Abstract
Parvalbumin inhibitory interneurons (PVIs) are crucial for maintaining proper excitatory/inhibitory balance and high-frequency neuronal synchronization. Their activity supports critical developmental trajectories, sensory and cognitive processing, and social behavior. Despite heterogeneity in the etiology across schizophrenia and autism spectrum disorder, PVI circuits are altered in these psychiatric disorders. Identifying mechanism(s) underlying PVI deficits is essential to establish treatments targeting in particular cognition. On the basis of published and new data, we propose oxidative stress as a common pathological mechanism leading to PVI impairment in schizophrenia and some forms of autism. A series of animal models carrying genetic and/or environmental risks relevant to diverse etiological aspects of these disorders show PVI deficits to be all accompanied by oxidative stress in the anterior cingulate cortex. Specifically, oxidative stress is negatively correlated with the integrity of PVIs and the extracellular perineuronal net enwrap** these interneurons. Oxidative stress may result from dysregulation of systems typically affected in schizophrenia, including glutamatergic, dopaminergic, immune and antioxidant signaling. As convergent end point, redox dysregulation has successfully been targeted to protect PVIs with antioxidants/redox regulators across several animal models. This opens up new perspectives for the use of antioxidant treatments to be applied to at-risk individuals, in close temporal proximity to environmental impacts known to induce oxidative stress.
Similar content being viewed by others
Main
Proper cortical excitatory-inhibitory balance through GABAergic circuits is critical for supporting cognitive, affective and social functions.1, 2 Some of these inhibitory circuits are impacted in psychiatric disorders (for example, autism,3 schizophrenia and4 bipolar disorder5). In particular, alterations in parvalbumin-expressing interneurons (PVIs) are highly replicated findings in postmortem brains of schizophrenia patients,6, 7 and are reported in bipolar (BP)8 and autism spectrum disorders (ASD).9 Anomalies in PVIs therefore constitute a pathological hallmark common to several different psychiatric conditions. Elucidating mechanisms underlying the causes and consequences of these alterations may help to identify biomarkers and novel targets for the improved detection, prevention and treatment. Here, we review published data and present novel results which provide compelling evidence for the convergence of various genetic and environmental risk factors onto oxidative stress leading to PVI impairment. We propose this represents a 'common pathological pathway', which contributes to schizophrenia, and at least some forms of ASD.
Aberrant PVI circuits in psychiatric disorders
PVIs, which express parvalbumin (a high-affinity calcium-binding protein), are fast-spiking, non-adapting interneurons. Because their inhibitory terminals synapse onto the cell body and axon initial segment of their target neurons, PVIs control the output of postsynaptic neurons via feedforward and feedback inhibition. They coordinate the activity of neuronal assemblies, particularly in high-frequency synchrony.1 PVI-associated circuits shape the processing and flow of information required for sensory perception, working memory, attention, learning, memory and social behavior. They also drive the early refinement of cortical networks. Maturation of PVIs and their specialized extracellular matrix (the perineuronal net, PNN) envelo** most PVIs10 define critical periods of network plasticity during postnatal development.11
There is accumulating evidence for anomalies of PVI-associated circuits in schizophrenia, but also in BP and ASD. In schizophrenia, these alterations include reduced expression of parvalbumin, GAD67 (GABA synthesizing enzyme), Kv3.1-containing K+--channels, changes in their pre- and postsynaptic terminals,7, 12 and abnormal PNN13 known to promote interneuron maturation, synaptic/network stability14, 15 and protection against oxidative stress.16 Together with an immature developmental gene expression profile of PVIs in ASD and schizophrenia,17 this suggests a defect in the proper maturation of these interneurons. Dysfunction of PVI-associated networks may lead to impaired high-frequency neuronal synchrony,18 and contribute to altered sensory perception, social and cognitive deficits found in patients. Understanding the mechanism(s) driving PVI anomalies is therefore central to the development of novel therapeutic strategies, targeting in particular negative symptoms and cognition.
