Abstract
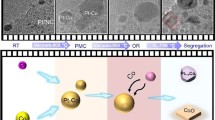
The catalytic performance of nanoparticles is primarily determined by the precise nature of the surface and near-surface atomic configurations, which can be tailored by post-synthesis annealing effectively and straightforwardly. Understanding the complete dynamic response of surface structure and chemistry to thermal treatments at the atomic scale is imperative for the rational design of catalyst nanoparticles. Here, by tracking the same individual Pt3Co nanoparticles during in situ annealing in a scanning transmission electron microscope, we directly discern five distinct stages of surface elemental rearrangements in Pt3Co nanoparticles at the atomic scale: initial random (alloy) elemental distribution; surface platinum-skin-layer formation; nucleation of structurally ordered domains; ordered framework development and, finally, initiation of amorphization. Furthermore, a comprehensive interplay among phase evolution, surface faceting and elemental inter-diffusion is revealed, and supported by atomistic simulations. This work may pave the way towards designing catalysts through post-synthesis annealing for optimized catalytic performance.
Similar content being viewed by others
Introduction
Pt-bimetallic (PtM where M=Fe, Co, Ni and so on) nanoparticles (NPs) have attracted great interest because of their outstanding activity for the oxygen reduction reaction (ORR) in fuel cell applications1,2. Such PtM NPs exhibit enhanced catalytic performance compared with commercial Pt/C fuel cell catalysts, with improvement factors varying from 2 × to greater than 20 × depending on the particle size, morphology, composition and surface structure. Each of these material parameters can influence and/or control the geometric and electronic structure of the particle surface, and therefore play a vital role in determining the catalytic performance of the PtM catalystsFull size image
Multiple representative Pt3Co NPs were selected to track the atomic-scale structural and compositional changes occurring during isothermal holds for times ranging from 10 to 80 min at several different temperatures (room temperature (RT) to 800 °C) during the in situ annealing sequence (Fig. 1a). A series of HAADF-STEM images of a single Pt3Co particle captured during in situ heating are shown in Fig. 2 (with the simultaneously acquired BF-STEM images shown in Supplementary Fig. 2). An initial composition of 3:1 for the Pt:Co NP shown in Fig. 2a was confirmed using energy dispersive X-ray spectroscopy (EDS; see Supplementary Fig. 3). As there is a relatively large difference in the atomic number of Pt and Co, the contrast present in the atomic-scale HAADF-STEM images can be used to estimate the elemental concentrations of Pt and Co comprising each atomic column projection in the image15,16.
(a–i) Atomic-resolution HAADF-STEM images of a single Pt3Co NP acquired at different temperature and annealing times. The (1–10), (111) and (001) surface facets are labelled for each annealing stage. The alternating planes of bright-dark contrast corresponding to the formation of the ordered Pt3Co structure, are marked by arrows in d, indicating the origin of transformation of fcc to L12 phase. Scale bar, 2 nm.
Surface platinum segregation
The HAADF-STEM image of the Pt3Co NP at RT (Fig. 2a) exhibits brighter contrast at the centre of the NP, which gradually changes to darker contrast towards the NP surface; this image contrast variation follows the thickness change across the spherical NP. The contrast uniformity within areas of the same NP thickness is consistent with a random distribution of Pt and Co atoms in the Pt3Co alloy NP. No distinct faceting was observed on the initial NP surface at RT (Fig. 2a). Increasing the temperature to 350 °C resulted in Pt segregation to the surface, as evidenced by the appearance of a slightly brighter contrast on the NP surface compared with that at RT (shown by arrows in Fig. 2b). Pt surface segregation is clearly demonstrated from image intensity profiles taken across the HAADF-STEM image of the NP (Fig. 3a–f), exploiting the sensitivity of HAADF-STEM imaging with respect to the atomic number5,16. As shown in the corresponding HAADF-STEM image and intensity profile, Fig. 3b,e, respectively, an increase in the image contrast was found directly at the surface of the NP annealed at 350°C compared with that at RT (Fig. 3a,d, respectively), supporting segregation of Pt atoms to the surface.
(a–c) HAADF-STEM images of the Pt3Co NP at different annealing temperatures, (a) RT, (b) 350 °C and (c) 550 °C, used for elemental segregation assessments; (d–f) corresponding intensity profiles of a–c across particle images, which reveals Pt surface segregation behaviour. The dashed boxes shown in images indicate locations where the intensity profiles were obtained. An obvious intensity increase at the NP surface in e marked with an arrow indicates surface segregation of Pt at 350 °C, which is absent at RT and 550 °C.
