionally activated IL-12 INDUKINE, releases IL-12 selectively in the tumor microenvironment to activate anti-tumor immune responses and induce regressions in mouse tumor models. Cancer Res. 81, 1716 (2021).
Italiano, A. et al. Clinical activity and safety of simlukafusp alfa, an engineered interleukin-2 variant targeted to fibroblast activation protein-α, combined with atezolizumab in patients with recurrent or metastatic cervical cancer. J. Clin. Oncol. 39, 5510–5510 (2021).
O’Neil, J. et al. Tumor-selective activity of XTX202, a protein-engineered IL-2, in mice without peripheral toxicities in nonhuman primates. J. Clin. Oncol. 39, 2563–2563 (2021).
Mirlekar, B. & Pylayeva-Gupta, Y. IL-12 family cytokines in cancer and immunotherapy. Cancers 13, 1–23 (2021).
Algazi, A. et al. Intratumoral delivery of tavokinogene telseplasmid yields systemic immune responses in metastatic melanoma patients. Ann. Oncol. 31, 532–540 (2020).
Algazi, A. P. et al. Phase II trial of IL-12 plasmid transfection and PD-1 blockade in immunologically quiescent melanoma. Clin. Cancer Res. 26, 2827–2837 (2020).
Hotz, C. et al. Local delivery of mRNA-encoded cytokines promotes antitumor immunity and tumor eradication across multiple preclinical tumor models. Sci. Transl. Med. 13, eabc7804 (2021).
Li, Y. et al. Multifunctional oncolytic nanoparticles deliver self-replicating IL-12 RNA to eliminate established tumors and prime systemic immunity. Nat. Cancer 1, 882–893 (2020).
Barrett, J. A. et al. Regulated intratumoral expression of IL-12 using a RheoSwitch Therapeutic System® (RTS®) gene switch as gene therapy for the treatment of glioma. Cancer Gene Ther. 25, 106–116 (2018).
Briukhovetska, D. et al. Interleukins in cancer: from biology to therapy. Nat. Rev. Cancer 21, 481–499 (2021).
Propper, D. J. & Balkwill, F. R. Harnessing cytokines and chemokines for cancer therapy. Nat. Rev. Clin. Oncol. 19, 237–253 (2022).
Garon, E. B. et al. Responses and durability in NSCLC treated with pegilodecakin and anti-PD-1. J. Clin. Oncol. 36, 9018 (2018).
Naing, A. et al. Efficacy and safety of pegylated human IL-10 (AM0010) in combination with an anti-PD-1 in renal cell cancer. J. Clin. Oncol. 35, 4567 (2017).
Naing, A. et al. PEGylated human IL-10 (AM0010) in combination with pembrolizumab in anti-PD1 and CTLA-4 refractory melanoma. J. Clin. Oncol. 35, 3084 (2017).
Derynck, R., Turley, S. J. & Akhurst, R. J. TGFβ biology in cancer progression and immunotherapy. Nat. Rev. Clin. Oncol. 18, 9–34 (2021).
Tauriello, D. V. F., Sancho, E. & Batlle, E. Overcoming TGFβ-mediated immune evasion in cancer. Nat. Rev. Cancer 22, 25–44 (2022).
Imai, K. et al. Inhibition of dendritic cell migration by transforming growth factor-β1 increases tumor-draining lymph node metastasis. J. Exp. Clin. Cancer Res. 31, 3 (2012).
Sato, K. et al. TGF-β1 Reciprocally Controls Chemotaxis of Human Peripheral Blood Monocyte-Derived Dendritic Cells Via Chemokine Receptors. J. Immunol. 164, 2285–2295 (2000).
Gujar, R. & Sen, P. Transforming growth factor-β1 impairs lymph node homing of dendritic cells by downregulating C-type lectin receptor-2 expression. Cytokine 110, 39–43 (2018).
Gonzalez-Junca, A. et al. Autocrine TGFβ is a survival factor for monocytes and drives immunosuppressive lineage commitment. Cancer Immunol. Res. 7, 306–320 (2019).
Kobie, J. J. et al. Transforming growth factor β inhibits the antigen-presenting functions and antitumor activity of dendritic cell vaccines. Cancer Res 63, 1860–1864 (2003).