PVIs are affected by many genetic and environmental risk factors of neurodevelopmental disorders
Schizophrenia and ASD are disorders with heterogeneous etiologies, involving a large array of genetic and environmental vulnerabilities. In preclinical models, environmental risk factors (for example, prenatal maternal stress, immune challenge, hypoxia, early-life iron deficiency, maternal separation and social isolation)19, 20, 21, 22, 23, 24 and manipulations of genes associated with these diseases (for example, DISC1, DTNB1, ERBB4, NRG1, SHANK3 and CNTAP2)25, 26, 27, 28 affect PVIs. Because of their positioning within cortical networks, their activity-dependent maturation and the plasticity of their associated networks, PVIs might be highly sensitive to abnormal cortical organization. Changes in expression of parvalbumin, GAD67, or GABA receptors may thus reflect homeostatic adaptations to alterations of neuronal wiring caused by diverse independent mechanisms of genetic and/or environmental origins. Alternatively, PVI alterations induced by a large variety of genetic and environmental factors may be attributed to a single or a subset of pathological mechanism(s) common to all these conditions. For example, PVI dysfunction in prefrontal cortex could be secondary to reduced glutamatergic transmission.7, 29
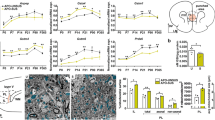
Another potential mechanism could be oxidative stress, for which there is considerable evidence in schizophrenia, BP and ASD.30, 31, 32, 33, 34, 35 Novel in vivo NAD+/NADH 31P-MRS technique has confirmed redox dysregulation in schizophrenia patients.36 Indeed, PVIs are vulnerable to redox dysregulation/oxidative stress.37, 38, 39, 40 To examine the hypothesis that oxidative stress is part of a common pathway leading to PVI abnormalities, we performed a comparative immunohistological analysis of oxidative stress and PVIs in the anterior cingulate cortex (ACC) of a panel of genetic and/or environmental models relevant to schizophrenia and ASD. Oxidative stress was detected with an antibody against 8-oxo-2'-deoxyguanosine (8-oxo-dG), a marker for DNA oxidation. PVI integrity was assessed with an antibody against parvalbumin and with the lectin, Wisteria floribunda agglutinin (WFA), to label the PNN39 (for brief methods, see legend of Figure 1). Models included genetically engineered mice-bearing human copy number variations linked to increased risk for schizophrenia and/or ASD (deletion in the 22q11, 15q13 or 1q21 loci) or knockout (KO) for genes associated with these disorders (FMR1, serine racemase (SRR) and GRIN2A), rodents with deficient glutathione (GSH) synthesis (GCLM KO mice, mice with conditional GCLC KO in PVIs, rats with transient GSH depletion during development), mouse models of gene-environment interactions (early postnatal oxidative challenge in GCLM and GRIN2A KO mice), an environmental 'two-hit' mouse model of schizophrenia (maternal immune challenge+peripubertal stress), and neurodevelopmental rat models relevant to schizophrenia (neonatal ventral hippocampal lesion (NVHL) and methylazoxymethanol acetate (MAM)). All analyses were performed in late adolescent/young adult animals (2–3 months old), and the results are summarized below and in Figure 1.
