Abstract
The frequent wildfires and extensive biochar application accumulate biochar colloids in soils. The transport of biochar colloids may influence the release of soil dissolved organic matter (DOM) due to their high mobility and active surface, posing a detrimental impact on carbon cycle, pollutants behaviors, and water quality. Here, we examined the effect of pristine and aged biochar colloids on soil DOM release in three types of soils (black soil, fluvisol, and paddy soil) through adsorption and column experiments combined with fluorescence excitation/emission matrix spectroscopy (EEM) and fourier transform ion cyclotron resonance mass spectrometry (FT-ICR-MS). Adsorption experiment showed that biochar exhibited little effect on soil DOM release, while transport experiment indicated that the downward movement of both pristine and aged biochar colloids significantly enhanced the release of soil DOM, especially black soil and fluvisol. EEM spectra indicated that a humic acid-like substance with large molecular weights was the primary DOM that enhanced release from black soil (10.9–12.0% enhancement) and fluvisol (6–8% enhancement) during biochar colloid transport. Condensed aromatic-like (22.8% and 19.4%) and tannin-like (18.4% and 18.9%) compounds are the primary molecules correlated to the enhanced release of humic acid-like substances. Although chemical aging resulted in a more negative surface of aged biochar colloids and slightly enhanced biochar colloid transport (up to 7.0%), it showed no distinct effect on the release of soil DOM. This study indicates that biochar colloids may cause an increase in DOM release when irrigation and rainfall occur, and more attention should be paid to the environmental impacts of biochar colloids from intensive biochar application and wildfires.
Graphical Abstract

Highlights
• The transport of pristine and aged biochar colloids enhanced release of soil DOM.
• The DOM released from soils was characterized by EEM and FT-ICR-MS.
• More lignin, tannin, and condensed aromatic compounds were in the released DOM.
• The released DOM showed less lability and larger molecular weights.
• Optical properties of the released DOM were associated with molecular compositions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
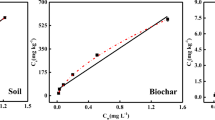
Dissolved organic matter (DOM) is one of the key variables that drive soil and aquatic ecologies. The change of DOM in quantity and composition can directly affect carbon cycle, pollutant behavior, and microbial activity (Lapierre et al. 2013; Lou et al. 2018; Stegen et al. 2018). Recently, the change of DOM due to the frequent wildfires in forest watersheds was reported to pose a threat to water quality and affect drinking water treatment (Hohner et al. 2016, 2019). The changes of DOM in quantity and composition could affect the water treatment process and increase the challenge of coagulation and filtration. The further formed disinfection byproducts that are regulated in drinking water may pose threat to human health. Pyrogenic carbon (e.g., biochar), pyrolyzed from biomass during the wildfires and production, has accumulated or applied in the soil and surrounding ecosystem, which gained broad interest due to its significant effect on carbon sequential and soil amendment (Rudolf et al. 2013; Bistarelli et al. 2021; Zhang et al. 2021). The pyrogenic carbon in the soil and aquatic system may affect the DOM pool. Since biochar is easy to produce and apply, biochar is often used to represent pyrogenic carbon in laboratory and field research. Biochar may change the characteristics of soil DOM. For example, in a batch experiment, pyrogenic carbon increased the release of DOM from the soil due to the increased pH, and the composition of the released DOM correlated with the size exclusion effect of pyrogenic carbon pore structure (Smebye et al. 2016). Similar results were confirmed in the agricultural field experiment (Zhang et al. 2017; Liu et al. 2011). For the black soil and fluvisol, the active surface of biochar colloids might have exchanged the humic acid-like DOM through physicochemical processes, after which the exchanged DOM was leached out from the soils.