Novitskiy, S. V. et al. Deletion of TGF-β signaling in myeloid cells enhances their anti-tumorigenic properties. J. Leukoc. Biol. 92, 641–651 (2012).
Gorelink, L. & Flavell, R. A. Immune-mediated eradication of tumors through the blockade of transforming growth factor-β signaling in T cells. Nat. Med. 7, 1118–1122 (2001).
Gunderson, A. J. et al. TGFβ suppresses CD8 + T cell expression of CXCR3 and tumor trafficking. Nat. Commun. 11, 1749 (2020).
Das, L. & Levine, A. D. TGF-β inhibits IL-2 production and promotes cell cycle arrest in TCR-activated effector/memory T cells in the presence of sustained TCR signal transduction. J. Immunol. 180, 1490–1498 (2008).
Yoon, J. H. et al. Activin receptor-like kinase5 inhibition suppresses mouse melanoma by ubiquitin degradation of Smad4, thereby derepressing eomesodermin in cytotoxic T lymphocytes. EMBO Mol. Med. 5, 1720–1739 (2013).
Thomas, D. A. & Massagué, J. TGF-β directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 8, 369–380 (2005).
Mackay, L. K. et al. T-box transcription factors combine with the cytokines TGF-β and IL-15 to control tissue-resident memory T cell fate. Immunity 43, 1101–1111 (2015).
Takimoto, T. et al. Smad2 and Smad3 are redundantly essential for the TGF-β–mediated regulation of regulatory T plasticity and Th1 development. J. Immunol. 185, 842–855 (2010).
Tone, Y. et al. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat. Immunol. 9, 194–202 (2008).
Fridlender, Z. G. et al. Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 16, 183–194 (2009).
Sagiv, J. Y. et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 10, 562–573 (2015).
Mantovani, A., Sozzani, S., Locati, M., Allavena, P. & Sica, A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 23, 549–555 (2002).
Wu, F. et al. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct. Target. Ther. 6, 218 (2021).
Chandra Jena, B., Sarkar, S., Rout, L. & Mandal, M. The transformation of cancer-associated fibroblasts: current perspectives on the role of TGF-β in CAF mediated tumor progression and therapeutic resistance. Cancer Lett. 520, 222–232 (2021).
Shima, T. et al. Infiltration of tumor-associated macrophages is involved in tumor programmed death-ligand 1 expression in early lung adenocarcinoma. Cancer Sci. 111, 727–738 (2020).
Wu, P. et al. Tumor cell–derived TGFβ1 attenuates antitumor immune activity of T cells via regulation of PD-1 mRNA. Cancer Immunol. Res. 8, 1470–1484 (2020).
Dodagatta-Marri, E. et al. α-PD-1 therapy elevates Treg/Th balance and increases tumor cell pSmad3 that are both targeted by α-TGFβ antibody to promote durable rejection and immunity in squamous cell carcinomas. J. Immunother. Cancer 7, 62 (2019).
Novartis. Novartis receives FDA Orphan Drug Designation for NIS793 in pancreatic cancer | Novartis. https://www.novartis.com/news/novartis-receives-fda-orphan-drug-designation-nis793-pancreatic-cancer (2021).
Melisi, D. et al. Safety and activity of the TGFβ receptor I kinase inhibitor galunisertib plus the anti-PD-L1 antibody durvalumab in metastatic pancreatic cancer. J. Immunother. Cancer 9, e002068 (2021).
Sanofi. Press Release: Strong 2021 sales and business EPS growth enabling increased investment in R&D—Sanofi. https://www.sanofi.com/en/media-room/press-releases/2022/2022-02-04-06-30-00-2379020 (2022).
Becker, C. et al. TGF-β suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity 21, 491–501 (2004).
Kim, B.-G. et al. Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature 441, 1015–1019 (2006).
Bhowmick, N. A. et al. TGF-β signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 303, 848–851 (2004).
Choi, G., Kim, B.-S., Chang, J.-H. & Chung, Y. Defining the role of transforming growth factor β1 in Foxp3+ T regulatory cells. Immunity 54, 393–394 (2021).
Velegraki, M., Salem, M., Ansa-Addo, E. A., Wu, B. X. & Li, Z. Autocrine transforming growth factor β1 in regulatory T cell biology—gone but not missed. Immunity 54, 395–396 (2021).
