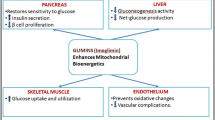
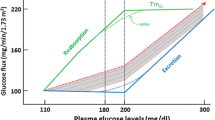
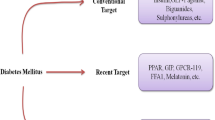
Abstract
World Health Organization (WHO) has identified diabetes as one of the fastest growing non-communicable diseases with 422 million patients around the world in 2014. Diabetes, a metabolic disease, is characterized primarily by hyperglycemia which results in various macrovascular and microvascular complications like cardiovascular disease and neuropathies which can significantly deteriorate the quality of life. The body either does not manufactures enough insulin (type 1 diabetes or T1DM) or becomes insensitive to physiologically secreted insulin or both (type 2 diabetes or T2DM). The majority of the diabetic population is affected by type 2 diabetes. Currently, hyperglycemia is treated by a broad range of molecules such as biguanides, sulfonylurea, insulin, thiazolidinediones, incretin mimetics, and DPP-4 inhibitors exerting different mechanisms. However, new drug classes have indeed come in the market such as SGLT-2 inhibitors and other are in the experimental stages such as GPR 40 agonists, GSK-3 inhibitors, GK activators and GPR21 inhibitors which definitely could be anticipated as safe and effective for diabetes therapy. This article reviews the general approach to currently approved therapies for type 2 diabetes and focusing on novel approaches that could be a panacea and might be useful in the future for diabetes patients.
Similar content being viewed by others
References
Diabetes: Fact sheet, 2016, Accessed from: http://www.who.int/mediacentre/factsheets/fs312/en/ (Accessed 4 November, 2016).
Kumar A, Bharti S, Kumar A. Diabetes mellitus type 2: the concerned complications and target organs. Apollo Med 2014;11(3):161–6.
American Diabetes Association. Classification and diagnosis of diabetes. Sec 2. Standards of medical care in diabetes. Diabetes Care 2016;39(Suppl. 1): S13–22.
Inzucchi SE, Majumdar SK. Current therapies for the medical management of diabetes. Obstet Gynecol 2016;127:780–94.
Rojas LBA, Gomes MB. Metformin: an old but still the best treatment for type 2 diabetes. Diabetol Metab Syndr 2013;5(1):6.
American Diabetes Association. Standards of medical care in diabetes approaches to glycemic treatment. Diabetes Care 2016;39(1):S52–9.
Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 2000;49:2063–9.
Ting RZ, Szeto CC, Chan MH, Ma KK, Chow KM. Risk factors of vitamin B12 deficiency in patients receiving metformin. Arch Intern Med 2006;166:1975–9.
Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab 2014;20(6):953–66.
Breathnach CS. Insulin. Ir J Med Sci 2012;181:15–8.
Tibaldi JM. Evolution of insulin development: focus on key parameters. Adv Ther 2012;29(7):590–619.
Insulin Basics, Accessed from: http://www.diabetes.org/living-with-diabetes/treatment-and-care/medication/insulin/insulin-basics.html (Accessed 4 November, 2016).
Nasrallah SN, Reynolds LR. Insulin degludec, the new generation basal insulin or just another basal insulin? Clin Med Insights Endocrinol Diabetes 2012;5:31–7.
Harper W, Clement M, Goldenberg R, Hanna A, Main A, Retnakaran R, et al. Pharmacologic management of type 2 diabetes. Can J Diabetes 2013;37(1):S61–8.
Mosenzon O, Raz I. Intensification of insulin therapy for type 2 diabetic patients in primary care: basal-Bolus regimen versus premix insulin analogs. Diabetes Care 2013;36(2):S212–8.
Freeman JS. Insulin analog therapy improving the match with physiologic insulin secretion. J Am Osteopath Assoc 2009;109:26–36.
Enzmann HG, Weise M. Current european regulatory perspectives on insulin analogs. Diabetol Metab Syndr 2011;3:14.
Shukla A, Grisouard J, Ehemann V, Hermani A, Enzmann H, Mayer D. Analysis of signaling pathways related to cell proliferation stimulated by insulin analogs in human mammary epithelial cell lines. Endocr Relat Cancer 2009;16:429–41.
Weinstein D, Simon M, Yehezkel E, Laron Z, Werner H. Insulin analogs display IGF-I-like mitogenic and anti-apoptotic activities in cultured cancer cells. Diabetes Metab Res Rev 2009;25:41–9.