Sand column experiments were conducted to verify the mechanisms of enhanced release of humic acid-like substance during biochar colloid transport. Compared with the sand column with only HA transport, the DOC of the sand column with HA transport followed by pristine biochars injection was increased from 3.71 mg L−1 to 5.31 mg L−1 (Fig. 3a). The result indicated that more HA was released during biochar colloid transport. In addition, the SEM images also showed that after biochar colloid transport (Fig. 3c), less HA was retained on the sand surface than that without biochar colloids (Fig. 3b) transport, revealing the enhanced release of HA. The paddy soil had a high content of Fe element (Table S2), with a high isoelectric point (Wang et al. 2012), making the paddy soil surface less negative than the black soil and fluvisol. Due to the relatively weaker electrostatic repulsion between biochar colloids and paddy soil, the biochar colloids showed weak transport in the paddy soil column and exhibited little effect on DOM release. In addition, the large amount of Fe oxides in the paddy soil has a strong precipitation and immobilization ability for soil DOM, which might have also reduced the release of DOM. The sand column experiment also showed that Fe oxides decreased the release of HA. The DOC content in the Fe sand column was lower than that in the clean sand column (5.31 mg L−1 < 2.35 mg L−1) (Fig. 3a), which indicated that iron oxides decreased the release of HA. The SEM image also showed that much HA was retained on the sand surface in the column with Fe oxides (Fig. 3d). The less negative and rougher sand surface with Fe oxides than that without iron oxides decreased the release of HA.
The dissolved organic carbon (DOC) in the effluent (a) and the SEM images of quartz sand (b-d) in sand column experiments at pH 7. A: 10 mg L−1 humic acid (HA) in clean sand; B: 100 mg L−1 biochar colloids in clean sand; C: 10 mg L−1 HA followed by 100 mg L−1 pristine biochar colloids in clean sand; D: 10 mg L−1 HA followed by 100 mg L−1 pristine biochar colloids in iron oxides coated sand (Fe sand)
3.3.3 Molecular characterization of released DOM
The FT-ICR-MS can provide detailed structural information on every molecule and the dynamics of the DOM released from soils (Sun et al. 2020; Liu et al. 2021; Hu et al. 2023). In biochar colloid transport experiments, relatively higher O/C, DBE, AImod, and NOSC values, and lower H/C values were observed in all soil leachate (Table 1), indicating that the transport of biochar colloids increased the unsaturation, aromaticity, and oxidation of the leached DOM. All formulas of the molecules mainly contained four subcategories based on their elemental composition: CHO, CHON, CHOS, and CHONS. CHO (relative abundance of 53%–64%) and CHON (relative abundance of 22%–38%) were the dominant formulas in the soil leachate (Fig. 4a and Table S4).
The van-Krevelen diagram was used to present a graphic plot of compound distribution (Fig. S6), which revealed the changes in DOM molecules caused by the transport of biochar colloids. Lipid-like, protein-like, carbohydrate-like, lignin-like, tannin-like, condensed aromatic-like, and unsaturated hydrocarbon-like components were detected in the van-Krevelen diagram. The lignin-like component had the highest relative abundance in all soil leachate probably because of the degradation of terrestrial-plants sources (Fig. 4b; Ohno et al. 2010; Liu et al. 2021). The transport of pristine and aged biochar colloids increased the relative abundance of condensed aromatic-like and tannin-like compounds in the leachate from the black soil and fluvisol. The relative abundance of condensed aromatic-like compounds in the BS soil leachate increased from 9.44% to 15.24% and 15.69% with the transport of pristine and aged biochar colloids, respectively (Fig. 4b and Table S5). In the paddy soil, the relative abundance of the seven compounds showed minimal change, consistent with the slight change shown by the EEM spectra. Nevertheless, the relative abundance of condensed aromatic-like and tannin-like compounds in the paddy soil leachate slightly increased from 3.31% to 4.43% and 5.71%, and from 5.51% to 6.04% and 8.48% for pristine and aged biochar colloids, respectively. These results revealed that the transport of both pristine and aged biochar colloids enhanced the leaching of condensed aromatic-like and tannin-like compounds in all soils.
The condensed aromatic-like and tannin-like compounds could originate from biochar colloids and soils. To elucidate the sources of the increased DOM compounds in the leachate, we compared the number and types of molecules before and after biochar colloid transport in the three soils. Compared with the column experiments without biochar colloid transport, the molecule number of DOM in the leachate from pristine and aged biochar colloid transport experiments showed 1898, 1602, 2561, 1315, 1888, and 1768 newly emerged molecules, 1929, 1705, 2583, 2031, 1811, and 1472 vanished molecules, and 6724, 5923, 6336, 6026, 6369, and 7105 resistant molecules (Fig. S7). These molecules plotted in van Krevelen diagrams are shown in Fig. 5a-f. Since the molecules in pristine and aged biochar colloids were removed during the selection processes, these “New/Increased” and “Vanished/Decreased” molecules were from soils, not biochar colloids.