Martin, C. J. et al. Selective inhibition of TGFβ1 activation overcomes primary resistance to checkpoint blockade therapy by altering tumor immune landscape. Sci. Transl. Med. 12, eaay8456 (2020).
Lan, Y. et al. Colocalized targeting of TGF-β and PD-L1 by bintrafusp alfa elicits distinct antitumor responses. J. Immunother. Cancer 10, e004122 (2022).
Gulley, J. L. et al. Dual inhibition of TGF‐β and PD‐L1: a novel approach to cancer treatment. Mol. Oncol. 16, 2117–2134 (2022).
Lan, Y. et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci. Transl. Med. 10, eaan5488 (2018).
Cho, B. C. et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in advanced squamous cell carcinoma of the head and neck: results from a phase I cohort. J. Immunother. Cancer 8, e000664 (2020).
Strauss, J. et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with human papillomavirus-associated malignancies. J. Immunother. Cancer 8, e001395 (2020).
Yoo, C. et al. Phase I study of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with pretreated biliary tract cancer. J. Immunother. Cancer 8, e000564 (2020).
Kang, Y.-K. et al. Safety and tolerability of bintrafusp alfa, a bifunctional fusion protein targeting TGFβ and PD-L1, in Asian patients with pretreated recurrent or refractory gastric cancer. Clin. Cancer Res. 26, 3202–3210 (2020).
Paz-Ares, L. et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in second-line treatment of patients with NSCLC: results from an expansion cohort of a phase 1 trial. J. Thorac. Oncol. 15, 1210–1222 (2020).
Strauss, J. et al. Phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGFβ, in advanced solid tumors. Clin. Cancer Res. 24, 1287–1295 (2018).
Merck. Bintrafusp Alfa Update—News | Merck. https://www.merckgroup.com/en/news/bintrafusp-alfa-update-23-08-2021.html (2021).
Merck. Merck reports topline data for bintrafusp alfa as second-line monotherapy treatment | Merck. https://www.merckgroup.com/en/news/bintrafusp-topline-data-biliary-tract-cancer-16-03-2021.html (2021).
Search of: bintrafusp alfa—List Results—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?cond=&term=bintrafusp+alfa&cntry=&state=&city=&dist= (2023).
Ozga, A. J., Chow, M. T. & Luster, A. D. Chemokines and the immune response to cancer. Immunity 54, 859–874 (2021).
Kohli, K., Pillarisetty, V. G. & Kim, T. S. Key chemokines direct migration of immune cells in solid tumors. Cancer Gene Ther. 29, 10–21 (2022).
Nagarsheth, N., Wicha, M. S. & Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 17, 559–572 (2017).
Highfill, S. L. et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci. Transl. Med. 6, 237ra67 (2014).
Yang, J. et al. Targeted deletion of CXCR2 in myeloid cells alters the tumor immune environment to improve antitumor immunity. Cancer Immunol. Res. 9, 200–213 (2021).
Zhang, M. et al. Interferon gamma inhibits CXCL8-CXCR2 axis mediated tumor-associated macrophages tumor trafficking and enhances anti-PD1 efficacy in pancreatic cancer. J. Immunother. Cancer 8, e000308 (2020).
Daniel, S. K., Seo, Y. D. & Pillarisetty, V. G. The CXCL12-CXCR4/CXCR7 axis as a mechanism of immune resistance in gastrointestinal malignancies. Semin. Cancer Biol. 65, 176–188 (2020).
Fei, L., Ren, X., Yu, H. & Zhan, Y. Targeting the CCL2/CCR2 axis in cancer immunotherapy: one stone, three birds? Front. Immunol. 12, 771210 (2021).
Xu, M., Wang, Y., **a, R., Wei, Y. & Wei, X. Role of the CCL2-CCR2 signalling axis in cancer: mechanisms and therapeutic targeting. Cell Prolif. 54, e13115 (2021).
Lin, C. et al. Tumour-associated macrophages-derived CXCL8 determines immune evasion through autonomous PD-L1 expression in gastric cancer. Gut 68, 1764–1773 (2019).
Li, Z. et al. Cancer-associated fibroblasts promote PD-L1 expression in mice cancer cells via secreting CXCL5. Int. J. Cancer 145, 1946–1957 (2019).
Feig, C. et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti–PD-L1 immunotherapy in pancreatic cancer. Proc. Natl Acad. Sci. USA 110, 20212–20217 (2013).