Hemkens LG, Grouven U, Bender R, Günster C, Gutschmidt S, Selke GW, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogs: a cohort study. Diabetologia 2009;52:1732–44.
Żełobowska K, Gumprecht J, Grzeszczak W. Mitogenic potency of insulin glargine. Endokrynol Pol 2009;60(1):34–9.
Varewijck AJ, Janssen JA. Insulin and its analogs and their affinities for the IGF1 receptor. Endocr Relat Cancer 2012;19:F63–75.
Proks P, Reimann F, Green N, Gribble F, Ashcroft F. Sulfonylurea stimulation of insulin secretion. Diabetes 2002;51(3):S368–76.
Roumie CL, Hung AM, Greevy RA, Grijalva CG, Liu X, Murff HJ, et al. Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus. Ann Intern Med 2012;157:601–10.
Corrao G, Romio SA, Zambon A, Merlino L, Bosi E, Scavini M. Multiple outcomes associated with the use of metformin and sulphonylureas in type 2 diabetes: a population-based cohort study in Italy. Eur J Clin Pharmacol 2011;67:289–99.
Landgraf R. Meglitinide analogs in the treatment of type 2 diabetes mellitus. Drugs Aging 2000;17(5):411–25.
Plosker GL, Figgit DP. Repaglinide: a pharmacoeconomic review of its use in type 2 diabetes mellitus. Pharmacoeconomics 2004;22(6):389–411.
Konya H, Katsuno T, Tsunoda T, Yano Y, Kamitani M, Miuchi M, et al. Effects of combination therapy with mitiglinide and voglibose on postprandial plasma glucose in patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes 2013;6:317–25.
Gelman L, Feige JN, Desvergne B. Molecular basis of selective PPARγ modulation for the treatment of Type 2 diabetes. Biochim Biophys Acta 2007;1771:1094–107.
Greenfield JR, Chisholm DJ. Thiazolidinediones-mechanisms of action. Aust Prescr 2004;27:67–70.
Gross B, Staels B. PPAR agonists: multimodal drugs for the treatment of type-2 diabetes. Best Pract Res Clin Endocrinol Metab 2007;21(4):687–710.
Staels B, Fruchart JC. Therapeutic roles of peroxisome proliferator-activated receptor agonists. Diabetes 2005;54:2460–70.
Selvin E, Bolen S, Yeh HC, Wiley C, Wilson LM, Marinopoulos SS, et al. Cardiovascular outcomes in trials of oral diabetes medications: a systematic review. Arch Intern Med 2008;168(19):2070–80.
Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA 2007;298(10):1189–95.
Nissen SE, Wolsky K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457–71.
Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA 2007;298(10):1180–8.
Beltowski J, Rachanczyk J, Wlodarczyk M. Thiazolidinedione-Induced fluid retention: recent insights into the molecular mechanisms. PPAR Res 2013;628628.
Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Circulation 2003;108:2941–8.
van de Laar FA, Lucassen PL, Akkermans RP, van de Lisdonk EH, Rutten GE, van Weel C. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care 2005;28(1):154–63.
Standl E, Schnell O. Alpha-glucosidase inhibitors 2012-cardiovascular considerations and trial evaluation. Diab Vasc Dis Res 2012;9(3):163–9.
Kalra S, Sahay RK, Schnell O, Sheu WH, Grzeszczak W, Watada H, et al. Alpha-glucosidase inhibitor, acarbose, improves glycemic control and reduces body weight in type 2 diabetes: findings on Indian patients from the pooled data analysis. Indian J Endocrinol Metab 2013;17(1):S307–9.
Kitano D, Chiku M, Li Y, Okumura Y, Fukamachi D, Takayama T, et al. Miglitol improves postprandial endothelial dysfunction in patients with acute coronary syndrome and new-onset postprandial hyperglycemia. Cardiovasc Diabetol 2013;12:92.
Derosa G, Maffioli P. α-Glucosidase inhibitors and their use in clinical practice. Arch Med Sci 2012;8(5):899–906.
Arakawa M, Ebato C, Mita T, Fujitani Y, Shimizu T, Watada H, et al. Miglitol suppresses the postprandial increase in interleukin 6 and enhances active glucagon-like peptide 1 secretion in viscerally obese subjects. Metabolism 2008;57:1299–306.
Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 2012;8:728–42.
Marre M, Penfornis A. GLP-1 receptor agonists today. Diabetes Res Clin Pract 2011;93(3):317–27.
Ahren B. GLP-1 for type 2 diabetes. Exp Cell Res 2011;317(9):1239–45.
Holst JJ, Vilsboll T, Deacon CF. The incretin system and its role in type 2 diabetes mellitus. Mol Cell Endocrinol 2009;297(1–2):127–36.
IMPORTANT SAFETY INFORMATION for BYETTA® (exenatide) injection, Accessed from: https://www.byetta.com/learn-about-byetta/important-safety-information (Accessed 24 November, 2016).
Selected Important Safety Information, Accessed from: https://www.victoza.com/important-safety-information.html (Accessed 24 November, 2016).
Thornberry NA, Gallwitz B. Mechanism of action of inhibitors of dipeptidyl-peptidase-4 (DPP-4). Best Pract Res Clin Endocrinol Metab 2009;47(4):9–86.
Capuano A, Sportiello L, Maiorino MI, Rossi F, Giugliano D, Esposito K. Dipeptidyl peptidase-4 inhibitors in type 2 diabetes therapy-focus on alogliptin. Drug Des Devel Ther 2013;7:989–1001.
Important Safety Information About JANUVIA, Accessed from: https://www.januvia.com/safety-information/ (Accessed 4 December, 2016).
Morishita R, Nakagami H. Teneligliptin: expectations for its pleiotropic action. Expert Opin Pharmacother 2015;16(3):417–26.
Panacea Biotec Launches-TENEPAN, Accessed from: http://www.panaceabiotec.com/press_releases/PR28062016.pdf (Accessed 24 December, 2016).
FDA Drug Safety Communication: FDA investigating reports of possible increased risk of pancreatitis and pre-cancerous findings of the pancreas from incretin mimetic drugs for type 2 diabetes. 2016 Accessed from: http://www.fda.gov/Drugs/DrugSafety/ucm343187.htm; (Accessed 25 December 2016).
FDA Drug Safety Communication: FDA warns that DPP-4 inhibitors for type 2 diabetes may cause severe joint pain. 2016 Accessed from: http://www.fda.gov/Drugs/DrugSafety/ucm459579.htm; (Accessed 25 December 2016).
FDA Drug Safety Communication: FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. 2016 http://www.fda.gov/Drugs/DrugSafety/ucm486096.htm; (Accessed 25 December 2016).
Nair S, Wilding JP. Sodium glucose cotransporter 2 inhibitors as a new treatment for diabetes mellitus. J Clin Endocrinol Metab 2010;95(1):34–42.
Tahara A, Takasu T, Yokono M, Imamura M, Kurosaki E. Characterization and comparison of sodium-glucose cotransporter 2 inhibitors in pharmacokinetics: pharmacodynamics, and pharmacologic effects. J Pharmacol Sci 2016;130(3):159–69.
Important Safety Information, Accessed from: https://www.jardiance.com/#isi (Accessed 28 December, 2016).
FDA Drug Safety Communication: FDA strengthens kidney warnings for diabetes medicines canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, **gduo XR), Accessed from: http://www.fda.gov/Drugs/DrugSafety/ucm505860.htm (Accessed 3 March, 2017).
FDA Drug Safety Communication: FDA revises label of diabetes drug canagliflozin (Invokana, Invokamet) to include updates on bone fracture risk and new information on decreased bone mineral density, Accessed from: http://www.fda.gov/Drugs/DrugSafety/ucm461449.htm (Accessed 3 March, 2017).
Idris I, Donnelly R. Sodium-glucose co-transporter-2 inhibitors: an emerging new class of oral antidiabetic drug. Diabetes Obes Metab 2009;11(2):79–88.
Feng XT, Leng J, **e Z, Li SL, Zhao W, Tang QL. GPR40: a therapeutic target for mediating insulin secretion. Int J Mol Med 2012;30(6):1261–6.
Yabuki C, Komatsu H, Tsujihata Y, Maeda R, Ito R, Matsuda-Nagasumi K, et al. A novel antidiabetic drug, fasiglifam/TAK-875, acts as an ago-allosteric modulator of FFAR1. PLoS One 2013;8(10):e76280.