The van Krevelen diagrams (a-f), the number of “Vanished/Decreased” (g) and “New/Increased” (h) molecule in soil column leachate after biochar colloid transport. “New”, “Increased”, “Decreased”, and “Vanished” represent the newly emerged, intensity increased, intensity decreased, and vanished molecule after biochar colloid transport; BS, FS, and PS represent black soil, fluvisol, and paddy soil, respectively
The “New/Increased” molecules with high O/C and low H/C ratios and “Vanished/Decreased” molecules with low O/C and high H/C ratios revealed the increased release of molecules with large molecular weight. The molecules released from soils with low H/C ratio suggested their low lability (Liu et al. 2009; Gu et al. 2019), which could change the soil organic carbon pool and alter the microbial communities since carbon lability affects microbial metabolic process. In addition, the results revealed that the transport of biochar colloids increased the release of soil DOM molecules with large molecular weight. This was different from the results in the previous studies, which reported that molecules with low molecular weights were easily leached out during precipitation and irrigation (Sun et al. 2022; Hu et al. 2023). This might be because the biochar colloids change the soil DOM toward molecules with larger sizes by sorbing smaller aliphatic species (Smebye et al. 2016). The elemental composition and compound distribution showed 191 “Vanished/Decreased” molecules (Fig. 5g) and 520 “New/Increased” molecules (Fig. 5h) in all soils after biochar colloid transport. CHO and CHON were the main formulas in “New/Increased” and “Vanished/Decreased” molecules. The lignin-like compound was the most abundant in “New/Increased” and “Vanished/Decreased” molecules. Condensed aromatic-like and tannin-like compounds had more “New/Increased” molecules with few “Vanished/Decreased” molecules, indicating that they were the most released DOM compounds during biochar colloid transport.
The optical characteristics of DOM in the EEM spectra were related to its molecular composition in the FT-ICR-MS. The EEM-PARAFAC model decomposed the EEM spectra into three individual characterized fluorescence components of DOM: C1 (at Ex/Em of 250 nm/ < 450 nm), C2 (at Ex/Em of < 250 nm/ < 450 nm), and C3 (at Ex/Em of < 270 nm/480 nm). C1 and C2 were microbial humic acid-like species with anthropogenic origin usually found in agriculturally dominated watersheds, while C3 was a typical terrestrial humic acid-like component primarily found in soil-derived DOM (Murphy et al. 2014; Peleato et al. 2016). The correlations between the three components (C1, C2, and C3) and the relative abundance of “New & Increased” and “Vanished & Decreased” formulas are presented in Fig. S10. There were 491, 281, and 421 “New & Increased” formulas significantly correlated to C1, C2, and C3 components, of which 64.6%, 6.1%, and 92.9% were positively correlated to C1, C2, and C3 components, respectively. This indicated that these formulas were mainly positively correlated with C1 and C3 components. For the C1 and C3 components, the positively correlated formulas were mainly distributed in the regions with high O/C and low H/C ratios and consisted of lignin-like (57.0% and 60.1%), condensed aromatic-like (22.8% and 19.4%), and tannin-like (18.4% and 18.9%) compounds. There were 297, 251, and 191 “Vanished & Decreased” formulas significantly correlated to C1, C2, and C3 components, of which 44.1%, 96.0%, and 2.1% were positively correlated to C1, C2, and C3 components, respectively. This showed that the formulas were mainly positively correlated with C1 and C2 components. For C1 and C2 components, the positively correlated formulas were mainly distributed in the regions with low O/C and high H/C ratios and contained lignin-like compounds (79.4% and 77.2%). The strong correlation between the optical characteristics and the molecular composition of DOM indicated the increased leaching of humic acid-like substances that mainly consisted of lignin-like, condensed aromatic-like, and tannin-like compounds. The decreased leaching of humic acid-like substance showed that the main component was a lignin-like compound.