Relationship between oxidative stress and PVI integrity in the ACC of 2–3 month-old animal models relevant to schizophrenia, autism and/or redox dysregulation. (a) Oxidative stress (assessed by the immunoreactivity intensity against 8-oxo-2'-deoxyguanosine (8-oxo-dG), a marker of mitochondrial DNA oxidation), number of PV-IR cells (PV cells) and number of PV cells enwrapped with a WFA-labeled PNN. # indicates models for which the presented data are already published elsewhere.39, 41 The references indexed below provide detailed descriptions of each investigated model and its control. 22q11: mice with a 22q11.2 deletion (LgDel/+)42 (n=7 animals per group); 15q13.3: mice with a 15q13.3 deletion (Df[h15q13]/+)43 (n=5, 7); 1q21: mice with a 1q21 deletion (Df[h1q21]/+) (from M Didriksen) (n=4 per group), SRR: serine racemace KO mice44 (n=5, 7); FMR1: FMR1 KO mice45 (n=7, 8); PV-GCLC: mice with conditional KO of GCLC (catalytic subunit of the key synthesizing enzyme of GSH) in PVIs40 (n=5 per group); GRIN2A: GRIN2A KO mice46 (n=7 per group); GCLM: GCLM KO mice47 (n=5 per group); GCLM GBR: GCLM KO mice treated with dopamine uptake inhibitor GBR12909 during postnatal development (P10-20)39 (n=5 per group); GRIN2A GBR: GRIN2A KO mice treated with GBR12909 during postnatal development (P10-20) (n=7 per group); ODS BSO: ODS rats treated with the specific inhibitor of GSH synthesis (BSO) during postnatal development (P5-16)38 (n=4 per group); NVHL: rats with a neonatal ventral hippocampal lesion41 (n=6, 7); MAM: rats treated on gestation day GD17 with MAM48 (n=4, 6); Poly(I:C): mice with a sub-threshold prenatal immune challenge (on GD9) with poly(I:C); +Str: poly(I:C)-treated mice stressed at preadolescence (P30-40)49 (n=5 per group). Data are depicted by the mean±s.d. (in red: animal models; in blue: their respective controls). *** P<0.001; ** P<0.01, * P<05. (b) Quantile density contours with linear regression (red) and smoothing spline (green) plots illustrating the relationships between changes in oxidative stress (8-oxo-dG-IR), in number of PV cells, and in number of PV cells with WFA-labeled PNN (PV cells+PNN) for all animal models relative to their respective controls (JMP11, SAS Institute, Cary, NC, USA). (c) As in (b) but illustrating the relationship between changes in number of PV cells+PNN and in number of PV cells for all animal models relative to their respective controls. Brief method description: perfused fixed brains from all animal models were sent to Lausanne where immunohistological preparation, image acquisition and analyses were performed blindly using the methods described previously.39 Three to four sections per animal were used for the analyses. Analyses of 8-oxo-dG-IR intensity, numbers of PV-IR cells and PV-IR cells surrounded with a WFA-labeled PNN were done in a region of interest comprising all layers of the ACC. Oxidative stress was assessed in all cells of ROI. Each animal model was compared with its own control animals. Only males were analyzed, except for the GRIN2A model where individuals from both sexes were used. On the basis of previously analyzed data,39 sample size was choosen to detect ~25% change in number of PV-IR cells and ~75% change in 8-oxo-dG intensity with a power of 80% at a significant α-value set to P=0.05. Statistical significance was tested by comparing means of the different models with their respective controls using the Dunnett’s test. When variances were not equal, we used the Welch’s test to give confidence and confirm the Dunnett’s test outcome. ACC, anterior cingulate cortex; BSO, buthionine sulphoximine; KO, knockout; MAM, methylazoxymethanol acetate; NVHL, neonatal ventral hippocampal lesion; ODS, osteogenic disorder Shionogi; PNN, perineuronal net; PV, parvalbumin; PV-IR, parvalbumin-immunoreactive; PVI, parvalbumin inhibitory interneuron; WFA, Wisteria floribunda agglutinin.
Oxidative stress and PVI integrity in preclinical models of genetic and environmental risks
Mice with the human 22q11.2 deletion (LgDel/+)
The 22q11.2 deletion represents the most common microdeletion, and causes craniofacial, cardiovascular abnormalities, immunodeficiency and cognitive dysfunctions.50 Microdeletion of this region accounts for ~0.3% of persons diagnosed with schizophrenia,51 and leads to structure and connectivity abnormalities in various brain regions including the cortex, hippocampus, and cerebellum. Mice modeling the human 22q11.2 deletion show cognitive deficits, impaired sensorimotor gating and fear conditioning.52 In addition, they have disrupted cortical neurogenesis, abnormal interneuron migration/placement, reduced density of parvalbumin-immunoreactive (PV-IR) synapses and abnormal formation of excitatory synapses.52 Our analysis shows that, relative to their control wild-type (WT) mice, adult LgDel/+ mice displayed higher oxidative stress (+125% 8-oxo-dG immunoreactivity intensity), decreased number of PV-IR cells (−22%), and PV-IR cells surrounded by WFA-labeled PNN (-38%).