Yashiro H, Tsujihata Y, Takeuchi K, Hazama M, Johnson PR, Rorsman P. The effects of TAK-875: a selective G protein-coupled receptor 40/free fatty acid 1 agonist, on insulin and glucagon secretion in isolated rat and human islets. J Pharmacol Exp Ther 2012;340(2):483–9.
News Releases, Accessed from: http://www.takeda.com/news/2013/20130516_5780.html (Accessed 4 January, 2017).
Ali A, Hoeflich KP, Woodgett JR. Glycogen synthase kinase-3: properties, functions, and regulation. Chem Rev 2001;101:2527–40.
Finkelman HE. Glycogen synthase kinase 3: An emerging therapeutic target. Trends Mol Med 2002;8(3):126–32.
Nikoulina SE, Ciaraldi TP, Mudaliar S, Mohideen P, Carter L, Henry RR. Potential role of glycogen synthase kinase-3 in skeletal muscle insulin resistance of type 2 diabetes. Diabetes 2000;49:263–71.
Forde JE, Dale TC. Glycogen synthase kinase 3: A key regulator of cellular fate. Cell Mol Life Sci 2007;64:1930–44.
Meijer L, Flajolet M, Greengard P. Pharmacological inhibitors of glycogen synthase kinase 3. Trends Pharmacol Sci 2004;25(9):471–80.
Ring DB, Johnson KW, Henriksen EJ, Nuss JM, Goff D, Kinnick TR, et al. Selective glycogen synthase kinase 3 inhibitors potentiate insulin activation of glucose transport and utilization in vitro and In vivo. Diabetes 2003;52:588–95.
Lee SC, Kim HT, Park CH, Lee DY, Chang HJ, Park S, et al. Design, synthesis and biological evaluation of novel imidazopyridines as potential antidiabetic GSK3b inhibitors. Bioorg Med Chem Lett 2012;22:4221–4.
Seto S, Yumoto K, Okada K, Asahina Y, Iwane A, Iwago M, et al. Quinolone derivatives containing strained spirocycle as orally active glycogen synthase kinase 3b (GSK-3b) inhibitors for type 2 diabetics. Bioorg Med Chem 2012;20:1188–200.
Motawi TM, Bustanji Y, El-Maraghy SA, Taha MO, Al Ghussein MA. Naproxen and cromolyn as new glycogen synthase kinase 3β inhibitors for amelioration of diabetes and obesity: an investigation by docking simulation and subsequent in Vitro/In vivo biochemical evaluation. J Biochem Mol Toxicol 2013;27:425–36.
Sarabu R, Taub R, Grimsby J. Glucokinase activation-a strategy for T2D therapy: recent developments. Drug Discov Today 2007;4(2):111–5.
Arden C, Harbottle A, Baltrusch S, Tiedge M, Agius L. Glucokinase is an integral component of the insulin granules in glucose-responsive insulin secretory cells and does not translocate during glucose stimulation. Diabetes 2004;53:2346–52.
Matschinsky FM, Zelent B, Doliba N, Li C, Vanderkooi JM, Naji A, et al. Glucokinase activators for diabetes therapy. Diabetes Care 2011;34(2):S236–43.
Pal M. Recent advances in Glucokinase activators for the treatment of type 2 diabetes. Drug Discov Today 2009;14(15/16):784–92.
Perseghin G. Exploring the in vivo mechanisms of action of glucokinase activators in type 2 diabetes. J Clin Endocrinol Metab 2010;95(11):4871–3.
Bonadonna RC, Heise T, Arbet-Engels C, Kapitza C, Avogaro A, Grimsby J, et al. Piragliatin (RO4389620), a novel glucokinase activator, lowers plasma glucose both in the postabsorptive state and after a glucose challenge in patients with type 2 diabetes mellitus: a mechanistic study. J Clin Endocrinol Metab 2010;95:5028–36.
Ericsson H, Sjöberg F, Heijer M, Dorani H, Johansson P, Wollbratt M, et al. The Glucokinase activator AZD6370 decreases fasting and postprandial glucose in type 2 diabetes mellitus patients with effects influenced by dosing regimen and food. Diab Res Clin Prac 2012;98:436–44.
Ericsson H, Röshammar D, Wollbratt M, Heijer M, Persson M, Ueda S, et al. Tolerability, pharmacokinetics, and pharmacodynamics of the glucokinase activator AZD1656, after single ascending doses in healthy subjects during euglycemic clamp. Int J Clin Pharmacol Ther 2012;50(11):765–77.
