Abstract
VprBP (also known as DCAF1) is a recently identified kinase that is overexpressed in cancer cells and serves as a major determinant for epigenetic gene silencing and tumorigenesis. The role of VprBP in driving target gene inactivation has been largely attributed to its ability to mediate histone H2A phosphorylation. However, whether VprBP also phosphorylates non-histone proteins and whether these phosphorylation events drive oncogenic signaling pathways have not been explored. Here we report that serine 367 phosphorylation (S367p) of p53 by VprBP is a key player in attenuating p53 transcriptional and growth suppressive activities. VprBP catalyzes p53S367p through a direct interaction with the C-terminal domain of p53. Mechanistically, VprBP-mediated S367p inhibits p53 function in the wake of promoting p53 proteasomal degradation, because blocking p53S367p increases p53 protein levels, thereby enhancing p53 transactivation. Furthermore, abrogation of VprBP-p53 interaction by p53 acetylation is critical for preventing p53S367p and potentiating p53 function in response to DNA damage. Together, our findings establish VprBP-mediated S367p as a negative regulator of p53 function and identify a previously uncharacterized mechanism by which S367p modulates p53 stability.
Similar content being viewed by others
Introduction
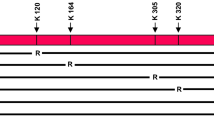
p53 is an important tumor suppressor controlling a wide range of DNA damage response processes, and its functional inactivation is the most frequent alteration in human cancers [1, 2]. Although some p53 effects may involve nontranscriptional mechanisms, major roles played by p53 are mediated through its action as a transcription factor that regulates the expression of downstream target genes such as p21, BTG2, and PUMA [3,4,5,6,7,8]. Structurally and functionally, p53 can be divided into four major domains: the N-terminal transactivation domain (residues 1–80), the central DNA binding domain (residues 100–290), the C-terminal tetramerization domain (residues 323–355), and the C-terminal regulatory domain (residues 364–393) [9,10,11]. All these domains are uniquely dedicated to a rapid and precise action of p53 on its target genes. In unstressed cells, p53 levels are very low owing to rapid degradation via ubiquitin-proteasome pathways in the cytoplasm. In the event of DNA damage, p53 becomes stabilized and activated to control the expression of target genes in the nucleus [12]. The exact mechanism of the enhancement of p53 function upon DNA damage is not yet fully understood, but accumulating evidence indicates that p53 activity is influenced by posttranslational modifications [13,14,15,16,17]. Among all known modifications, phosphorylation and acetylation are so far the most intensively studied modifications of p53 [15, 18,19,20,21,22,23,24]. They are dynamically regulated after DNA damage and widely accepted to be crucial for controlling the stability and sequence specific DNA binding capacity of p53, thereby affecting p53-dependent transcriptional program. Moreover, while initially thought to act independently, accumulating evidence suggest that p53 phosphorylation and acetylation can work together in a mutually exclusive or cooperative manner [14, 25,26,27,28,29,30,31]. Thus, it seems of importance to unravel molecular mechanisms and signaling pathways that govern the actions of these modifications in establishing thresholds for p53 activation and target gene expression in response to DNA damage.
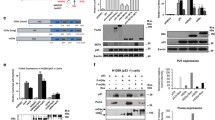
The HIV-1 Vpr Binding Protein (VprBP), also known as DDB1 and CUL4 Associated Factor 1, is a nuclear-localized protein and was originally identified owing to its interaction with HIV-1 Vpr in the context of the HIV-1 life cycle [32, 33]. Since its discovery, VprBP has been mainly characterized as the substrate recognition component of E3 ubiquitin ligase complexes and implicated in regulating cell cycle progression and DNA replication [34, 35]. Of special relevance to the current study, our recent investigation unexpectedly revealed that VprBP participates in establishing a transcriptionally inactive chromatin state and impairs p53-dependent transactivation of target genes [1B). Consistent with the mass spectrometric data, alanine substitution of S367 (S367A) almost completely abrogated VprBP-mediated p53 phosphorylation (Fig. 1C). To confirm and analyze in more detail p53S367 phosphorylation (p53S367p), we raised a rabbit polyclonal antibody that reacts with p53S367p. The specificity of the purified antibody was verified by dot blot and peptide competition assays using p53 unmodified and S367p peptides (Supplementary Fig. S1). In Western blot analysis of the kinase reactions, this p53S367p antibody reacted strongly with p53 wild-type, but not with p53S367A (Fig. 1D, VprBP). Moreover, inclusion of VprBP inhibitor B32B3 in kinase reactions efficiently blocked p53S367p, indicating that VprBP is responsible for the observed p53S367p (Fig. 1D, VprBP + B32B3).