Bockorny, B. et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the COMBAT trial. Nat. Med. 26, 878–885 (2020).
Choueiri, T. K. et al. 1134PD—A phase Ia/IIb trial of the CXCR4 inhibitor X4P-001 and nivolumab for advanced renal cell carcinoma (RCC) that is unresponsive to nivolumab monotherapy. Ann. Oncol. 29, viii402–viii403 (2018).
Saxton, R. A., Glassman, C. R. & Garcia, K. C. Emerging principles of cytokine pharmacology and therapeutics. Nat. Rev. Drug Discov. 22, 21–37 (2023).
Deckers, J. et al. Engineering cytokine therapeutics. Nat. Rev. Bioeng. 1, 286–303 (2023).
Zhao, R. et al. HHLA2 is a member of the B7 family and inhibits human CD4 and CD8 T-cell function. Proc. Natl Acad. Sci. USA 110, 9879–9884 (2013).
Zhu, Y. et al. B7-H5 costimulates human T cells via CD28H. Nat. Commun. 4, 2043 (2013).
Janakiram, M. et al. Expression, clinical significance, and receptor identification of the newest B7 family member HHLA2 protein. Clin. Cancer Res. 21, 2359–2366 (2015).
Wei, Y. et al. KIR3DL3-HHLA2 is a human immunosuppressive pathway and a therapeutic target. Sci. Immunol. 6, eabf9792 (2021).
Bhatt, R. S. et al. KIR3DL3 is an inhibitory receptor for HHLA2 that mediates an alternative immunoinhibitory pathway to PD1. Cancer Immunol. Res. 9, 156–169 (2021).
De Louche, C. D. & Roghanian, A. Human inhibitory leukocyte Ig-like receptors: from immunotolerance to immunotherapy. JCI Insight 7, e151553 (2022).
Trowsdale, J., Jones, D. C., Barrow, A. D. & Traherne, J. A. Surveillance of cell and tissue perturbation by receptors in the LRC. Immunol. Rev. 267, 117–136 (2015).
Lin, A. & Yan, W.-H. HLA-G/ILTs targeted solid cancer immunotherapy: opportunities and challenges. Front. Immunol. 12, 1–14 (2021).
Nishide, M. & Kumanogoh, A. The role of semaphorins in immune responses and autoimmune rheumatic diseases. Nat. Rev. Rheumatol. 14, 19–31 (2018).
Kumanogoh, A. & Kikutani, H. Immunological functions of the neuropilins and plexins as receptors for semaphorins. Nat. Rev. Immunol. 13, 802–814 (2013).
Chuckran, C. A., Liu, C., Bruno, T. C., Workman, C. J. & Vignali, D. A. Neuropilin-1: a checkpoint target with unique implications for cancer immunology and immunotherapy. J. Immunother. Cancer 8, e000967 (2020).
Boelaars, K. & van Kooyk, Y. Targeting myeloid cells for cancer immunotherapy: siglec-7/9/10/15 and their ligands. Trends Cancer 10, 230–241 (2024).
van de Wall, S., Santegoets, K. C. M., van Houtum, E. J. H., Büll, C. & Adema, G. J. Sialoglycans and siglecs can shape the tumor immune microenvironment. Trends Immunol. 41, 274–285 (2020).
Smith, B. A. H. & Bertozzi, C. R. The clinical impact of glycobiology: targeting selectins, Siglecs and mammalian glycans. Nat. Rev. Drug Discov. 20, 217–243 (2021).
Rigau, M., Uldrich, A. P. & Behren, A. Targeting butyrophilins for cancer immunotherapy. Trends Immunol. 42, 670–680 (2021).
Sebestyen, Z., Prinz, I., Déchanet-Merville, J., Silva-Santos, B. & Kuball, J. Translating gammadelta (γδ) T cells and their receptors into cancer cell therapies. Nat. Rev. Drug Discov. 19, 169–184 (2020).
Mayassi, T., Barreiro, L. B., Rossjohn, J. & Jabri, B. A multilayered immune system through the lens of unconventional T cells. Nature 595, 501–510 (2021).
Ribot, J. C., Lopes, N. & Silva-Santos, B. γδ T cells in tissue physiology and surveillance. Nat. Rev. Immunol. 21, 221–232 (2021).
