Abstract
Light and gibberellins (GAs) antagonistically regulate hypocotyl elongation in plants. It has been demonstrated that DELLAs, which are negative regulators of GA signalling, inhibit phytochrome-interacting factors 3 and 4 (PIF3 and PIF4) by sequestering their DNA-recognition domains. However, it is unclear whether there are other mechanisms of regulatory crosstalk between DELLAs and PIFs. Here, we demonstrate that DELLAs negatively regulate the abundance of four PIF proteins through the ubiquitin–proteasome system. Reduction of PIF3 protein abundance by DELLAs correlates closely with reduced hypocotyl elongation. Both sequestration and degradation of PIF3 by DELLAs contribute to a reduction in PIF3 binding to its target genes. Thus, we show that promotion of PIF degradation by DELLAs is required to coordinate light and GA signals, and the dual regulation of transcription factors by DELLAs by both sequestration and degradation may be a general mechanism.
Similar content being viewed by others
Introduction
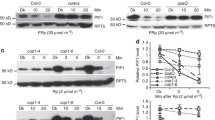
Light promotes plant photomorphogenesis, giving rise to open and expanded cotyledons, and short hypocotyls in light-grown Arabidopsis seedlings. In the dark, seedlings undergo skotomorphogenesis/etiolation, as characterized by closed cotyledons and elongated hypocotyls1. A subset of basic helix-loop-helix (bHLH) transcription factors, known as phytochrome-interacting factors (PIFs), has been reported to have a key role in etiolation and light-regulated plant development. PIF3 was the first characterized member of this gene family, identified by yeast two-hybrid screen using phytochrome B as the bait2. Mutation of PIF3 resulted in short hypocotyls in red light, indicating that PIF3 is a negative regulator of light signal transduction3,4. Several other homologous PIF proteins, including PIF1, PIF4, and PIF5, have also been reported to regulate photomorphogenesis5,6,7. A quadruple pif mutant (lacking PIF1, PIF3, PIF4, and PIF5) exhibits a striking constitutively photomorphogenic phenotype in the dark, which indicates that these four PIF factors act redundantly to promote etiolated growth8,9,10. In the dark, PIF proteins accumulate and directly regulate thousands of genes to maintain skotomorphogenesis11,12,13. Upon illumination, the photoactived phytochromes trigger PIFs’ rapid phosphorylation and subsequently proteasome-mediated degradation14,15,16,17, leading to a cascade of transcriptional changes to promote photomorphogenesis12,18,19,20.
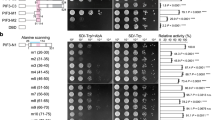
During seedling development, gibberellins (GAs) promote etiolated growth, thus showing the opposite effect to light on photomorphogenic programme21. Deficiency of GA (as in ga1-3 mutant) induces partial constitutive photomorphogenesis phenotype in darkness, resulting in a loss of apical hook, open cotyledons and shortened hypocotyls21. DELLA proteins are the key repressors of almost all GA responses22. There are five DELLA proteins in Arabidopsis GA INSENSITIVE (GAI), REPRESSOR OF ga1-3 (RGA), RGA-LIKE 1 (RGL1), RGL2 and RGL3, which have both distinct and overlap** functions22,23,24,25,26,27,28,29,30. In darkness, constitutively photomorphogenic phenotypes of ga1-3 mutants can be almost fully suppressed by rga and gai null alleles, indicating that RGA and GAI are the two main DELLA members involved in GA-dependent repression of photomorphogenic growth in seedlings21,31,32. When GA is present, GA receptor GID1 binds to the DELLAs to form GID1–GA–DELLA complex, which triggers the ubiquitination and subsequent degradation of DELLA proteins by the 26S proteasome33,34,35. DELLAs have a conserved DELLA domain at the N terminus that is essential for GA-triggered protein degradation22. Deletion of the DELLA domain results in stabilization of these proteins and leads to a GA-unresponsive dwarf phenotype22.