3.4 Environmental implications
The increased release and downward movement of DOM may affect the soil carbon pool, microbial communities, and water quality, which is tightly related to composition and structure of DOM (Kellerman et al. 2015; Roth et al. 2019). Generally, the DOM enriched in components with a high H/C ratio (e.g., proteins and sugars) is considered more labile, whereas DOM composed of high O/C ratio structures (e.g., aromatic-like) is less labile (Sun et al. 2020). The most labile fractions of DOM are usually consumed firstly by the microbes, followed by less labile fractions. Our study showed that the DOM components with higher aromaticity were released during the transport of biochar colloids (Fig. 6), and these released DOM could not be metabolized by microbe or further change the microbial community in short term. However, the long-term effect of DOM with higher aromaticity should not be neglected. For example, an incubation experiment showed that after the initial faster consumption of labile DOM, the aromatic DOM tends to be more selective for the microbial community (Zhou et al. 2021). In addition, the degradation processes of DOM in lakes preferentially remove aromatic compounds rather than reduced and aliphatic compounds (Kellerman et al. 2015), and the light penetrates water preferentially degrading aromatic compounds (Stubbins et al. 2010). Thus, the different degradation process in aquatic systems compared with soils indicates that more attention should be paid to the watershed and water quality in regions with biochar application and frequent wildfires. Additionally, the high aromaticity and strong electron transfer ability of released DOM could change the transport and degradation of organic pollutants in aquatic systems (e.g., antibiotics) (Lou et al. 2018), and the preferential adsorption of aromatic compounds on mineral surfaces (Coward et al. 2019) may further affect pollutant behaviors and carbon sequestration in soils.
4 Conclusion
This study showed the optical and molecular evidence highlighting the vital role of biochar colloid transport in soil DOM release. Aging process alters the properties of biochar colloids and slightly enhances their transport in black soil and fluvisol. The significant DOM release in three soils differed slightly in response to pristine and aged biochar colloid transport, revealing the critical role of both pristine and aged biochar colloids in soil DOM release. The minor effect of chemical aging on soil DOM release enhanced by biochar colloids revealed that chemical aging was not a detrimental factor contributing to the increased soil DOM release. Various aging processes may coexist in the natural environment, and further research is needed to investigate their effect on soil DOM release. Our study could provide a reference for evaluating the environmental aging process on the soil DOM release enhanced by biochar colloids during rainfall and irrigation. The different results between the adsorption and transport experiments indicated that the prediction of DOM release based on adsorption experiments might be inadequate in real fields where precipitation and irrigation frequently occur. The effect of biochar application on soil DOM release may be underestimated in areas with high rainfall and irrigation since soil DOM release could be enhanced during biochar colloids downward movement. The increased release of humic acid-like substance had large molecular weights and contained lignin-like, condensed aromatic-like, and tannin-like compounds, which had important implications for the soil organic carbon pool, microbial communities, and groundwater systems. Thus, more attention should be focused on the increasing accumulation of biochar colloids in the soil and aquatic environments due to frequent wildfires and artificial biochar application. The long-term effect of biochar field application on DOM release remains largely unknown and deserves further attention.
Availability of data and materials
All data associated with this study can be found within the main manuscript and the Supplementary Material, and sources are stated.