Multi-slice simulations further proves that such a contrast change at the NP surface is a result of Pt segregation. Structural models of a Pt3Co NP, with and without Pt segregation, were calculated, and the corresponding HAADF-STEM images were simulated using multi-slice methods (Fig. 4). A particle size of 5.3 nm was used in the calculations to reduce the computing time. The structural models were based on a randomly alloyed Pt3Co with structural relaxations. The Pt3Co NP structure with Pt segregation (Fig. 4d) was obtained by energy-biased Metropolis Monte Carlo (MC) simulations at 350 °C. Detailed descriptions regarding the calculation and simulation methods are described in the Supplementary Information. The simulated HAADF-STEM images (Fig. 4b,e) show relatively clear atomic columns compared with the experimental images shown in Fig. 2 because of the smaller particle size used for the simulations. Nevertheless, an obvious difference in contrast was revealed in the intensity profiles from the surface atomic columns between the NP without and with Pt segregation, as shown in Fig. 4c,f, respectively, which are consistent with the NP behaviour observed experimentally (Fig. 3).
Multi-slice simulations of HAADF-STEM images of relaxed alloyed Pt3Co NPs performed for a particle size of 5.3 nm. (a,d) Calculated structure models without and with Pt surface segregation. Corresponding simulated images (b,e) and intensity profiles across simulated images (c,f). Dashed boxes shown in b and e indicate the locations where the intensity profiles were measured. Arrows in e and f mark the contrast increase, which results from Pt surface segregation.
The Pt-enriched surfaces disappear when the annealing temperature is increased from 350 to 550 °C (Figs 2c and 3c,f), behaviour that was also observed for other Pt3Co NPs (Supplementary Fig. 4) in the same sample. These results show that Pt-segregation to the NP surface is extremely sensitive to the annealing temperature and is explained by the interplay between surface segregation and the compositional disorder present in the as-synthesized Pt3Co NPs. The segregation of Pt to the NP surface is mainly driven by the decrease in free energy owing to a negative surface segregation enthalpy17, which will lead to a decrease in configurational entropy of the system by inducing surface compositional ordering. Although surface-segregation free energy eventually turns positive because of the increased entropic contribution, increasing the annealing temperature results in the observed disappearance of the Pt-segregated surface. The surface segregation behaviour of Pt was confirmed by EDS map** of the NPs at 350 and 550 °C, as shown in Fig. 5a–d for a different annealed Pt3Co NP from the same sample. The Pt3Co NP exhibits a homogenous random elemental distribution at RT; Pt surface enrichment is observed when the NP is heated to 350 °C (Fig. 5e–h), but vanishes when the temperature is further increased to 550 °C (Fig. 5i–l). Careful selection of the NP annealing temperature is critical to form a Pt-segregated surface architecture for enhanced catalytic activity.
HAADF-STEM images and corresponding EDS elemental maps of a Pt3Co nanoparticle, which was annealed at different temperatures: RT (a–d), 350 °C (e–h) and 550 °C (i–l). The intensity profiles of Pt and Co (rightmost column) are aligned to the maximum of Pt and Co intensities in each sub-figure and demonstrate that Pt segregates to the particle surface at 350 °C and diminishes when NP is annealed at 550 °C. These intensity profiles are the average of intensity profiles at perpendicular and horizontal directions (schematically shown as dashed boxes in the Co map at RT). Scale bar, 4 nm.
Surface faceting accompanies the elemental diffusion observed for the NPs. Distinct {110} surface facets form at 350 °C (shown by arrows in Fig. 2b), the development of which are coincident with the observed Pt surface segregation. Surface faceting occurs as a combined result of Pt segregation to the surface and the initiation of atomic surface reconstruction during the early stage of annealing, which is consistent with previous theoretical calculations11. Well-defined facets are observed at 550 °C compared with those formed at lower temperatures (350 °C), which is likely a result of enhanced elemental diffusion at the elevated temperature. Interestingly, {001} faceting was more prominent, whereas {110} facets shrink, when the temperature is increased from 350 to 550 °C, indicating a lower surface energy of {001} compared with {110} at the higher temperature. {111} faceting is minimal in the low-temperature regime and nearly vanishes when the temperature is increased to 550 °C. In fact, clearly defined {111} facets did not form until the NPs were annealed at 600 °C for 40 min. As the NP surface electronic structure is dependent on the atomic coordination, directly observing the evolution of surface faceting on alloy NPs provides valuable insight for designing NPs with desirable surface configurations.
Nucleation and development of ordered framework
When the sample temperature was increased to 600 °C and held for 20 min, alternating bands of bright-dark intensity on the {001} planes appeared at the {110} surface facets of the NP (shown by arrows in Fig. 2d). This alternating contrast is associated with the initiation of elemental ordering, that is, nucleation of the ordered Pt3Co phase on {001}. The brighter bands correspond to Pt-rich {001} planes, whereas the darker {001} planes have higher Co concentrations, for example, formation of the ordered superlattice. The observation that Pt-Co ordering initiates at the {110} surfaces suggests that this surface has the lowest activation energy for the phase transformation from simple disordered face-centered cubic Pt3Co to the ordered primitive L12 Pt3Co phase. This is the first direct observation of the surface initiation of the disorder-to-order phase transformation in a single NP, whereby the specific surface associated with the transformation is identified experimentally. However, a critical question is raised: why does the disorder-to-order phase transformation initiate on the Pt3Co NP {110} surface facets? First-principles density functional theory (DFT) calculations were performed to evaluate the formation energy between the ordered and disordered phases for a Pt3Co bulk crystal, and the dominant surface facets observed for the Pt3Co NPs, e.g., {100} and {110}. Energy calculations for these crystallographic surfaces were performed using surface slab models with a thickness of eight atomic layers. The ordered configurations had lower formation energies for all three pairs of comparative structures, 14.4, 11.1 and 1.5 meV per atom for the (110), Pt3Co bulk crystal and (100) surfaces, respectively (Fig. 6). Consequently, the energetic driving force for the disorder-to-order transition is the highest for the {110} surfaces of the Pt3Co NPs, which matches the experimental observation that atomic ordering of the Pt3Co NP initiates on the {110} facets as compared with the {100} facets and the bulk Pt3Co NP core.