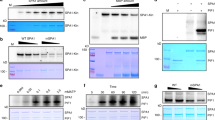
How light and GAs antagonistically regulate hypocotyl elongation has been extensively analyzed in the past two decades. Two independent studies demonstrated that DELLAs can physically interact with and block PIF3 and PIF4 activities by sequestering the transcription factors from binding to their targets, which ultimately results in inhibition of hypocotyl elongation36,37. Further study showed that DELLAs also interact with the bHLH proteins PIF1 (also known as PIL5), PIF6 (also known as PIL2) and SPATULA(SPT)38. The sequestration of PIFs by DELLAs provided an important molecular link between light and GA signalling in regulating photomorphogenesis. Later, the model of sequestration was demonstrated to be a general mechanism for DELLAs to regulate transcription factors involved in other signalling pathways39,40,41,42,40, 41, 42, 43, 44). These results suggest that sequestration is a general mechanism by which DELLAs inhibit transcription factors. Among these transcription factors, BZR1 was the only one for which DELLA was reported to promote a reduction in protein levels41. Here, we showed that DELLAs can promote the degradation of PIF1, PIF3, PIF4 and PIF5. Extending from this finding, we propose that controlling protein abundance of transcription factors represents another general mechanism of how DELLAs may regulate their targets. To test this hypothesis, we examined EIN3 from the ethylene signalling pathway. A previous study showed that DELLAs sequester EIN3, but GA3 did not markedly affect EIN3 protein abundance42. Consistently, GA treatments did not noticeably affect the abundance of EIN3-GFP and EIN3-FLAG proteins in our experiment; however, PAC treatments dramatically reduced their abundance (Supplementary Fig. 9a,b). It is possible that EIN3 accumulates to a high level in darkness, and is not sensitive to exogenous GA, which decreases DELLA levels but could not further increase EIN3 abundance. On the other hand, highly elevated levels of DELLAs after PAC treatment may promote EIN3 degradation. Taken together, our data suggests that the inhibition of transcription factors by DELLAs is mediated through both sequestration and degradation.
Methods
Plant materials and growth conditions
The wild-type Arabidopsis ecotypes used in this study were Columbia-0 (Col-0), Landsberg erecta (Ler) and Wassilewskija (WS). The mutants and transgenic lines were described previously: pif3-3 (ref. 4), 35S: PIF1-Myc, 35S: PIF3-Myc, 35S: PIF4-Myc and 35S: PIF5-Myc (ref. 48), rga-24 (ref. 25), rga-Δ17 (ref. 26), della (gai-t6 rga-t2 rgl1-1 rgl2-1 rgl3-1) (ref. 37), sly1-10 gai-t6, sly1-10 rga-24, sly1-10 gai-t6 rga-24 (ref. 34), phyB-9 (ref. 51), lrb123 (ref. 16), cop1-4 (ref. 52), det1-1(ref. 53), 35S: EIN3-GFP/ein3 eil1 (ref. 54) and 35S: EIN3-FLAG/ein3 eil1 (ref. 55). Since homozygous rga-Δ17 is sterile26, the homozygous rga-Δ17 seedlings segregated from the progenies of heterozygous rga-Δ17 were used for experiments. gai (Col background) is a gift from **angdong Fu of IGDB. All seeds were surface-sterilized and sown on Murashige and Skoog (MS) medium containing 1% sugar. Seeds were cold-treated at 4 °C for 4 days in the dark before germination. The seedlings were grown in the dark, under continuous red light (0.5 μmol m−2s−1) or under short-day conditions (8 h white light (85 μmol m−2s−1) +16 h dark) for the indicated times, unless indicated otherwise. Manipulation of seedlings in darkness was performed under dim green light.
Generation of transgenic plants
DNA fragments containing two deletion mutants (RGAΔ17 and GAIΔ17) without stop codons were amplified and inserted into the XmaI and PstI restriction sites of a pBSK-derived plasmid containing triple HA-tag to make constructs pBSK-RGAΔ17-HA and pBSK-GAIΔ17-HA. Further, the fragments RGAΔ17-HA and GAIΔ17-HA were amplified from pBSK-RGAΔ17-HA or pBSK-GAIΔ17-HA, and these fragments were digested with the SalI and SpeI, and then ligated into the XhoI and SpeI digested binary vector pTA7002 (ref. 56) to generate pTA7002-RGAΔ17-HA and pTA7002-GAIΔ17-HA constructs. These binary constructs were introduced into the GV3101 strain of Agrobacterium and transformed into della mutant plants, using the floral dip transformation method57. The transformants were selected on MS medium containing 50 mg ml−1 hygromycin B (Sigma-Aldrich), and named as RGAΔ17-HA and GAIΔ17-HA, respectively. The primers are listed in Supplementary Table 2.