Although our in vitro modification assays confirmed the ability of VprBP to catalyze p53S367p, it is not clear whether VprBP kinase activity toward p53 requires its physical interaction with p53. To check this possibility, we conducted in vitro pull-down assays using His-tagged VprBP and glutathione S-transferase (GST)-fused p53. As shown in Fig. 1E, GST-p53 efficiently interacted with His-VprBP, whereas GST alone did not. When binding experiments were repeated with the N-terminal, central, and C-terminal domains of p53, VprBP interacted with p53 C-terminal multifunctional domain (residues 291-393), while no apparent interaction was observed with p53 N-terminal transactivation (residues 1-101) and central DNA binding (residues 102-290) domains (Fig. 1E). In map** p53-interacting region of VprBP, the direct binding of VprBP C-terminal domain (residues 910-1580) was readily detectable, but VprBP N-terminal (residues 1-750) and central (residues 751-909) domains showed no interaction with p53 (Fig. 1F), strongly supporting that the C-terminal domain plays a major role in VprBP binding to p53.
VprBP is the kinase responsible for cellular p53S367p and inactivation
Based on the capacity of VprBP to generate p53S367p in vitro, we next explored whether VprBP can also catalyze cellular p53S367p and, if so, S367p has any significance in regulating p53 function in response to DNA damage. As confirmed by Western blotting, VprBP is readily detectable in U2OS human osteosarcoma cells, and shows little changes in its levels following treatment with etoposide and doxorubicin which are topoisomerase II-targeted, DNA cleavage-inducing agents (Fig. 2A, Supplementary Fig. S2A, VprBP, Eto, and Dox). Contrarily, a reproducible increase in p53 protein levels was observed in response to the etoposide/doxorubicin-induced DNA damage (p53, Eto, and Dox). In Western blot analysis with p53S367p antibody, high levels of p53S367p were detected in undamaged control cells, but its marked decrease was evident 24 h after etoposide/doxorubicin treatment (p53S367p, Eto, and Dox). Because p53S367p was almost completely disappeared in VprBP-depleted cells, and because p53 protein levels were increased after VprBP knockdown, VprBP seems to directly participate in generating p53S367p and destabilizing p53 in U2OS cells. Moreover, the fact that p53S367p and p53 protein levels returned to original levels after the expression of VprBP wild-type, but not VprBPK194R kinase-dead mutant, in VprBP-depleted U2OS cells (Fig. 2A, Supplementary Fig. S2A) strongly argues that VprBP kinase activity is critical for modulating the S367p and stability of p53. The treatment of U2OS cells with VprBP inhibitor B32B3 at the final concentration of 0.5 µM also led to a significant reduction in p53S367p and showed detectable effects on p53 protein levels following etoposide-induced DNA damage (Eto + B32B3). The observed correlation between p53S367p and p53 protein levels suggests the possibility that p53S367p carries out its negative effects on p53 function by controlling the intrinsic stability of p53.
A Control, VprBP-depleted, and VprBP/VprBPK194R-rescued U2OS cells were treated with etoposide (40 µM) in the presence or absence of B32B3 (0.5 µM) for 24 h. Whole cell lysates were prepared and subjected to Western blotting using p53, p53S367p and VprBP antibodies. B Whole cell lysates from (A) were subjected to immunoprecipitation using p53 DO-1 antibody. The binding of VprBP to p53 was analyzed by Western blotting. C Total RNA was isolated from the cells treated as in (A), and subjected to RT-qPCR analysis with p21 and BTG2 specific primers listed in Supplementary Table S1. Data represent the means ± SD of three independent experiments. P values were calculated using two-way ANOVA with post-hoc Tukey’s test for multiple comparisons. ***P < 0.001 versus Ctrl sh; #P < 0.05 and ###P < 0.001 versus Eto. (See also Supplementary Fig. S4A). D ChIP assays were performed in the U2OS cells treated as in (A) using p53 DO-1 antibody. Precipitated DNA was amplified with primers listed in Supplementary Table S2. Data are represented as mean ± SD of three independent experiments. P values were calculated using two-way ANOVA with post-hoc Tukey’s test for multiple comparisons. *P < 0.05 and ***P < 0.001 versus Ctrl sh; #P < 0.05 and ###P < 0.001 versus Eto. (See also Supplementary Fig. S4B). E U2OS cells were treated as in (A) and their growth was assessed after 72 h culture using the cell proliferation reagent WST-1. Data are represented as mean ± SD of three independent experiments. P values were calculated using two-way ANOVA with post-hoc Tukey’s test for multiple comparisons. *P < 0.05 and ***P < 0.001 versus Ctrl sh; ##P < 0.01 and ###P < 0.001 versus Eto.