Mice with the human 15q13.3 deletion (Df[h15q13]/+)
Deletion or duplication of the chromosome region, 15q11-13, cause severe neurological phenotypes. The 15q13.3 deletion confers high risk for schizophrenia,53 but also epileptic seizures,54 attention deficit, aggression, developmental delay and ASD.55 Mice with such microdeletion (Df[h15q13]/+) display several features resembling the clinical syndromes, including changes in schizophrenia relevant domains like reduced evoked gamma oscillations and seizure susceptibility.43 Relative to their control mice, adult Df[h15q13]/+ mice showed increased oxidative stress (+262% 8-oxo-dG immunolabeling intensity), lower number of PV-IR cells (-35%) and PV-IR cells enwrapped with PNN (-45%).
Mice with the human 1q21 deletion (Df[h1q21]/+)
The symptoms caused by a 1q21.1 microdeletion overlap with those seen in individuals with a 22q11.2 microdeletion. These include congenital heart defect, facial abnormalities, developmental delay, microcephaly and neuropsychiatric disorders,56 including schizophrenia.51 Compared to their control mice, adult Df[h1q21]/+ mice, whose full phenoty** is ongoing, did not show any significant oxidative stress, deficit in PV-IR cells and in WFA-labeled PNN.
FMR1 KO mice
The Fragile X syndrome results from the expansion of an unstable trinucleotide repeat, hypermethylation and transcriptional shutdown of the fragile X mental retardation 1 (FMR1) gene causing the lack of the FMR1 protein. Fragile X syndrome patients present a complex neuropsychiatric phenotype. Half of the patients meet the diagnostic criteria for ASD; many present anxiety, attention deficit, cognitive impairment and preservative behaviors.57 Rare mutations in the FRM1 gene are also associated with schizophrenia.58 FMR1 KO mice have phenotypes that resemble the human disorder45, 59 and display abnormalities in GABAergic circuits including reduced PV-IR cell density.60 In adult FMR1 KO mice, we observed a strong increase in oxidative stress (+307% 8-oxo-dG immunolabeling intensity relative to their control WT mice) together with a decreased number of PV-IR cells (-48%), and PV-IR cells surrounded by WFA-labeled PNN (−50%).
SRR KO mice
Genetic and biochemical findings suggest that SRR, the enzyme that converts L-serine to D-serine, and D-serine itself are reduced in schizophrenia.61 Low levels of D-serine, the dominant endogenous co-agonist of NMDA receptors (NMDAR) in forebrain, may thus cause NMDAR hypofunction as observed in SRR KO mice.44 Although a previous study reported no alteration of parvalbumin immunoreactivity in hippocampus, pre- and infralimbic cortices of SRR KO mice,62 our analysis revealed reduced number of PV-IR cells (−26% relative to their control WT mice) and PV-IR cells surrounded by PNN (−35%) in the ACC of adult SRR KO mice. This was accompanied by an increase in intensity of 8-oxo-dG immunoreactivity (+410%).
GRIN2A KO mice
A large genome-wide association study identified independent loci associated with schizophrenia63 that contain numerous brain-enriched genes involved in glutamatergic transmission, including GRIN2A coding for the NMDAR subunit NR2A. Mutations in GRIN2A gene have been identified in intellectual disabilities, epilepsy, ASD and schizophrenia.64 GRIN2A KO mice show learning deficits65 and altered emotional-like behaviors.66 Relative to their control WT mice, GRIN2A KO mice display neither significant oxidative stress nor PV-IR deficit. The number of PV-IR cells with PNN was however slightly reduced in KO mice (−11% relative to their control WT). However, when an additional oxidative challenge was applied during early postnatal development (from days 10 to 20), young adult GRIN2A KO mice showed significant oxidative stress (+159% relative to WT), reduced number of PV-IR cells (−17%) and PV-IR cells surrounded by WFA-labeled PNN (−36%). The oxidative insult consisted of 10-days administration of GBR12909 (GBR),39 a dopamine uptake inhibitor, leading to elevated extracellular dopamine levels and subsequent generation of reactive oxygen species (ROS) and reactive compounds.67 This treatment mimics to some extent dopamine release induced by environmental stress.68 Such GBR treatment did not have any significant effects however in WT mice.39 These findings indicate that an exogenous stress during development induces long-lasting oxidative stress along with PVI/PNN deficits in GRIN2A KO mice.