Plant treatments
GA3 (GA), paclobutrazol (PAC) and DEX were dissolved in ethanol. The proteasome inhibitor MG132 was dissolved in DMSO. For continuous GA3 and PAC treatments, after the seeds were induced by white light for germination on regular MS medium, they were transferred to medium containing GA3 (10 μM), PAC (0.5 μM) and ethanol (0.01% (v/v), as control), and then grown in the dark for 4 days. For the transient treatment by GA3, the seedlings were collected and vacuum-infiltrated with liquid MS medium containing 100 μM GA3 or ethanol alone (as control) for 10 min and then kept immersed in the same solution for the indicated times. For continuous DEX treatment, Arabidopsis seedlings were grown on the MS medium containing 1 μM DEX or ethanol alone (as control). For the transient induction by DEX, the 4-day-old seedlings were vacuum-infiltrated with liquid MS medium containing 10 μM DEX or ethanol alone (as control) for 10 min and then kept immersed in the same solution for the indicated times. For MG132 treatment, the 4-day-old seedlings were vacuum-infiltrated with liquid MS medium containing 100 μM MG132 (dissolved in DMSO) or DMSO alone (as control) for 10 min and kept immersed in the same solution for 4 h unless indicated otherwise.
Protein extraction and Immunoblots
Total proteins were extracted by homogenizing seedlings using denaturing buffer (100 mM NaH2PO4, 10 mM Tris–HCl pH 8.0, 8 M urea, 1 mM phenylmethylsulfonyl fluoride, and × 1 complete protease inhibitor mixture (Roche). Seedlings were growing in the dark for 4 days unless specific indications. Extracts were centrifuged at 16,000g for 10 min at 4 °C, and protein concentration in the supernatants was quantified by the Bradford assay. Aliquots of denatured total protein were separated on 8% SDS–PAGE gels and transferred to PVDF membranes. Anti-PIF3 purified antibody at 1:500 (v/v) dilution (ref. 48), anti-Myc polyclonal antibody (Sigma-Aldrich, Cat. No: C3956) at 1:1,000 (v/v) dilution, anti-HA antibody (Sigma-Aldrich, Cat. No: H9658) at 1:1,000 (v/v) dilution, anti-RGA antibody (Agrisera, Cat. No: AS111630) at 1:1,000 (v/v) dilution, anti-FLAG (Sigma-Aldrich, Cat. No: F3165) at 1:1,000 (v/v) dilution, anti-GFP (Abmart, Cat. No: M20004) at 1:1,000 (v/v) dilution, anti-RPN6 polyclonal antibody (ref. 58) at 1:2,000 (v/v) dilution, and anti-PRT5 polyclonal antibody (ref. 58) at 1:2,000 (v/v) dilution were used as primary antibodies. Each experiment was repeated at least three times, and one representative result was shown. Quantification results of immunoblots in Figs 3f, 4d, 6b, 6d-6f, were quantified by Image J software. Supplementary Figures 10–22 are original full versions of the immunoblot images.
RNA extraction and quantitative RT-PCR
Total RNA was extracted using the RNeasy plant mini kit (Qiagen). cDNA was synthesized by ReverTra Ace qPCR RT Master Mix (TOYOBO). The quantitative RT-PCR analysis was performed using SYBR Premix Ex Taq (Takara) in an ABI 7500 fast real-time instrument. Each experiment was repeated with three biological samples, and RT-PCR reactions were performed with three technical replicates for each sample. The primers are listed in Supplementary Table 2.
Hypocotyl lengths measurement
After the indicated times of growth and treatment, at least 30 seedlings were laid on the agar plates, and digital pictures were taken. Then, the hypocotyl lengths were measured using Image J software.