The ordered structures that initiate on the {110} surfaces propagated on the {001} planes into the centre of the NP with further annealing at 600 °C (Fig. 2e). When the particle was annealed at 600 °C for longer times (Fig. 2d–g), elemental diffusion within the bulk progressed, as evidenced by the largely inhomogeneous image contrast intensity distributions (Fig. 2f). The ordered structures form on opposite sides of the NP (Fig. 2d–f), that is, those associated with {110} surfaces, eventually coalescing in the centre of the NP with continued annealing at 600 °C, resulting in an ordered Pt3Co NP structure (Fig. 2g). This phase transformation mechanism is consistently observed for multiple Pt3Co NPs under these annealing conditions. Figure 7 shows HAADF-STEM images of the annealed Pt3Co NPs and corresponding diffractograms (fast Fourier transforms—FFTs—of the images). The <110> oriented NP (Fig. 7a–c) exhibits a different ordering contrast compared with the NP in a <100> zone axis orientation (Fig. 7d–f) as a result of the distinctive elemental stacking in the projected viewing directions. When oriented along <100>, each Co column is surrounded by eight Pt columns, forming a checkerboard-type intensity pattern in the HAADF-STEM image (Fig. 7d,e). The {100} superlattice reflections are clearly observed in the corresponding FFTs (Fig. 7f). It should be noted that the disorder-to-order phase transformation temperature of 600 °C for the Pt3Co NPs is slightly lower than that reported for bulk Pt3Co (∼650 °C)18, which can be explained by a higher elemental inter-diffusion rate within NPs compared with that for the bulk material owing to the significantly increased surface area of the NPs19.
(a,d) HAADF-STEM images of the ordered phase shown for [110] and [100] NP zone axis orientations. (b,e) Corresponding projections of the perfectly ordered structure models (silver spheres represents Pt atoms and red spheres represent Co atoms). (c,f) FFTs from the HAADF-STEM images showing superlattice reflections indicative of structural/elemental ordering. Scale bar, 2 nm.
Initiation of amorphization
Continued annealing at 600 °C for another 20 min does not change the ordering architecture but leads to further elemental inter-diffusion (Fig. 2g). Complete ordering within the Pt3Co particle is realized when the temperature is increased to 700 °C and held for only 10 min. (Fig. 2h). The contrast difference between the ordered {001} planes (alternating Pt-rich and Co-rich layers) is obviously greater compared with that observed at 600 °C, indicating extensive Pt-Co inter-diffusion towards the optimized L12 ordered structure. No observable elemental diffusion or structure changes were observed with further annealing at 700 °C for 30 min, which suggests that the NP achieved a thermally stable L12 structure after 10 min. Our observations on the evolution of the ordered structure shows that an ordered framework is established first in the NPs followed by additional diffusion towards a stable ordered structure. When the Pt3Co NPs are further annealed to a temperature of 800 °C, the atomic columns in the HAADF-STEM image (Fig. 2i) become blurred as a result of increased atomic vibration during imaging at the elevated temperature, although the ordered structure is still vaguely visible. Longer annealing times at 800 °C results in amorphization (that is, pre-melting) of the NP and the morphology assumes a rounded shape and loss of surface faceting.
The progressive transition of the disordered alloy Pt3Co NP to the ordered L12 phase (200–700 °C) and subsequent loss of crystallinity (800 °C) is also shown by the evolution of the diffractograms (FFTs). Supplementary Fig. 5 shows a series of FFTs acquired from selected HAADF-STEM images shown in Fig. 2. The intensity of the superlattice reflections ({001} and {110}) can be used as a direct indicator of the extent of structural ordering in the Pt3Co NP. The superlattice reflections appeared initially for the NP annealed at 600 °C for 20 min (Supplementary Fig. 5c). Continued annealing results in the gradual increase in the intensity of the superlattice reflections (Supplementary Fig. 5c,d). When the NP is annealed at 700 °C for 10 min (Supplementary Fig. 5e), the superlattice reflections are sharp and intense, corresponding to a highly ordered L12 structure. Annealing to 800 °C results in diffuse diffraction spots (Supplementary Fig. 5f), which may result from the vibration of atomic columns before amorphization of the structure, although the NP might be slightly tilted away from [110] zone axis indicated by the loss of {011} superlattice reflections in the FFT (Supplementary Fig. 5f).