Our demonstration of VprBP binding to recombinant p53 persuasively support the notion that VprBP-mediated p53S367p requires a stable kinase-substrate docking interaction. However, it is not clear whether VprBP utilizes a similar docking mechanism to phosphorylate p53 in U2OS cells. In approaching this question, lysates from U2OS cells were immunoprecipitated with anti-p53 antibody and subjected to Western blot analysis. Our results showed that p53 can stably associate with VprBP in undamaged control U2OS cells. However, etoposide-induced DNA damage and B32B3 treatment significantly compromised the ability of p53 to associate with VprBP in U2OS cells. We also found that B32B3 treatments have only minimal effects on the observed attenuation of intracellular VprBP-p53 association in etoposide-treated damaged cells (Fig. 2B). In additional analysis, p53 was able to interact with ectopic VprBP wild-type and K194R kinase-dead mutant that were expressed in VprBP-depleted cells (Fig. 2B). It thus seems that, although other factors may be involved, cellular p53S367p by VprBP is likely mediated through a direct substrate docking mechanism.
Since p53 functions as a DNA binding protein that stimulates transcription from downstream target genes, we next wanted to investigate whether VprBP-mediated S367p plays any role in modulating p53 transcriptional activity. Toward this end, we first conducted in vitro transcription assays using a DNA template containing p53 response elements as described [2A), our Western blot analysis using the p53S367p antibody showed that p53S367 is phosphorylated in H1299 and T84 cells co-transfected with p53 and VprBP (Fig. 3A, Supplementary Fig. S6A, lane 3). Also, confirming the specificity of the p53S367p antibody and the VprBP target phosphorylation site, no p53S367p was detected in H1299 and T84 cells expressing p53S367A mutant and VprBP (lanes 5-8). The fact that VprBPK194R kinase-dead mutant was not capable of generating p53S367p (lane 4) strongly argues that VprBP is responsible for mediating the observed p53S367p. To determine whether S367p has a role in modulating p53 stability, we also compared the steady-state level of p53 wild-type to that of p53S367A mutant. Remarkably, combined expression of p53 wild-type and VprBP in H1299 and T84 cells resulted in a marked reduction in the p53 protein levels (lanes 2 and 3). The observed change was dependent of S367p status, as S367A phosphorylation-blocking mutation significantly compromised the p53-reducing effects of VprBP (lanes 3 and 7). Moreover, exposure to VprBP inhibitor B32B3 (lanes 9-12) or expression of VprBPK194R kinase-dead mutant (lane 4) restored p53 steady levels in H1299 and T84 cells, thus again implying that VprBP regulates p53 stability through its phosphorylation of S367.
A H1299 cells were transfected with VprBP and/or p53 expression plasmids for 48 h in the presence or absence of B32B3 (3 µM) as indicated on the top. Extracts were prepared in lysis buffer and Western blot analysis was performed with antibodies against p53, p53S367p, FLAG, and VprBP. B Whole cell lysates from (A) were subjected to immunoprecipitation using p53 DO-1 antibody. The binding of VprBP to p53 was analyzed by Western blotting. C Total RNA was isolated from the H1299 cells transfected with VprBP and/or p53 expression plasmids as in (A), and subjected to RT-qPCR analysis with p21 and BTG2 specific primers listed in Supplementary Table S1. Data represent the means ± SD of three independent experiments. P values were calculated using two-way ANOVA with post-hoc Tukey’s test for multiple comparisons. ***P < 0.001 versus p53; ###P < 0.001 versus p53 + FLAG-VprBP. (See also Supplementary Fig. S8A). D ChIP assays were performed in the H1299 cells transfected with VprBP and/or p53 expression plasmids as in (A) using p53 DO-1 antibody. Precipitated DNA was amplified with specific primers for p21 and BTG2 listed in Supplementary Table S2. Data are represented as mean ± SD of three independent experiments. P values were calculated using two-way ANOVA with post-hoc Tukey’s test for multiple comparisons. ***P < 0.001 versus p53; ###P < 0.001 versus p53 + FLAG-VprBP. (See also Supplementary Fig. S8B).