In vitro pull down assays
The constructs for expressing His-PIF1, His-PIF3, GST-PIF4 and GST-PIF5 were described previously48. Full-length RGA fragment was inserted into pMal-C2X vector to fuse with maltose-binding protein (MBP). All constructs were expressed in E. coli strain BL21 under the induction of 1 mM IPTG (isopropyl-β-D-thiogalactopyranoside). Two micrograms MBP or MBP–RGA proteins were mixed with 2 μg His- or GST-tagged proteins in 500 μl binding buffer (20 mM Tris–HCl, pH 7.5, 150 mM NaCl and 0.1% Nonidet P-40), and the mixture was rotated at 4 °C for 2 h. The amylase agarose beads were washed with binding buffer for three times and then added into the mixture. Then, the mixture was rotated at 4 °C for another 2 h. After being washed for five times with binding buffer, the MBP resin was boiled with protein loading buffer and analyzed by immunoblots. Anti-His (Sigma-Aldrich, Cat. No: H1029-.2ML) at 1:1,000 dilution, anti-GST (Sigma-Aldrich, Cat. No: G1160-.2ML) at 1:5,000 dilution and anti-MBP (New England Biolabs, Cat. No: E8032S) at 1:5,000 dilution were used for the western blots.
Bimolecular fluorescence complementation assay
The full-length cDNA of RGA was amplified and inserted into the SpeI and BamHI sites of pSY736 (YFPN) vector, resulting in plasmid YFPN-RGA. YFPC-PIF1, YFPC-PIF3, YFPC-PIF4 and YFPC-PIF5 plasmids were described previously48. The plasmids were extracted and concentrated to 2 mg ml−1. The particle-mediated transformation using onion epidermal cells was performed59. After 24 h of incubation, YFP signal was detected using a Zeiss LSM 710 confocal microscope. The primers used for plasmid construction were listed in Supplementary Table 2.
Chromatin immunoprecipitations assay
ChIP assays were performed as described previously37. 35S:PIF3-Myc seedlings were grown for 4 days on the MS medium containing 0.5 μM PAC or EtOH in darkness, and were collected and treated with DMSO or 100 μM MG132 for 4 h. RGAΔ17-HA seedlings were grown for 4 days in the dark, and were collected and infiltrated with or without 10 μM DEX for 24 h. The samples (2 g) were treated with 15 ml of 1% formaldehyde under vacuum infiltration for 15 min, and then 1 ml 2 M glycine was added to stop crosslinking for 5 min. For the ChIP analysis used 35S: PIF3-Myc seedlings, the solubilized chromatin was immunoprecipitated by 30 μl EZview Red Anti-c-Myc Affinity Gel (Sigma-Aldrich, Cat. No: E6654) at 4 °C for 5 h. For the ChIP analysis using RGAΔ17-HA seedlings, the solubilized chromatin was mixed with 10 μl anti-HA antibody (Sigma) and incubated at 4 °C for 1 h. Then, 40 μl Dynabeads Protein G (Life Technologies, Cat. No: 10003D) was added, and the sample was incubated overnight at 4 °C. The coimmunoprecipitated DNA was recovered and analyzed by quantitative PCR. All primers used in ChIP assays were listed in Supplementary Table 2.
Data availability
The authors declare that all data supporting the findings of this study are available within the article and its Supplementary Information files or are available upon request from the corresponding authors.
Additional information
How to cite this article: Li, K. et al. DELLA-mediated PIF degradation contributes to coordination of light and gibberellin signalling in Arabidopsis. Nat. Commun. 7:11868 doi: 10.1038/ncomms11868 (2016).
References
Von Arnim, A. & Deng, X. W. Light control of seedling development. Ann. Rev. Plant. Physiol. Mol. Biol. 47, 215–243 (1996).
Ni, M., Tepperman, J. M. & Quail, P. H. PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell 95, 657–667 (1998).
Kim, J. Y. et al. Functional characterization of phytochrome interacting factor 3 in phytochrorne-mediated light signal transduction. Plant Cell 15, 2399–2407 (2003).
Monte, E. et al. The phytochrome-interacting transcription factor, PIF3, acts early, selectively, and positively in light-induced chloroplast development. Proc. Natl Acad. Sci. USA 101, 16091–16098 (2004).
Huq, E. & Quail, P. H. PIF4, a phytochrome-interacting bHLH factor, functions as a negative regulator of phytochrome B signalling in Arabidopsis. EMBO J. 21, 2441–2450 (2002).
