Abstract
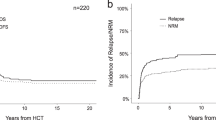
Despite the marked improvement in the overall survival (OS) for patients diagnosed with Wilms’ tumor (WT), the outcomes for those who experience relapse have remained disappointing. We describe the outcomes of 253 patients with relapsed WT who received high-dose chemotherapy (HDT) followed by autologous hematopoietic stem cell transplant (HCT) between 1990 and 2013, and were reported to the Center for International Blood and Marrow Transplantation Research. The 5-year estimates for event-free survival (EFS) and OS were 36% (95% confidence interval (CI); 29–43%) and 45% (95 CI; 38–51%), respectively. Relapse of primary disease was the cause of death in 81% of the population. EFS, OS, relapse and transplant-related mortality showed no significant differences when broken down by disease status at transplant, time from diagnosis to transplant, year of transplant or conditioning regimen. Our data suggest that HDT followed by autologous HCT for relapsed WT is well tolerated and outcomes are similar to those reported in the literature. As attempts to conduct a randomized trial comparing maintenance chemotherapy with consolidation versus HDT followed by stem cell transplant have failed, one should balance the potential benefits with the yet unknown long-term risks. As disease recurrence continues to be the most common cause of death, future research should focus on the development of consolidation therapies for those patients achieving complete response to therapy.


Similar content being viewed by others
References
Fernandez C, Geller JI, Ehrlich PF, Hill DA, Kalapurakal JA, Grundy PE et al. Renal Tumors. Principles and Practice of Pediatric Oncology. In: Pizzo PA, Poplack DG ( eds). Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2011.
Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF et al. SEER Cancer Statistics Review, 1975-2012, National Cancer Institute, based on November 2014 SEER data submission, posted to the SEER web site, April 2015. Available at http://seer.cancer.gov/csr/1975_2012/results_merged/sect_29_childhood_cancer_iccc.pdf. Accessed on 6 December 2015.
Grundy P, Breslow N, Green DM, Sharples K, Evans A, D'Angio GJ . Prognostic factors for children with recurrent Wilms' tumor: results from the Second and Third National Wilms' Tumor Study. J Clin Oncol 1989; 7: 638–647.
Pinkerton CR, Groot-Loonen JJ, Morris-Jones PH, Pritchard J . Response rates in relapsed Wilms' tumor. A need for new effective agents. Cancer 1991; 67: 567–571.
Pein F, Rey A, de Kraker J . Multivariate analysis of adverse prognostic factors (APF) in children with recurrent (Rec) Wilms' tumor (WT) after initial treatment according to SIOP-6 or SIOP-9 strategies. Med Pediatr Oncol 1999; 33: 170.
Tannous R, Giller R, Holmes E Intensive therapy for high risk (HR) relapsed Wilms' tumor (WT). A CCG-4921/POG-9445 Study Report. In: Proceeding of ASCO 200; 588a abstract 2315.
Dome JS, Liu T, Krasin M, Lott L, Shearer P, Daw NC et al. Improved survival for patients with recurrent Wilms tumor: the experience at St. Jude Children's Research Hospital. J Pediatr Hematol Oncol 2002; 24: 192–198.
Reinhard H, Schmidt A, Furtwangler R, Leuschner I, Rube C, Von Schweinitz D et al. Outcome of relapses of nephroblastoma in patients registered in the SIOP/GPOH trials and studies. Oncol 2008; 20: 463–467.
Hale J, Hobson R, Moroz V, Satori P Results of UK Children's Cancer and Leukemia Group (CCLG) protocol for relapsed Wilms tumor (UKWR): Unified relapse strategy improves outcome. In: 40th meeting of International Society of Paediatric Oncology 2008; pp 62.
Green DM, Cotton CA, Malogolowkin M, Breslow NE, Perlman E, Miser J et al. Treatment of Wilms tumor relapsing after initial treatment with vincristine and actinomycin D: a report from the National Wilms Tumor Study Group. Pediatr Blood Cancer 2007; 48: 493–499.
Malogolowkin M, Cotton CA, Green DM, Breskiw NE, Perlman E, Miser J et al. Treatment of Wilms tumor relapsing after initial treatment with vincristine, actinomycin D, and doxorubicin. a report from the National Wilms Tumor Study Group. Pediatr Blood Cancer 2008; 50: 236–241.