Since phosphorylation often acts as a signal to modulate protein stability, we also treated H1299 cells expressing p53 wild-type or S367A mutant with the protein synthesis inhibitor cycloheximide (CHX) for 2 h, and evaluated its impact on p53 protein levels by Western blotting. Intriguingly, our analysis detected an equivalent level of p53S367A mutant protein, but a fast disappearance of p53 wild-type protein, in CHX-treated H1299 cells (Supplementary Fig. S7A), an indicative of a possible role of VprBP-mediated S367p in decreasing p53 protein stability. Additionally, that treating p53-transfected H1299 cells with the proteasome inhibitor MG132 generated an apparent increase in p53 proteins levels argues strongly that the observed effects of VprBP-mediated S367p on p53 reflects an alteration of proteomic degradation processes (Supplementary Fig. S7B). To further understand the p53-stablizing process, the interaction of p53 with VprBP was tested by Western blot analysis of anti-p53 immunoprecipitates from lysates of H1299 cells that were transfected with combinations of plasmids encoding p53 and VprBP. As shown in Fig. 3B, when equal amounts of immunoprecipitated p53 were subjected to Western blotting, we found that p53 can generate a stable interaction with both VprBP wild-type and K194R kinase-dead mutant (lanes 2 and 3). In agreement with our observation in U2OS cells, additional binding experiments in which co-immunoprecipitation was carried out using lysates of p53S367A mutant-transfected cells disclosed that VprBP can interact with p53S367A mutant with affinity similar to that of p53 wild-type (Fig. 3B, lanes 2 and 5). Meanwhile, we found that B32B3 is unable to impact p53-VprBP interaction in H1299 cells (lanes 7-10), results supportive of the view that altered VprBP binding properties of p53 are not part of the mechanisms by which VprBP-mediated S367p regulates p53 cellular activity.
To assess the functional contributions made by p53S367p in the above results, we conducted RT-qPCR analysis of the four representative p53 target genes. The expression of ectopic p53 activated target gene transcription in H1299 and T84 cells, but co-expression of VprBP significantly reduced the transcription levels of p53 target genes (Fig. 3C, Supplementary Figs. S6B, S8A). In checking the effects of p53S367p, VprBPK194R kinase-dead mutant failed to diminish the extent of target gene induction in p53-transfected cells. Consistent with the concept of p53S367p as an essential process for VprBP function, we also noticed that VprBP is not effective in inhibiting p53 target gene transcription in H1299 and T84 cells expressing p53S367A phosphorylation-blocking mutant (Fig. 3C, Supplementary Figs. S6B, S8A). These results are in complete agreement with data indicating that treatment with VprBP inhibitor B32B3 could reactivate p53 target genes in H1299 and T84 cells expressing p53 and VprBP wild-type, but not p53S367A and VprBPK194R mutant.
In accordance with our RT-qPCR data, ChIP experiments using p53 antibody showed a stable occupancy of the response element regions of target genes by ectopic p53 in H1299 and T84 cells. On the contrary, when p53 was co-expressed with VprBP, the observed p53 enrichment in target genes was compromised, as indicated by a significant reduction in p53 ChIP signal (Fig. 3D, Supplementary Figs. S6C, S8B). Because VprBP regulates p53 function through its kinase activity, we also checked the levels of p53 at target genes after replacing VprBP wild-type with VprBPK194R kinase-dead mutant. Our analysis clearly demonstrated that target gene occupancy of p53 was not affected by VprBPK194R mutant in H1299 and T84 cells (Fig. 3D, Supplementary Figs. S6C, S8B). These results raised another question of whether the negative impact of VprBP on p53 enrichment at target genes is dependent on S367p. In fact, the predicted role for VprBP-mediated S367p is confirmed by the observation that p53 ChIP signal in p53S367A mutant-expressing cells was about 3-5 times higher compared to those obtained with p53S367 wild-type-expressing cells (Fig. 3D, Supplementary Figs. S6C, S8B). These observations were further corroborated by additional ChIP assays showing that chromatin prepared from B32B3-treated, VprBP-transfected cells generates a higher p53 ChIP signal relative to untreated VprBP-transfected cells (Fig. 3D, Supplementary Figs. S6C, S8B).
p53 acetylation prevents its interaction with VprBP and S367p-induced destabilization
Because p53 acetylation at the C-terminal regulatory domain promotes p53 stability and function in response to DNA damage, our finding that VprBP drives p53 degradation suggests that p53 acetylation may be vital to the control of VprBP-mediated p53S367p in damaged cell nuclei. To check this possibility, cytoplasmic and nuclear fractions were prepared from untreated and etoposide-treated U2OS cell extracts, and their equivalent proportions were probed with p53-K373ac/K382ac antibody by Western blotting. A noteworthy observation emerged from these experiments was that the acetylation levels of p53 were significantly enhanced in the nucleus, while acetylated p53 was not detectable in the cytoplasm, 24 h following etoposide treatment (Fig. 4A, DMSO and Eto). Also, no detectable changes in p53 acetylation levels after VprBP knockdown and rescue expression indicate that p53 acetylation is not affected by VprBP-mediated S367p (Eto). Consistent with these results, treatment of U2OS cells with VprBP inhibitor B32B3 failed to show any effects on p53 acetylation in response to etoposide-induced DNA damage (Eto + B32B3).