Huq, E. et al. PHYTOCHROME-INTERACTING FACTOR 1 is a critical bHLH regulator of chlorophyll biosynthesis. Science 305, 1937–1941 (2004).
Khanna, R. et al. A novel molecular recognition motif necessary for targeting photoactivated phytochrome signalling to specific basic helix-loop-helix transcription factors. Plant Cell 16, 3033–3044 (2004).
Leivar, P. et al. Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr. Biol. 18, 1815–1823 (2008).
Shin, J. et al. Phytochromes promote seedling light responses by inhibiting four negatively-acting phytochrome-interacting factors. Proc. Natl Acad. Sci. USA 106, 7660–7665 (2009).
Leivar, P. et al. Dynamic antagonism between phytochromes and PIF family basic helix-loop-helix factors induces selective reciprocal responses to light and shade in a rapidly responsive transcriptional network in Arabidopsis. Plant Cell 24, 1398–1419 (2012).
Leivar, P. et al. Definition of early transcriptional circuitry involved in light-induced reversal of PIF-imposed repression of photomorphogenesis in young Arabidopsis seedlings. Plant Cell 21, 3535–3553 (2009).
Leivar, P. & Quail, P. H. PIFs: pivotal components in a cellular signalling hub. Trends Plant Sci. 16, 19–28 (2011).
Zhang, Y. et al. A quartet of PIF bHLH factors provides a transcriptionally centered signalling hub that regulates seedling morphogenesis through differential expression-patterning of shared target genes in Arabidopsis. PLoS Genet. 9, e1003244 (2013).
Al-Sady, B., Ni, W., Kircher, S., Schafer, E. & Quail, P. H. Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol. Cell 23, 439–446 (2006).
Shen, Y., Khanna, R., Carle, C. M. & Quail, P. H. Phytochrome induces rapid PIF5 phosphorylation and degradation in response to red-light activation. Plant Physiol. 145, 1043–1051 (2007).
Ni, W. M. et al. A mutually assured destruction mechanism attenuates light signalling in Arabidopsis. Science 344, 1160–1164 (2014).
Shen, H. et al. Light-induced phosphorylation and degradation of the negative regulator phytochrome-interacting factor1 from Arabidopsis depend upon its direct physical interactions with photoactivated phytochromes. Plant Cell 20, 1586–1602 (2008).
Castillon, A., Shen, H. & Huq, E. Phytochrome Interacting Factors: central players in phytochrome-mediated light signalling networks. Trends Plant Sci. 12, 514–521 (2007).
Jiao, Y. L., Lau, O. S. & Deng, X. W. Light-regulated transcriptional networks in higher plants. Nat. Rev. Genet. 8, 217–230 (2007).
Bae, G. & Choi, G. Decoding of light signals by plant phytochromes and their interacting proteins. Annu. Rev. Plant Biol. 59, 281–311 (2008).
Alabadi, D., Gil, J., Blazquez, M. A. & Garcia-Martinez, J. L. Gibberellins repress photomorphogenesis in darkness. Plant Physiol. 134, 1050–1057 (2004).
Sun, T. P. Gibberellin metabolism, perception and signalling pathways in Arabidopsis. Arabidopsis book 6, e0103 (2008).
Koornneef, M. et al. A gibberellin insensitive mutant of Arabidopsis thaliana. Physiol. Plantarum 65, 33–39 (1985).
Peng, J. R. et al. The Arabidopsis GAI gene defines a signalling pathway that negatively regulates gibberellin responses. Gene Dev. 11, 3194–3205 (1997).
Silverstone, A. L., Mak, P. Y. A., Martinez, E. C. & Sun, T. P. The new RGA locus encodes a negative regulator of gibberellin response in Arabidopsis thaliana. Genetics 146, 1087–1099 (1997).
Dill, A., Jung, H. S. & Sun, T. P. The DELLA motif is essential for gibberellin-induced degradation of RGA. Proc. Natl Acad. Sci. USA 98, 14162–14167 (2001).
Silverstone, A. L. et al. Repressing a repressor: gibberellin-induced rapid reduction of the RGA protein in Arabidopsis. Plant Cell 13, 1555–1565 (2001).