Garaventa A, Hartmann O, Bernard JL, Zucker JM, Pardo N, Castel V et al. Autologous bone marrow transplantation for pediatric Wilms' tumor: the experience of the European Bone Marrow Transplantation Solid Tumor Registry. Med Pediatr Oncol 1994; 22: 11–14.
Pein F, Michon J, Valteau-Couanet D, Quintana E, Frappaz D, Vannier JP et al. High-dose melphalan, etoposide, and carboplatin followed by autologous stem-cell rescue in pediatric high-risk recurrent Wilms' tumor: a French Society of Pediatric Oncology study. J Clin Oncol 1998; 16: 3295–3301.
Kremens B, Gruhn B, Klingebiel T, Hasan C, Laws HJ, Koscielniak E et al. High-dose chemotherapy with autologous stem cell rescue in children with nephroblastoma. Bone Marrow Transplant 2002; 30: 893–898.
Campbell AD, Cohn SL, Reynolds M, Seshadri R, Morgan E, Geissler G et al. Treatment of relapsed Wilms' tumor with high-dose therapy and autologous hematopoietic stem-cell rescue: the experience at Children's Memorial Hospital. J Clin Oncol 2008; 22: 2885–2890.
Spreafico F, Bisogno G, Collini P, Jankner A, Gandola L, D’Angelo P et al. Treatment of high-risk relapsed Wilms tumor with dose-intensive chemotherapy, marrow-ablative chemotherapy, and autologous hematopoietic stem cell support: experience by the Italian Association of Pediatric Hematology and Oncology. Pediatr Blood Cancer 2008; 51: 23–28.
Dallorso S, Dini G, Faraci M, Spreafico F . SCT for Wilms' tumour. Bone Marrow Transplant 2008; 41: S128–S130.
Klein J . Survival analysis: techniques of censored and truncated data 2nd edn. Springer Verlag: New York, NY, USA, 2003.
Gooley TA, Leisenring W, Crowley J, Storer BE . Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999; 18: 695–706.
Kaplan EL . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Ha TC, Spreafico F, Graf N, Dallorso S, Dome JS, Malogolowkin M et al. An international strategy to determine the role of high dose therapy in recurrent Wilms’ tumour. Eur J Cancer 2013; 49: 194–210.
Presson A, Moore TB, Kempert P . Efficacy of high-dose chemotherapy and autologous stem cell transplant for recurrent Wilms’ tumor: a meta-analysis. J Pediatr Hematol Oncol 2010; 32: 454–461.
Acknowledgements
CIBMTR Support List: The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-15-1-0848 and N00014-16-1-2020 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals, Inc.; Alexion; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma USA; AstraZeneca; Atara Biotherapeutics, Inc.; Be the Match Foundation; *Bluebird Bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; Cellular Dynamics International, Inc.; Cerus Corporation; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Genentech, Inc.; Genzyme Corporation; Gilead Sciences, Inc.; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Jeff Gordon Children’s Foundation; The Leukemia & Lymphoma Society; Medac, GmbH; MedImmune; The Medical College of Wisconsin; *Merck & Co, Inc.; *Mesoblast; MesoScale Diagnostics, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Otsuka America Pharmaceutical, Inc.; Otsuka Pharmaceutical Co, Ltd.—Japan; PCORI; Perkin Elmer, Inc.; Pfizer, Inc; *Sanofi USA; *Seattle Genetics; *Spectrum Pharmaceuticals, Inc.; St Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; University of Minnesota; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the USA Government. *Corporate Members
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Malogolowkin, M., Hemmer, M., Le-Rademacher, J. et al. Outcomes following autologous hematopoietic stem cell transplant for patients with relapsed Wilms’ tumor: a CIBMTR retrospective analysis. Bone Marrow Transplant 52, 1549–1555 (2017). https://doi.org/10.1038/bmt.2017.178
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2017.178
- Springer Nature Limited
This article is cited by
-
Allogenic perinatal tissue for musculoskeletal regenerative medicine applications: a systematic review protocol
Journal of Orthopaedic Surgery and Research (2022)
-
High dose chemotherapy and autologous hematopoietic cell transplantation for Wilms tumor: a study of the European Society for Blood and Marrow Transplantation
Bone Marrow Transplantation (2020)