Payne, K. K. et al. BTN3A1 governs antitumor responses by coordinating ab and gd T cells. Science 369, 942–949 (2020).
Yu, X. et al. Reducing affinity as a strategy to boost immunomodulatory antibody agonism. Nature 614, 539–547 (2023).
Liu, D. et al. Tumor microenvironment‐responsive nanoparticles amplifying STING signaling pathway for cancer immunotherapy. Adv. Mater. 36, 2304845 (2024).
Liang, Y., Duan, L., Lu, J. & **a, J. Engineering exosomes for targeted drug delivery. Theranostics 11, 3183–3195 (2021).
Jiang, Y. et al. Fine-tuning bacterial cyclic di-AMP production for durable antitumor effects through the activation of the STING pathway. Research 6, 0102 (2023).
Ma, G., Gong, T. & Liu, Z. Targeting aberrant histone posttranscription modification machinery in esophageal squamous cell carcinoma: current findings and challenges. Research 2022, 9814607 (2022).
O’Donnell, J. S., Teng, M. W. L. & Smyth, M. J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 16, 151–167 (2019).
Han, D. & Xu, M. M. RNA Modification in the Immune System. Annu. Rev. Immunol. 41, 73–98 (2023).
Cui, L. et al. RNA modifications: importance in immune cell biology and related diseases. Signal Transduct. Target. Ther. 7, 334 (2022).
Tang, Q. et al. RNA modifications in cancer. Br. J. Cancer 129, 204–221 (2023).
Berraondo, P. et al. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 120, 6–15 (2019).
Cytokines in the balance. Nat. Immunol. 20, 1557–1557 (2019).
Robert, C. et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J. Clin. Oncol. 36, 1668–1674 (2018).
Miura, J. T. & Zager, J. S. Neo-DREAM study investigating Daromun for the treatment of clinical stage IIIB/C melanoma. Future Oncol. 15, 3665–3674 (2019).
Philogen. NidlegyTM Phase III PIVOTAL trial meets the study’s primary objective in patients with locally advanced fully resectable melanoma (Price Sensitive)—Philogen Spa. https://www.philogen.com/2023/10/16/nidlegy-phase-iii-pivotal-trial-meets-the-studys-primary-objective-in-patients-with-locally-advanced-fully-resectable-melanoma-price-sensitive/ (2023).
Sorin, M. et al. Single-cell spatial landscapes of the lung tumour immune microenvironment. Nature 614, 548–554 (2023).
Pulanco, M. C., Madsen, A. T., Tanwar, A., Corrigan, D. T. & Zang, X. Recent advancements in the B7/CD28 immune checkpoint families: new biology and clinical therapeutic strategies. Cell. Mol. Immunol. 20, 694–713 (2023).
Gay, C. M. et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell 39, 346–360.e7 (2021).
Nabet, B. Y. et al. Immune heterogeneity in small-cell lung cancer and vulnerability to immune checkpoint blockade. Cancer Cell 42, 429–443.e4 (2024).
Chen, H.-Y. et al. Regulation of neuroendocrine plasticity by the RNA-binding protein ZFP36L1. Nat. Commun. 13, 4998 (2022).
Augert, A. et al. Targeting NOTCH activation in small cell lung cancer through LSD1 inhibition. Sci. Signal. 12, 2922 (2019).
Patel, M. et al. 313 A phase 1 evaluation of tebotelimab, a bispecific PD-1 x LAG-3 DART® molecule, in combination with margetuximab in patients with advanced HER2+ neoplasms. J. Immunother. Cancer 8, A193.1–A193 (2020).
Halbrook, C. J., Lyssiotis, C. A., Pasca di Magliano, M. & Maitra, A. Pancreatic cancer: advances and challenges. Cell 186, 1729–1754 (2023).
Llovet, J. M. et al. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 19, 151–172 (2022).
El-Khoueiry, A. B. et al. Nivolumab (NIVO) plus ipilimumab (IPI) combination therapy in patients (Pts) with advanced hepatocellular carcinoma (aHCC): Long-term results from CheckMate 040. J. Clin. Oncol. 39, 269–269 (2021).
Abou-Alfa, G. K. et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 1, EVIDoa2100070 (2022).