Wen, C. K. & Chang, C. Arabidopsis RGL1 encodes a negative regulator of gibberellin responses. Plant Cell 14, 87–100 (2002).
Lee, S. C. et al. Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibition. Gene Dev. 16, 646–658 (2002).
Tyler, L. et al. DELLA proteins and gibberellin-regulated seed germination and floral development in Arabidopsis. Plant Physiol. 135, 1008–1019 (2004).
Reed, J. W., Foster, K. R., Morgan, P. W. & Chory, J. Phytochrome B affects responsiveness to gibberellins in Arabidopsis. Plant Physiol. 112, 337–342 (1996).
Achard, P. et al. DELLAs contribute to plant photomorphogenesis. Plant Physiol. 143, 1163–1172 (2007).
McGinnis, K. M. et al. The Arabidopsis SLEEPY1 gene encodes a putative F-box subunit of an SCF E3 ubiquitin ligase. Plant Cell 15, 1120–1130 (2003).
Dill, A., Thomas, S. G., Hu, J., Steber, C. M. & Sun, T. P. The Arabidopsis F-box protein SLEEPY1 targets gibberellin signalling repressors for gibberellin-induced degradation. Plant Cell 16, 1392–1405 (2004).
Fu, X. et al. The Arabidopsis mutant sleepy1gar2-1 protein promotes plant growth by increasing the affinity of the SCFSLY1 E3 ubiquitin ligase for DELLA protein substrates. Plant Cell 16, 1406–1418 (2004).
de Lucas, M. et al. A molecular framework for light and gibberellin control of cell elongation. Nature 451, 480–484 (2008).
Feng, S. et al. Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 451, 475–479 (2008).
Gallego-Bartolome, J. et al. Transcriptional diversification and functional conservation between DELLA proteins in Arabidopsis. Mol. Biol. Evol. 27, 1247–1256 (2010).
Bai, S. et al. PIF3 is involved in the primary root growth inhibition of Arabidopsis induced by nitric oxide in the light. Mol. Plant 7, 616–625 (2014).
Bai, M. Y. et al. Brassinosteroid, gibberellin and phytochrome im**e on a common transcription module in Arabidopsis. Nat. Cell Biol. 14, 810–U878 (2012).
Li, Q. F. et al. An interaction between BZR1 and DELLAs mediates direct signalling crosstalk between brassinosteroids and gibberellins in Arabidopsis. Sci. Signal 5, ra72 (2012).
An, F. et al. Coordinated regulation of apical hook development by gibberellins and ethylene in etiolated. Arabidopsis seedlings. Cell. Res. 22, 915–927 (2012).
Hou, X. L., Lee, L. Y. C., **a, K. F., Yen, Y. Y. & Yu, H. DELLAs modulate jasmonate signalling via competitive binding to JAZs. Dev. Cell. 19, 884–894 (2010).
Qi, T. et al. Arabidopsis DELLA and JAZ proteins bind the WD-Repeat/bHLH/MYB complex to modulate gibberellin and jasmonate signalling synergy. Plant Cell 26, 1118–1133 (2014).
Arana, M. V., Marin-de la Rosa, N., Maloof, J. N., Blazquez, M. A. & Alabadi, D. Circadian oscillation of gibberellin signalling in Arabidopsis. Proc. Natl Acad. Sci. USA 108, 9292–9297 (2011).
Soy, J. et al. Phytochrome-imposed oscillations in PIF3 protein abundance regulate hypocotyl growth under diurnal light/dark conditions in Arabidopsis. Plant J. 71, 390–401 (2012).
Bauer, D. et al. Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signalling in Arabidopsis. Plant Cell 16, 1433–1445 (2004).
Dong, J. et al. Arabidopsis DE-ETIOLATED1 represses photomorphogenesis by positively regulating phytochrome-interacting factors in the dark. Plant Cell 26, 3630–3645 (2014).
Zentella, R. et al. Global analysis of della direct targets in early gibberellin signalling in Arabidopsis. Plant Cell 19, 3037–3057 (2007).
Park, J., Nguyen, K. T., Park, E., Jeon, J. S. & Choi, G. DELLA proteins and their interacting RING finger proteins repress gibberellin responses by binding to the promoters of a subset of gibberellin-responsive genes in Arabidopsis. Plant Cell 25, 927–943 (2013).