A Control, VprBP-depleted, VprBP/VprBPK194R-rescued U2OS cells were treated with etoposide (40 µM) in the presence or absence of B32B3 (0.5 µM) for 24 h. Cytoplasmic extracts, and nuclear extracts were prepared and analyzed by Western blotting with antibodies against p53, p53S367p and p53ac. B H1299 cells were transfected with FLAG-VprBP and/or p53, p53S367A or p536KQ for 48 h in the presence or absence of B32B3 (3 µM). Cytoplasmic extracts and nuclear extracts were prepared and analyzed by Western blotting with indicated antibodies. C Total RNA was isolated from the H1299 cells transfected with VprBP/p53 expression plasmids as in (B), and subjected to RT-qPCR analysis with p21 and BTG2 specific primers listed in Supplementary Table S1. Data represent the means ± SD of three independent experiments. P values were calculated using two-way ANOVA with post-hoc Tukey’s test for multiple comparisons. ***P < 0.001 versus p53; ###P < 0.001 versus p53 + FLAG-VprBP. (See also Supplementary Fig. S10A). D ChIP assays were performed in the H1299 cells transfected with VprBP/p53 expression plasmids as in (B) using p53 DO-1 antibody. Precipitated DNA was amplified with specific primers for p21 and BTG2 listed in Supplementary Table S2. Data are represented as mean ± SD of three independent experiments. P values were calculated using two-way ANOVA with post-hoc Tukey’s test for multiple comparisons. ***P < 0.001 versus p53; ###P < 0.001 versus p53 + FLAG-VprBP. (See also Supplementary Fig. S10B).
As a more direct approach to study the role of p53 acetylation in controlling VprBP-mediated S367p, we also analyzed nuclear and cytoplasmic fractions that were prepared from H1299 cells expressing ectopic p53 and VprBP. The results correlated well with those obtained from etoposide-treated U2OS cells and demonstrated that the steady-state level of acetylated p53 was maintained mainly in the nuclei of H1299 cells expressing p53 wild-type (Fig. 4B, Supplementary S9A, p53). Moreover, the finding that the acetylation status of p53 was not affected by VprBP expression and S367D mutation reconfirmed that p53 acetylation acts as an upstream regulator of VprBP-mediated p53S367p (Supplementary Fig. S9A, B). To further support these results, our analysis was repeated with nuclear and cytoplasmic fractions of H1299 cells transfected with p536KQ mutant mimicking constitutive acetylation. Unexpectedly, we found that the p53ac antibody used in our assays cross-reacted with ectopic p536KQ mutant and generated a nonspecific band in our analyses (p536KQ). Whereas similar cross-reactivity was observed with a p53ac antibody from another source (Abcam), these commercial p53ac antibodies were not reactive against p536KR and other p53 mutants (not shown), supporting the specificity of the antibodies. As shown in Fig. 4B, our studies with p53 and p53S367p antibodies revealed a distinct increase in p53 protein levels and an almost complete loss of p53S367p after the expression of p536KQ acetylation mimicking mutant (p536KQ). Along with these p536KQ mutant results, the fact that p536KR acetylation blocking mutant displays a stable accumulation to high levels in the cytoplasm clearly supports the view that p53 acetylation could attenuate S367p-induced p53 degradation (Supplementary Fig. S9A). The observed effects of p53 mutations were dependent of proteomic degradation process, because those mutations generated little to no changes in p53 protein levels in MG132-treated H1299 cells (Supplementary Fig. S9B).