Sano, Y. et al. Combination of T cell-redirecting bispecific antibody ERY974 and chemotherapy reciprocally enhances efficacy against non-inflamed tumours. Nat. Commun. 13, 1–17 (2022).
Foà, R. et al. Long-term results of the dasatinib-blinatumomab protocol for adult philadelphia-positive ALL. J. Clin. Oncol. 42, 881–885 (2024).
Jabbour, E., Haddad, F. G., Short, N. J. & Kantarjian, H. Treatment of adults with philadelphia chromosome-positive acute lymphoblastic leukemia-from intensive chemotherapy combinations to chemotherapy-free regimens: a review. JAMA Oncol. 8, 1340–1348 (2022).
Chen, C. J. J., Choi, M. Y. & Heyman, B. M. Targeted therapy in follicular lymphoma: towards a chemotherapy-free approach. Cancers 15, 4483 (2023).
Olszewski, A. J. et al. Single-agent mosunetuzumab is a promising safe and efficacious chemotherapy-free regimen for elderly/unfit patients with previously untreated diffuse large B-cell lymphoma. Blood 136, 43–45 (2020).
Casey, D. L. & Cheung, N.-K. V. Immunotherapy of pediatric solid tumors: treatments at a crossroads, with an emphasis on antibodies. Cancer Immunol. Res. 8, 161–166 (2020).
Quail, D. F. & Joyce, J. A. The microenvironmental landscape of brain tumors. Cancer Cell 31, 326–341 (2017).
Butler, E. et al. Recent progress in the treatment of cancer in children. CA Cancer J. Clin. 71, 315–332 (2021).
van den Bent, M. J. et al. Primary brain tumours in adults. Lancet 402, 1564–1579 (2023).
Thompson, E. M. et al. Recombinant polio–rhinovirus immunotherapy for recurrent paediatric high-grade glioma: a phase 1b trial. Lancet Child Adolesc. Health 7, 471–478 (2023).
Park, J. A. & Cheung, N.-K. V. Targets and Antibody Formats For Immunotherapy Of Neuroblastoma. J. Clin. Oncol. 38, 1836–1848 (2020).
Qiu, B. & Matthay, K. K. Advancing therapy for neuroblastoma. Nat. Rev. Clin. Oncol. 19, 515–533 (2022).
Bogani, G. et al. Adding immunotherapy to first-line treatment of advanced and metastatic endometrial cancer. Ann. Oncol. https://doi.org/10.1016/j.annonc.2024.02.006 (2024).
Odunsi, K. Immunotherapy in ovarian cancer. Ann. Oncol. 28, viii1–viii7 (2017).
Herzog, T. J. et al. ARTISTRY-7: phase III trial of nemvaleukin alfa plus pembrolizumab vs chemotherapy for platinum-resistant ovarian cancer. Future Oncol. 19, 1577–1591 (2023).
O’Cearbhaill, R. E. et al. 754 P Ubamatamab (MUC16xCD3 bispecific antibody) with cemiplimab (anti-PD-1 antibody) in recurrent ovarian cancer: Phase I dose-escalation study. Ann. Oncol. 34, S516–S517 (2023).
Monk, B. J. et al. Integration of immunotherapy into treatment of cervical cancer: Recent data and ongoing trials. Cancer Treat. Rev. 106, 102385 (2022).
Lorusso, D. et al. Pembrolizumab or placebo with chemoradiotherapy followed by pembrolizumab or placebo for newly diagnosed, high-risk, locally advanced cervical cancer (ENGOT-cx11/GOG-3047/KEYNOTE-A18): a randomised, double-blind, phase 3 clinical trial. Lancet https://doi.org/10.1016/S0140-6736(24)00317-9 (2024).
Oaknin, A. et al. 520MO Safety And Efficacy Of nivolumab (NIVO) ± ipilimumab (IPI) in patients (pts) with recurrent/metastatic cervical cancer (R/M Cx Ca) in checkmate 358. Ann. Oncol. 33, S782 (2022).
Jogalekar, M. P. et al. CAR T-cell-based gene therapy for cancers: new perspectives, challenges, and clinical developments. Front. Immunol. 13, 925985 (2022).
Cappell, K. M. & Kochenderfer, J. N. Long-term outcomes following CAR T cell therapy: what we know so far. Nat. Rev. Clin. Oncol. 20, 359–371 (2023).