Reed, J. W., Nagpal, P., Poole, D. S., Furuya, M. & Chory, J. Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5, 147–157 (1993).
McNellis, T. W. et al. Genetic and molecular analysis of an allelic series of cop1 mutants suggests functional roles for the multiple protein domains. Plant Cell 6, 487–500 (1994).
Chory, J., Peto, C., Feinbaum, R., Pratt, L. & Ausubel, F. Arabidopsis thaliana mutant that develops as a light-grown plant in the absence of light. Cell 58, 991–999 (1989).
He, W. et al. A small-molecule screen identifies L-kynurenine as a competitive inhibitor of TAA1/TAR activity in ethylene-directed auxin biosynthesis and root growth in Arabidopsis. Plant Cell 23, 3944–3960 (2011).
An, F. et al. Coordinated regulation of apical hook development by gibberellins and ethylene in etiolated Arabidopsis seedlings. Cell. Res. 22, 915–927 (2012).
Aoyama, T. & Chua, N. H. A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J. 11, 605–612 (1997).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Peng, Z. et al. The cellular level of PR500, a protein complex related to the 19S regulatory particle of the proteasome, is regulated in response to stresses in plants. Mol. Biol. Cell 12, 383–392 (2001).
von Arnim, A. Subcellular localization of GUS- and GFP-tagged proteins in onion epidermal cells. CSH Protoc. 2007, pdb.prot4689 (2007).
Acknowledgements
We thank P.H. Quail for lrb123, 35S:PIF1-Myc, 35S:PIF3-Myc, 35S:PIF4-Myc, and 35S:PIF5-Myc seeds; H. Guo for 35S:EIN3-GFP/ein3 eil1 and 35S:EIN3-Flag/ein3 eil1 seeds; T.P. Sun for sly1-10, sly1-10 gai-t6, sly1-10 rga-24, and sly1-10 gai-t6 rga-24 seeds; X. Fu for gai seed; G. Gusmaroli for assistance in generation of RGAΔ17-HA and GAIΔ17-HA transgenic plants; Y. Wang for preliminary experiments. We also thank S. Feng of UCLA for critical reading and comments on the manuscript. This work was supported by grants from the National Natural Science Foundation of China (31330048 to X.W.D. and 31271294 to H.C.), National Program on Key Basic Research Project of China (2012CB910900 to X.W.D.), NIH of United States (GM-47850 to N.W.), Peking-Tsinghua Center for Life Sciences, and State Key Laboratory of Protein and Plant Gene Research.
Author information
Authors and Affiliations
Contributions
H.C. and X.W.D. conceived and supervised the research. K.L., L.-M.F., H.C. and X.W.D. designed the experiments. K.L. performed most of the experiments. R.Y. generated and purified the PIF3 antibody. K.L., N.W., H.C. and X.W.D. wrote the manuscript. All authors discussed the results and made contributions to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1 - 22 and Supplementary Tables 1 and 2 (PDF 7355 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, K., Yu, R., Fan, LM. et al. DELLA-mediated PIF degradation contributes to coordination of light and gibberellin signalling in Arabidopsis. Nat Commun 7, 11868 (2016). https://doi.org/10.1038/ncomms11868
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms11868
- Springer Nature Limited
This article is cited by
-
Petunia PHYTOCHROME INTERACTING FACTOR 4/5 transcriptionally activates key regulators of floral scent
Plant Molecular Biology (2024)
-
A gibberellin-deficient maize mutant exhibits altered plant height, stem strength and drought tolerance
Plant Cell Reports (2023)
-
Transcriptomic Analysis Reveals Suppression of Photosynthesis and Chlorophyll Synthesis Following Gibberellic Acid Treatment on Oil Palm (Elaies guineensis)
Journal of Plant Growth Regulation (2023)
-
Shade avoidance syndrome in soybean and ideotype toward shade tolerance
Molecular Breeding (2023)
-
SUPPRESSOR OF PHYTOCHROME B-4 #3 reduces the expression of PIF-activated genes and increases expression of growth repressors to regulate hypocotyl elongation in short days
BMC Plant Biology (2022)