Meininger GE, Scott R, Alba M, Shentu Y, Luo E, Amin H, et al. Effects of MK-0941, a novel glucokinase activator, on glycemic control in insulin-treated patients with type 2 diabetes. Diabetes Care 2011;34:2560–6.
Park K. Identification of YH-GKA: a novel benzamide glucokinase activator as therapeutic candidate for type 2 diabetes mellitus. Arch Pharm Res 2012;35(12):2029–33.
Park K, Lee BM, Kim YH, Han T, Yi W, Lee DH, et al. Discovery of a novel phenylethyl benzamide glucokinase activator for the treatment of type 2 diabetes mellitus. Bioorg Med Chem Lett 2013;23:537–42.
Amin NB, Aggarwal N, Pall D, Paragh G, Denney WS, Le V, et al. Two dose-ranging studies with PF-04937319: a systemic partial activator of glucokinase, as add-on therapy to metformin in adults with type 2 diabetes. Diabetes Obes Metab 2015;17(8):751–9.
Peter A, Stefan N, Cegan A, Walenta M, Wagner S, Königsrainer A, et al. Hepatic glucokinase expression is associated with lipogenesis and fatty liver in humans. J Clin Endocrinol Metab 2011;96(7):E1126–30.
De Ceuninck F, Kargar C, Ilic C, Caliez A, Rolin JO, Umbdenstock T, et al. Small molecule glucokinase activators disturb lipid homeostasis and induce fatty liver in rodents: a warning for therapeutic applications in humans. Br J Pharmacol 2013;168(2):339–53.
Tsumura Y, Tsushima Y, Tamura A, Hasebe M, Kanou M, Kato H, et al. TMG-123, a novel glucokinase activator, exerts durable effects on hyperglycemia without increasing triglyceride in diabetic animal models. PLoS One 2017;12(2):e0172252.
Young A, Pittner R, Gedulin B, Vine W, Rink T. Amylin regulation of carbohydrate metabolism. Biochem Soc Trans 1995;23(2):325–31.
Schmitz O, Brock B, Rungby J. Amylin agonists: a novel approach in the treatment of diabetes. Diabetes 2004;53(Suppl. 3):S233.
Edelman SV. Optimizing diabetes treatment using an amylin analog. Diabetes Educ 2008;34:4S–10S.
Ryan G, Briscoe TA, Jobe L. Review of pramlintide as adjunctive therapy in treatment of type 1 and type 2 diabetes. Drug Des Dev Ther 2008;2:203–14.
Lutz K. Safety of pramlintide added to mealtime insulin in patients with type 1 or type 2 diabetes: a large observational study. Diab Obes Metab 2010;12:548–51.
Gardner J, Wu S, Ling L, Danao J, Li Y, Yeh WC, et al. G-protein-coupled receptor GPR21 knockout mice display improved glucose tolerance and increased insulin response 418. Biochem Biophys Res Commun 2012;1:1–5.
Osborn O, Oh DY, McNelis J, Sanchez-Alavez M, Talukdar S, Lu M, et al. G protein-coupled receptor 21 deletion improves insulin sensitivity in diet-induced obese mice. J Clin Invest 2012;122(7):2444–53.
Wang J, Pan Z, Baribault H, Chui D, Gundel C, Véniant M. GPR21 KO mice demonstrate no resistance to high fat diet induced obesity or improved glucose tolerance. Version 2 F1000 Res 2016;5:136.
Leonard S, Kinsella GK, Benetti E, Findlay JB. Regulating the effects of GPR21, a novel target for type 2 diabetes. Sci Rep 2016;6:27002.
Bharti SK, Kumar A, Sharma NK, Prakash O, Jaiswal SK, Krishnan S, et al. Tocopherol from seeds of Cucurbita pepo against diabetes: validation by in vivo experiments supported by computational docking. J Formos Med Assoc 2013;112(11):676–90.
Liang B, Guo Z, **e F, Zhao A. Antihyperglycemic and antihyperlipidemic activities of aqueous extract of Hericium erinaceus in experimental diabetic rats. BMC Complement Altern Med 2013;13:253.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, A., Bharti, S.K. & Kumar, A. Therapeutic molecules against type 2 diabetes: What we have and what are we expecting?. Pharmacol. Rep 69, 959–970 (2017). https://doi.org/10.1016/j.pharep.2017.04.003
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.pharep.2017.04.003