Since the observed changes in p53 protein and S367p levels after 6KQ acetylation mimicking mutations might be functionally significant, we also examined the transcriptional states of target genes. The results of these efforts showed that VprBP is not capable of suppressing the expression of p53 target genes in p536KQ mutant-transfected cells (Fig. 4C, Supplementary Fig. S10A). Congruent with these data, when ChIP assays were conducted using H1299 cells transiently transfected with p536KQ mutant, a larger accumulation of p536KQ mutant around response element regions was observed as compared with p53 wild-type (Fig. 4D, Supplementary Fig. S10B). To further confirm these results and evaluate the effects of p53 acetylation in other cell types, we repeated Western blot analysis and RT/ChIP-qPCR assays with T84 colon cancer cells; similar results were obtained from these parallel experiments (Supplementary Fig. S11). Collectively, these observations are consistent with those of earlier studies of p53-VprBP interaction [40,41,42]. Notably, and consistent with the primary mechanisms of action of p53S367p and p53 acetylation in regulating p53 protein stability, S367A and acetylation-mimicking mutations failed to influence the transcriptional activity of recombinant p53 proteins in our in vitro assays. Defining the molecular basis for how VprBP binding and kinase activity toward p53 is inhibited by p53 acetylation is beyond the scope of this report. However, it would be of particular interest to examine whether the C-terminal acetylation may induce a distinct local structural feature of p53. Related, our previous study showed that VprBP can interact with unmodified histone H3 N-terminal tails, but not with acetylated H3 N-terminal tails, to establish and maintain target genes in an inactive state [36]. In this regard, one obvious possibility is that p53 acetylation acts in an analogous manner to modulate the strength of p53-VprBP interaction, thereby precluding undesired p53S367p as well as leading to p53 nuclear stabilization and transactivation. Although functional relevance of each specific lysine acetylation in p53 C-terminal domain in disabling p53 from interacting with VprBP remains to be determined, it seems clear that p53 acetylation allows for very tight regulation of VprBP-induced disturbance of p53 protein stability and transcriptional activity. Since the expression levels of VprBP are much higher in cancer cells with respect to those in normal cells, our results are also of significance in relation to the key role of VprBP in disrupting p53 signaling pathway during cancer development. Besides acetylation, other types of posttranslational modifications such as ubiquitination and methylation are known to influence p53 activity contributing toward cell cycle arrest and DNA repair [15, 43]. Thus, an additional interesting question to be addressed in future studies should concern a cooperative or antagonistic action of those modifications in controlling VprBP-mediated p53S367p in damaged cells. Apart from the possible contribution of other modifications, specific phosphatases might also participate in reversing the effects of VprBP-mediated p53S367p and initiating DNA damage-induced upregulation and functional activation of p53. Discerning whether this is the case and how S367p deposited by VprBP is selectively removed by phosphatases will help define whether a distinct mechanism is linked to the regulation of DNA damage response at the level of p53 protein stability.
In light of our data presented here, at least two VprBP-dependent events appear to be associated with the control of p53 protein stability, VprBP-mediated S367p, which promotes p53 degradation, and C-terminal acetylation, which protects p53 from degradation (Fig. 6C). Upon DNA damage, the acetylation overrides p53S367p and proteasomal degradation by preventing p53-VprBP interaction. It is not surprising that such a tightly regulated process has evolved for the fine-tuning of tumor suppressive functions of p53 and oncogenic activities of VprBP in response to DNA damage. Therefore, structural investigations of VprBP-bound p53 will provide information on the nature of p53-VprBP interaction and how p53S367p and acetylation regulate p53 signaling pathways. Moreover, the finding that VprBP-mediated p53S367p serves as a crucial step for p53 degradation helps us to develop the strategy of how VprBP can be pharmacologically targeted for the reactivation of p53 in cancer cells.
Materials and methods
Kinase assay
In vitro phosphorylation assays were performed with recombinant p53 and VprBP wild-type/mutant in kinase buffer (50 mM Tris-HCl [pH 7.5], 20 mM EGTA, 10 mM MgCl2, 1 mM DTT, and 1 mM β-glycerophosphate) containing 10 µCi of [γ-32P] ATP (PerkinElmer, Waltham, MA, USA) and 4 mM ATP as recently described [37]. Following incubation at 30 °C for 30 min, p53 and VprBP proteins from each reaction were separated by 5–20% SDS-PAGE, and phosphorylated p53 proteins were visualized by autoradiography.
RT-qPCR
Total RNA was isolated from U2OS, H1299 and T84 cells using a RNeasy Mini kit (Qiagen, Hilden, Germany) and converted to first-strand cDNA using the SuperScript III First-Strand System Kit (Thermo Fisher Scientific, Waltham, MA, USA). Real-time RT-PCR was carried out with SYBR Green Real-time PCR Master Mixes (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. The primers used for RT-qPCR are listed in Supplementary Table S1. All reactions were run in triplicate, and results were normalized to β-actin mRNA levels.
ChIP-qPCR
Chromatin immunoprecipitation (ChIP) assays with U2OS, H1299 and T84 cells were performed using the ChIP Assay Kit (Millipore Sigma, St. Louis, MO, USA) as recently described [44]. After reversing the protein–DNA cross-links, immunoprecipitated DNA was purified and analyzed by quantitative real-time PCR (qPCR) using the primers that amplify p53 response element region of p21, BTG2, Reprimo and PUMA genes (Supplementary Table S2). Specificity of amplification was determined by melting curve analysis, and all samples were run in triplicate.