Saez-Ibañez, A. R. et al. Landscape of cancer cell therapies: trends and real-world data. Nat. Rev. Drug Discov. 21, 631–632 (2022).
Stadtmauer, E. A. et al. CRISPR-engineered T cells in patients with refractory cancer. Science 367, eaba7365 (2020).
Lu, Y. et al. Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer. Nat. Med. 26, 732–740 (2020).
Haabeth, O. A. W. et al. Local delivery of Ox40l, Cd80, and Cd86 mRNA kindles global anticancer immunity. Cancer Res 79, 1624–1634 (2019).
Fan, T. et al. Therapeutic cancer vaccines: advancements, challenges, and prospects. Signal Transduct. Target. Ther. 8, 450 (2023).
Lin, M. J. et al. Cancer vaccines: the next immunotherapy frontier. Nat. Cancer 3, 911–926 (2022).
Katsikis, P. D., Ishii, K. J. & Schliehe, C. Challenges in develo** personalized neoantigen cancer vaccines. Nat. Rev. Immunol. 24, 213–227 (2024).
Ott, P. A. et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547, 217–221 (2017).
Kruit, W. H. J. et al. Selection of immunostimulant AS15 for active immunization with MAGE-A3 protein: results of a randomized phase II study of the European Organisation for Research and Treatment of Cancer Melanoma Group in Metastatic Melanoma. J. Clin. Oncol. 31, 2413–2420 (2013).
Mittendorf, E. A. et al. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide GP2 vaccine in breast cancer patients to prevent recurrence. Oncotarget 7, 66192–66201 (2016).
Odunsi, K. et al. Vaccination with an NY-ESO-1 peptide of HLA class I/II specificities induces integrated humoral and T cell responses in ovarian cancer. Proc. Natl Acad. Sci. USA 104, 12837–12842 (2007).
Ott, P. A. et al. A phase Ib trial of personalized neoantigen therapy plus anti-PD-1 in patients with advanced melanoma, non-small cell lung cancer, or bladder cancer. Cell 183, 347–362.e24 (2020).
Sahin, U. et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226 (2017).
Cheng, K. et al. Bioengineered bacteria-derived outer membrane vesicles as a versatile antigen display platform for tumor vaccination via plug-and-display technology. Nat. Commun. 12, 2041 (2021).
Luo, M. et al. A STING-activating nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 12, 648–654 (2017).
Zheng, B. et al. Bacterium-mimicking vector with enhanced adjuvanticity for cancer immunotherapy and minimized toxicity. Adv. Funct. Mater. 29, 1901437 (2019).
Chen, F. et al. Acid-ionizable iron nanoadjuvant augments STING activation for personalized vaccination immunotherapy of cancer. Adv. Mater. 35, e2209910 (2023).
Zhang, R. et al. Manganese salts function as potent adjuvants. Cell Mol. Immunol. 18, 1222–1234 (2021).
Acknowledgements
This work was supported by grants from 1·3·5 project for disciplines of excellence, West China Hospital, Sichuan University (No. ZYJC21003), the National Natural Science Foundation of China (Grant Nos. 82373021), the National Clinical Research Center for Geriatrics (Z2021JC001), Sichuan Provincial Research Foundation (24NSFSC6862), and Outstanding Youth Talent Foundation for Science and Technology of Sichuan Province (2022JDJQ0056).
Author information
Authors and Affiliations
Contributions
J.X. conceived the study. N.Y., X.L. and S.X. collected the literature. N.Y., X.L. and X.Z. co-wrote the manuscript. N.Y. and X.L. prepared the figures and tables. G.N., Y.L., and Y.C. provided critical suggestions on the preparation of the manuscript during the pre- and post-submission stages. N.Y., X.L. and X.Z. took the lead in revising the manuscript. G.N. assisted with language and scientific editing. J.X. and Y.L. supervised the project. All authors have read and approved the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests. Y.C. is the editorial board member of Signal Transduction and Targeted Therapy, but has not been involved in the process of the manuscript handling.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yin, N., Li, X., Zhang, X. et al. Development of pharmacological immunoregulatory anti-cancer therapeutics: current mechanistic studies and clinical opportunities. Sig Transduct Target Ther 9, 126 (2024). https://doi.org/10.1038/s41392-024-01826-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-024-01826-z
- Springer Nature Limited