Abstract
Objective:
In infants <35 weeks’ gestation, we sought to define the transcutaneous bilirubin (TcB) levels at which a total serum bilirubin (TSB) level suggesting the need for phototherapy is unlikely to occur and a TSB measurement can, therefore, be avoided.
Study Design:
Nursing staff performed 896 TcB measurements within 1 h of a TSB on 225 neonates 26 0/7–34 6/7 weeks’ postmenstrual age (PMA). Generalized linear models were fit with generalized estimating equations (GEEs) to model the probability of having a TSB level at or above the phototherapy initiation cutpoint as a function of the TcB; these methods allow for multiple tests per infant.
Results:
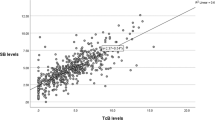
The mean difference between TcB and TSB measurements was <1 mg dl−1 for each PMA category. When the TcB was at least 3 mg dl−1 below the TSB cutpoint for phototherapy, there was a ⩾98% probability that the TSB was not at, or above, the recommended phototherapy level. The single exception to this was a phototherapy level of 6 mg dl−1 for infants of 28 0/7–29 6/7 weeks’ PMA, where a TcB of 4 mg dl−1 below the phototherapy level (ie a TcB ⩽2 mg dl−1) was necessary to achieve ⩾98% probability.
Conclusion:
Our data support the use of routine TcB screening for infants 28–34 6/7 weeks’ gestation. TcB screening in the neonatal intensive care unit can identify infants who require a TSB to confirm or exclude the need for phototherapy.



Similar content being viewed by others
References
De Luca D, Engle W, Jackson G . Transcutaneous Bilirubinometry: hepatology Research and Clinical Developments. Nova Biomedical: New York, NY, USA, 2013.
Nagar G, Vandermeer B, Campbell S, Kumar M . Reliability of transcutaneous bilirubin devices in preterm infants: a systematic review. Pediatrics 2013; 132: 871–881.
Maisels MJ, Watchko JF, Bhutani VK, Stevenson DK . An approach to the management of hyperbilirubinemia in the preterm infant less than 35 weeks of gestation. J Perinatol 2012; 32 (9): 660–664.
Maisels MJ, Engle W, Wainer S, Jackson GL, McManus S, Artinian F . Transcutaneous bilirubin levels in an outpatient and office population. J Perinatol 2011; 31: 621–624.
Schmidt ET, Wheeler CA, Jackson GL, Engle WD . Evaluation of transcutaneous bilirubinometry in preterm neonates. J Perinatol 2009; 29: 564–569.
De Luca D, Zecca E, De Turrist P, Barbato G, Marras M, Romagnoli C . Using bilicheck for preterm neonates in a sub-intensive unit: diagnostic usefulness and suitability. Early Hum Dev 2007; 83: 313–317.
Willems WA, van den Berg LM, de Wit H, Molendijk A . Transcutaneous bilirubinometry with the bilicheck in very premature newborns. J Matern Fetal Neonatal Med 2004; 16: 209–214.
Ebbesen F, Rasmussen LM, Wimberley PD . A new transcutaneous bilirubinometer, BiliCheck, used in the neonatal intensive care unit and the maternity ward. Acta Paediatr 2002; 91: 203–211.
Maisels MJ, Ostrea E Jr, Touch S et al. Evaluation of a new transcutaneous bilirubinometer. Pediatrics 2004; 113: 1628–1635.
Yaser A, Tooke L, Rhoda N . Interscapular site for transcutaneous bilirubin measurement in preterm infants: a better and safer screening site. J Perinatol 2014; 34: 209–212.
Acknowledgements
We are profoundly grateful to the nursing staff of our NICU for obtaining and documenting the TcB measurements on these infants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr. Maisels is an unpaid consultant to Draeger Medical Inc., the US supplier of the JM-103. The other authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Maisels, M., Coffey, M. & Kring, E. Transcutaneous bilirubin levels in newborns <35 weeks’ gestation. J Perinatol 35, 739–744 (2015). https://doi.org/10.1038/jp.2015.34
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2015.34
- Springer Nature America, Inc.
This article is cited by
-
Transcutaneous bilirubin levels in extremely preterm infants less than 30 weeks gestation
Journal of Perinatology (2023)
-
Transcutaneous bilirubin measurements: useful, but also reproducible?
Pediatric Research (2021)
-
Screening methods for neonatal hyperbilirubinemia: benefits, limitations, requirements, and novel developments
Pediatric Research (2021)
-
Reliability of transcutaneous bilirubin determination based on skin color determined by a neonatal skin color scale of our own
European Journal of Pediatrics (2021)
-
Repetitive bilirubin measurements in preterm infants prior to phototherapy: is it wise to use the rate of rise?
Pediatric Research (2020)