Data availability
All relevant data are available from the authors upon request.
References
Baugh EH, Ke H, Levine AJ, Bonneau RA, Chan CS. Why are there hotspot mutations in the TP53 gene in human cancers? Cell Death Differ. 2018;25:154–60.
Hainaut P, Pfeifer GP. Somatic TP53 Mutations in the Era of Genome Sequencing. Cold Spring Harbor Perspect Med. 2016;6:a026179.
Fields S, Jang SK. Presence of a potent transcription activating sequence in the p53 protein. Science. 1990;249:1046–9.
Raycroft L, Wu HY, Lozano G. Transcriptional activation by wild-type but not transforming mutants of the p53 anti-oncogene. Science. 1990;249:1049–51.
Farmer G, Bargonetti J, Zhu H, Friedman P, Prywes R, Prives C. Wild-type p53 activates transcription in vitro. Nature. 1992;358:83–86.
el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–25.
Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–94.
Rouault JP, Falette N, Guehenneux F, Guillot C, Rimokh R, Wang Q, et al. Identification of BTG2, an antiproliferative p53-dependent component of the DNA damage cellular response pathway. Nat Genet. 1996;14:482–6.
Joerger AC, Fersht AR. Structural biology of the tumor suppressor p53 and cancer-associated mutants. Adv Cancer Res. 2007;97:1–23.
Wang P, Reed M, Wang Y, Mayr G, Stenger JE, Anderson ME, et al. p53 domains: structure, oligomerization, and transformation. Mol Cell Biol. 1994;14:5182–91.
Ahn J, Prives C. The C-terminus of p53: the more you learn the less you know. Nature structural biology. 2001;8:730–2.
Brooks CL, Gu W. p53 ubiquitination: Mdm2 and beyond. Mol Cell. 2006;21:307–15.
Liu Y, Tavana O, Gu W. p53 modifications: exquisite decorations of the powerful guardian. J Mol Cell Biol. 2019;11:564–77.
Meek DW, Anderson CW. Posttranslational modification of p53: cooperative integrators of function. Cold Spring Harb Perspect Biol. 2009;1:a000950.
Bode AM, Dong Z. Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer. 2004;4:793–805.
Dai C, Gu W. p53 post-translational modification: deregulated in tumorigenesis. Trends Mol Med. 2010;16:528–36.
Kruse JP, Gu W. Modes of p53 regulation. Cell. 2009;137:609–22.
Maclaine NJ, Hupp TR. The regulation of p53 by phosphorylation: a model for how distinct signals integrate into the p53 pathway. Aging. 2009;1:490–502.
Tang Y, Zhao W, Chen Y, Zhao Y, Gu W. Acetylation is indispensable for p53 activation. Cell. 2008;133:612–26.
Tang Y, Luo J, Zhang W, Gu W. Tip60-dependent acetylation of p53 modulates the decision between cell-cycle arrest and apoptosis. Molecular cell. 2006;24:827–39.
Sykes SM, Mellert HS, Holbert MA, Li K, Marmorstein R, Lane WS, et al. Acetylation of the p53 DNA-binding domain regulates apoptosis induction. Mol Cell. 2006;24:841–51.
Jenkins LM, Durell SR, Mazur SJ, Appella E. p53 N-terminal phosphorylation: a defining layer of complex regulation. Carcinogenesis. 2012;33:1441–9.
Wu Y, Lin JC, Piluso LG, Dhahbi JM, Bobadilla S, Spindler SR, et al. Phosphorylation of p53 by TAF1 inactivates p53-dependent transcription in the DNA damage response. Mol Cell. 2014;53:63–74.
Rokudai S, Laptenko O, Arnal SM, Taya Y, Kitabayashi I, Prives C. MOZ increases p53 acetylation and premature senescence through its complex formation with PML. Proc Natl Acad Sci USA. 2013;110:3895–900.
Sakaguchi K, Herrera JE, Saito S, Miki T, Bustin M, Vassilev A, et al. DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev. 1998;12:2831–41.
Lambert PF, Kashanchi F, Radonovich MF, Shiekhattar R, Brady JN. Phosphorylation of p53 serine 15 increases interaction with CBP. J Biol Chem. 1998;273:33048–53.
Dornan D, Hupp TR. Inhibition of p53-dependent transcription by BOX-I phospho-peptide mimetics that bind to p300. EMBO Rep. 2001;2:139–44.
Polley S, Guha S, Roy NS, Kar S, Sakaguchi K, Chuman Y, et al. Differential recognition of phosphorylated transactivation domains of p53 by different p300 domains. J Mol Biol. 2008;376:8–12.