GCLM KO mice and other models of GSH deficiency
Altered levels of enzymatic and non-enzymatic antioxidants, including decrease in GSH, are reported in blood or brain of some patients suffering from schizophrenia, BP or ASD. Polymorphisms and copy number variations in genes related to the GSH synthesis and metabolism are associated with schizophrenia.50, 69, 70, 71, 72, 73, 74, 75 Moreover, subjects with high-risk genotype of the key GSH synthesizing gene GCLC presents low prefrontal GSH levels.24, 31 maternal separation in rats;19, 31 early-life iron deficiency;20, 88 hypoxia;21, 31 DTNB1 mutant mice; and25, 89 PCP.90, 91 Mice with COX10 KO in PVIs (PV-COX10)92 and PGC1-α KO mice83, 93 are models of mitochondria impairment which likely display redox dysregulation/oxidative stress. Dotted boxes, animal models with PVI deficit for which redox dysregulation/oxidative stress may be expected based on literature data: CHRNA7 KO mice;94, 95 mice with disrupted NRG1/ErbB4 signaling. KO, knockout; NVHL, neonatal ventral hippocampal lesion; PCP, postnatal treatment with phencyclidine; PNN, perineuronal net; PV, parvalbumin; PV-IR, parvalbumin-immunoreactive; PVI, parvalbumin inhibitory interneuron.26, 96, 97
A causal effect of oxidative stress on PVI integrity or phenotype is strongly supported by the fact that oxidative stress precedes PVI deficit in the hippocampus47 and by the protective effect of antioxidants on PVIs in several models (GBR-treated GCLM mice,39 ketamine-treated mice,37 isolated rat,23 isolated mice with deletion of NR1 in forebrain interneurons,83 social defeated rats and84 NVHL rats41). Many early-life stressors relevant to environmental risk factors for schizophrenia (maternal separation, hypoxia and early-life iron deficiency) also lead to PVI deficit in medial prefrontal cortex and/or hippocampus of rodents19, 20, 21, 24 (Figure 2). Although the link between PVI deficit and oxidative stress has not been investigated in the above studies, it is a reasonable hypothesis that oxidative stress contributes to prefrontal and hippocampal PVI deficit induced by these environmental insults known to generate oxidative stress31, 88 and/or neuroinflammation.19, 98, 99
As fast-spiking neurons, PVIs contain many mitochondria to meet their high-energy demand.100 Therefore, they are vulnerable to weakened antioxidant defenses,39, 40, 87 excessive production of ROS37 and diminished mitochondrial support.92, 93 However, PVIs are also particularly susceptible to redox dysregulation/oxidative stress during their postnatal maturation when their fast-spiking properties are not yet fully developed101 and the formation of the protective PNN enwrap** them are not yet complete.16 Based on the fact that a peripubertal antioxidant treatment can abolish oxidative stress and rescue PVI/PNN deficit in the NVHL rat model,41 we propose a hypothesis in which redox dysregulation/oxidative stress halts normal PVI maturation in the prefrontal cortex without causing their death,82 possibly via epigenetic mechanisms.102, 103The deleterious effect of oxidative stress on PNN integrity16 may be also central to the failure of PV circuits to properly mature as PNN is crucial for PVI maturation and synapse formation. As consequence, oxidative stress might impair the PVI-associated network plasticity, that is, activity-dependent regulation of the number of excitatory and inhibitory synapses on PVIs.103, 104 This would be consistent with the observation of an immature-like transcriptome in PVIs of schizophrenia and autism patients.17