Abbreviations
- AImod:
-
Modified aromaticity index
- BC:
-
Biochar colloid
- BS:
-
Black soil
- DBE:
-
Double bond equivalent
- DOC:
-
Dissolved organic carbon
- DOM:
-
Dissolved organic matter
- EEM:
-
Three-dimension excitation/emission matrix
- FT-ICR-MS:
-
Fourier transform ion cyclotron resonance mass spectrometry
- FS:
-
Fluvisol
- H/C:
-
Ratio of hydrogen to carbon
- HA:
-
Humic acid
- NOSC:
-
Nominal oxidation state of carbon
- O/C:
-
Ratio of oxygen to carbon
- PS:
-
Paddy soil
- PV:
-
Pore volume
- SSA:
-
Specific surface area
- T H :
-
Hydrodynamic torque
- T A :
-
Resisting adhesive torque
References
Bistarelli LT, Poyntner C, Santin C, Doeer SH, Talluto MV, Singer G, Sigmund G (2021) Wildfire-derived pyrogenic carbon modulates riverine organic matter and biofilm enzyme activities in an in situ flume experiment. ACS EST Water 1:1648–1656
Bradford SA, Torkzaban S, Wiegmann A (2011) Pore-Scale simulations to determine the applied hydrodynamic torque and colloid immobilization. Vadose Zone J 10:252–261
Bradford SA, Torkzaban S, Shapiro AA (2013) Theoretical analysis of colloid attachment and straining in chemically heterogeneous porous media. Langmuir 29:6944–6952
Chrysikopoulos CV, Aravantinou AF (2012) Virus inactivation in the presence of quartz sand under static and dynamic batch conditions at different temperatures. J Hazard Mater 233–234:148–157
Coward EK, Ohno T, Sparks DL (2019) Direct evidence for temporal molecular fractionation of dissolved organic matter at the iron oxyhydroxide interface. Environ Sci Technol 53:642–650
Dong XL, Singh BP, Li GT, Lin QM, Zhao XR (2019) Biochar has little effect on soil dissolved organic carbon pool 5 years after biochar application under field condition. Soil Use Manage 35:466–477
Eykelbosh AJ, Johnson MS, Couto EG (2015) Biochar decreases dissolved organic carbon but not nitrate leaching in relation to vinasse application in a Brazilian sugarcane soil. J Environ Manage 149:9–16
Frberg M, Jardine PM, Hanson PJ, Swanston CW, Todd DE, Tarver JR, Garten CT (2007) Low dissolved organic carbon input from fresh litter to deep mineral soils. Soil Sci Soc Am J 71:347–354
Georg G, Andrej R, Olga S, Matthias G, Olega K, Hans F, Natalia M, Galina Z, Heiner F (2008) Storage and mobility of black carbon in permafrost soils of the forest tundra ecotone in Northern Siberia. Glob Change Biol 14:1367–1381
Gu WL, Huang SB, Lei S, Yue J, Su ZX, Si F (2019) Quantity and quality variations of dissolved organic matter (DOM) in column leaching process from agricultural soil: hydrochemical effects and DOM fractionation. Sci Total Environ 691:407–416
Hohner AK, Cawley K, Oropeza J, Summers RS, Rosario-Ortiz FL (2016) Drinking water treatment response following a Colorado wildfire. Water Res 105:187–198
Hohner AK, Rhoades CC, Wilkerson P, Rosario-Ortiz FL (2019) Wildfires alter forest watersheds and threaten drinking water quality. Acc Chem Res 52:1234–1244
Hu TC, Luo M, Qi YL, He D, Chen LY, Xu YP, Chen DF (2023) Molecular evidence for the production of labile, sulfur-bearing dissolved organic matter in the seep sediments of the South China Sea. Water Res 233:119732
Kaiser K, Kalbitz K (2012) Cycling downwards - dissolved organic matter in soils. Soil Biol Biochem 52:29–32
Kellerman AM, Kothawala DN, Dittmar T, Tranvik LJ (2015) Persistence of dissolved organic matter in lakes related to its molecular characteristics. Nat Geosci 8:454–457
Lapierre JF, Guillemette F, Berggren M, Giorgio PA (2013) Increases in terrestrially derived carbon stimulate organic carbon processing and CO2 emissions in boreal aquatic ecosystems. Nat Commun 4(2972):1–7
Leng LJ, Huang HJ, Li H, Li J, Zhou WG (2019) Biochar stability assessment methods: a review. Sci Total Environ 647:210–222