Feng H, Jenkins LM, Durell SR, Hayashi R, Mazur SJ, Cherry S, et al. Structural basis for p300 Taz2-p53 TAD1 binding and modulation by phosphorylation. Structure. 2009;17:202–10.
Lee CW, Arai M, Martinez-Yamout MA, Dyson HJ, Wright PE. Map** the interactions of the p53 transactivation domain with the KIX domain of CBP. Biochemistry. 2009;48:2115–24.
Teufel DP, Bycroft M, Fersht AR. Regulation by phosphorylation of the relative affinities of the N-terminal transactivation domains of p53 for p300 domains and Mdm2. Oncogene. 2009;28:2112–8.
Zhang S, Feng Y, Narayan O, Zhao LJ. Cytoplasmic retention of HIV-1 regulatory protein Vpr by protein-protein interaction with a novel human cytoplasmic protein VprBP. Gene. 2001;263:131–40.
Zhao LJ, Mukherjee S, Narayan O. Biochemical mechanism of HIV-I Vpr function. Specific interaction with a cellular protein. J Biol Chem. 1994;269:15577–82.
Hrecka K, Gierszewska M, Srivastava S, Kozaczkiewicz L, Swanson SK, Florens L, et al. Lentiviral Vpr usurps Cul4-DDB1[VprBP] E3 ubiquitin ligase to modulate cell cycle. Proc Natl Acad Sci USA. 2007;104:11778–83.
McCall CM, Miliani de Marval PL, Chastain PD 2nd, Jackson SC, He YJ, Kotake Y, et al. Human immunodeficiency virus type 1 Vpr-binding protein VprBP, a WD40 protein associated with the DDB1-CUL4 E3 ubiquitin ligase, is essential for DNA replication and embryonic development. Mol Cell Biol. 2008;28:5621–33.
Kim K, Heo K, Choi J, Jackson S, Kim H, **ong Y, et al. Vpr-binding protein antagonizes p53-mediated transcription via direct interaction with H3 tail. Mol Cell Biol. 2012;32:783–96.
Kim K, Kim JM, Kim JS, Choi J, Lee YS, Neamati N, et al. VprBP has intrinsic kinase activity targeting histone H2A and represses gene transcription. Mol cell. 2013;52:459–67.
Ghate NB, Kim S, Spiller E, Kim S, Shin Y, Rhie SK, et al. VprBP directs epigenetic gene silencing through histone H2A phosphorylation in colon cancer. Mol Oncol. 2021;15:2801–17.
Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606.
Barlev NA, Liu L, Chehab NH, Mansfield K, Harris KG, Halazonetis TD, et al. Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol Cell. 2001;8:1243–54.
Avantaggiati ML, Ogryzko V, Gardner K, Giordano A, Levine AS, Kelly K. Recruitment of p300/CBP in p53-dependent signal pathways. Cell. 1997;89:1175–84.
Luo J, Li M, Tang Y, Laszkowska M, Roeder RG, Gu W. Acetylation of p53 augments its site-specific DNA binding both in vitro and in vivo. Proc Natl Acad Sci USA. 2004;101:2259–64.
Ivanov GS, Ivanova T, Kurash J, Ivanov A, Chuikov S, Gizatullin F, et al. Methylation-acetylation interplay activates p53 in response to DNA damage. Mol Cell Biol. 2007;27:6756–69.
Kim K, Choi J, Heo K, Kim H, Levens D, Kohno K, et al. Isolation and characterization of a novel H1.2 complex that acts as a repressor of p53-mediated transcription. J Biol Chem. 2008;283:9113–26.
Acknowledgements
This work was supported by NIH Grant CA201561 awarded to W.A. The study was also partly supported by award number P30CA014089 from the National Cancer Institute. We are grateful to Dr. Carol Prives, Department of Biological Sciences, Columbia University, USA for providing p536KQ and p536KR expression plasmid; Dr. Adam E. Snook, Thomas Jefferson University for T84 cell line.
Funding
Open access funding provided by SCELC, Statewide California Electronic Library Consortium.
Author information
Authors and Affiliations
Contributions
NBG, SK, and WA conceived and designed the study. NBG, SK, YS, and KK performed the experiments with contributions of WA. NBG, SK, RM, YS, KK, and WA analyzed data. NBG, SK, and WA wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghate, N.B., Kim, S., Mehmood, R. et al. VprBP/DCAF1 regulates p53 function and stability through site-specific phosphorylation. Oncogene 42, 1405–1416 (2023). https://doi.org/10.1038/s41388-023-02685-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-023-02685-8
- Springer Nature Limited