Li H, Lu X, Xu Y, Liu H (2019) How close is artificial biochar aging to natural biochar aging in fields? A meta-analysis. Geoderma 352:96–103
Liu L, Song CY, Yan ZG, Li FS (2009) Characterizing the release of different composition of dissolved organic matter in soil under acid rain leaching using three-dimensional excitation-emission matrix spectroscopy. Chemosphere 77:15–21
Liu C, Wang HL, Li PH, **an QS, Tang XY (2019) Biochar’s impact on dissolved organic matter (DOM) export from a cropland soil during natural rainfalls. Sci Total Environ 650:1988–1995
Liu T, Wang X, Zhu EX, Liu ZG, Zhang XY, Guo JJ, Liu XQ, He C, Hou SJ, Fu PQ, Shi Q, Feng XL (2021) Evolution of the dissolved organic matter composition along the upper Mekong (Lancang) River. ACS Earth Space Chem 5:319–330
Lou YY, Ye ZL, Chen SH, Wei QS, Zhang JQ, Ye X (2018) Influences of dissolved organic matters on tetracyclines transport in the process of struvite recovery from swine wastewater. Water Res 134:311–326
Martinez-Perez AM, Nieto-Cid M, Osterholz H, Catala TS, Reche I, Dittmar T, Alvarez-Salgado XA (2017) Linking optical and molecular signatures of dissolved organic matter in the Mediterranean Sea. Sci Rep 7:3436
Murphy KR, Bro R, Stedmon CA (2014) Chemometric analysis of organic matter fluorescence. Aquat Org Matter Fluoresc 261:339–375
Ohno T, He ZQ, Sleighter RL, Honeycutt CW, Hatcher PG (2010) Ultrahigh resolution mass spectrometry and indicator species analysis to identify marker components of soil- and plant biomass-derived organic matter fractions. Environ Sci Technol 44:8594–8600
Peleato NM, Mckie M, Taylor-Edmonds L, Andrews SA, Legge RL, Andrews RC (2016) Fluorescence spectroscopy for monitoring reduction of natural organic matter and halogenated furanone precursors by biofiltration. Chemosphere 153:155–161
Roth VN, Lange M, Simon C, Hertkorn N, Bucher S, Goodall T, Griffiths RI, Mellado-Vázquez PG, Mommer L, Oram NJ, Weigelt A, Dittmar T, Gleixner G (2019) Persistence of dissolved organic matter explained by molecular changes during its passage through soil. Nat Geosci 12:755–761
Rudolf J, Niggemann J, Vhtalo AV, Stubbins A, Spencer RGM, Campbell J, Dittmar T (2013) Global charcoal mobilization from soils via dissolution and riverine transport to the oceans. Science 340(6130):345–347
Shen CY, Huang YF, Li BG, ** Y (2010) Predicting attachment efficiency of colloid deposition under unfavorable attachment conditions. Water Resour Res 46:100–103
Sigmund G, Jiang CJ, Hofmann T, Chen W (2018) Environmental transformation of natural and engineered carbon nanoparticles and implications for the fate of organic contaminants. Environ Sci-Nano 5:2500–2518
Smebye A, Ailing V, Vogt RD, Gadmar TC, Mulder J, Cornelissen G, Hale SE (2016) Biochar amendment to soil changes dissolved organic matter content and composition. Chemosphere 142:100–105
Spokas KA, Novak JM, Masiello CA, Johnson MG, Colosky EC, Ippolito JA, Trigo C (2014) Physical disintegration of biochar: an overlooked process. Environ Sci Tech Let 1:326–332
Stegen JC, Johnson T, Fresrickson JK, Wilkins MJ, Konopka AE, Nelson WC, Arntzen EV, Chrisler WB, Chu RK, Fansler SJ, Graham EB, Kennedy DW, Resch CT, Tfaily M, Zachara J (2018) Influences of organic carbon speciation on hyporheic corridor biogeochemistry and microbial ecology. Nat Commun 9(585):1–11
Stubbins A, Spencer RGM, Chen HM, Hatcher PG, Mopper K, Hernes PL, Mwamba VL, Mangangu AM, Wabakanghanzi JN, Six J (2010) Illuminated darkness: molecular signatures of Congo River dissolved organic matter and its photochemical alteration as revealed by ultrahigh precision mass spectrometry. Limnol Oceanogr 55:1467–1477
Stubbins A, Lapierre JF, Berggren M, Prairie YT, Dittmar T, del Giorgio PA (2014) What’s in an EEM? Molecular signatures associated with dissolved organic fluorescence in Boreal Canada. Environ Sci Technol 48:10598–10606
Sun K, Han LF, Yang Y, **a XH, **ng BS (2020) Application of hydrochar altered soil microbial community composition and the molecular structure of native soil organic carbon in a paddy soil. Environ Sci Technol 54:2715–2725
Sun YZ, Li XF, Li XM, Wang J (2022) Deciphering the fingerprint of dissolved organic matter in the soil amended with biodegradable and conventional microplastics based on optical and molecular signatures. Environ Sci Technol 56:15746–15759
Tip** E, Chamberlain PM, Frberg M, Hanson PJ, Jardine PM (2011) Simulation of carbon cycling, including dissolved organic carbon transport, in forest soil locally enriched with 14C. Biogeochemistry 108:91–107
Treumann S, Torkzaban S, Bradford SA, Visalakshan RM, Page D (2014) An explanation for differences in the process of colloid adsorption in batch and column studies. J Contam Hydrol 164:219–229
Wang DJ, Bradford SA, Harvey RW, Gao B, Cang L, Zhou DM (2012) Humic acid facilitates the transport of ARS-Labeled hydroxyapatite nanoparticles in iron oxyhydroxide-coated sand. Environ Sci Technol 46:2738–2745
Wang DJ, Zhang W, Zhou DM (2013) Antagonistic effects of humic acid and iron oxyhydroxide grain-coating on biochar nanoparticle transport in saturated sand. Environ Sci Technol 47(10):5154–5161
Wang DJ, Jaisi DP, Yan J, ** Y, Zhou DM (2015) Transport and retention of polyvinylpyrrolidone-coated silver nanoparticles in natural soils. Vadose Zone J 14(7):1–13
Wang LW, O’Connor D, Rinklebe J, Ok YS, Tsang DCW, Shen ZT, Hou DY (2020) Biochar aging: mechanisms, physicochemical changes, assessment, and implications for feld application. Environ Sci Technol 54:14797–14814
Ward CP, Sleighter RL, Hatcher PG, Cory RM (2014) Insights into the complete and partial photooxidation of black carbon in surface waters. Environ Sci Proc Impacts 16:721–731
Wu D, He L, Ge Z, Tong M, Kim H (2018) Different electrically charged proteins result in diverse bacterial transport behaviors in porous media. Water Res 143:425–435
Xu ZB, Xu XY, Tao XY, Yao CB, Tsang DCW, Cao XD (2019) Interaction with low molecular weight organic acids affects the electron shuttling of biochar for Cr (VI) reduction. J Hazard Mater 378:120705
Yang W, Bradford SA, Wang Y, Sharma P, Shang JY, Li BG (2019) Transport of biochar colloids in saturated porous media in the presence of humic substances or proteins. Environ Pollut 246:855–863
Zhang AF, Zhou X, Li M, Wu HM (2017) Impacts of biochar addition on soil dissolved organic matter characteristics in a wheat-maize rotation system in Loess Plateau of China. Chemosphere 186:986–993
Zhang HY, Wu L, Qian W, Ni JZ, Wei R, Qi ZC, Chen WF (2021) Spectral characteristics of dissolved organic carbon derived from biomass-pyrogenic smoke (SDOC) in the aqueous environment and its solubilization effect on hydrophobic organic pollutants. Water Res 203:117515
Zhao K, Gao L, Zhang QR, Shang JY (2021) Accumulation of sulfamethazine and ciprofloxacin on grain surface decreases the transport of biochar colloids in saturated porous media. J Hazard Mater 417:125908
Zhao K, Shang JY (2023) Transport of biochar colloids under unsaturated flow condition: Roles of chemical aging and cation type. Sci Total Environ 859:160415
Zhao K, Wang X, Li BG, Shang JY (2023) The roles of Fe oxyhydroxide coating and chemical aging in pyrogenic carbon nanoparticle transport in unsaturated porous media. Environ Pollut 317:120776
Zhou L, Zhou YQ, Tang XM, Zhang YL, Jang KS, Székely AJ, Jeppesen E (2021) Resource aromaticity affects bacterial community successions in response to different sources of dissolved organic matter. Water Res 190:116776
Acknowledgements
Not applicable.
Funding
This research was supported by National Natural Science Foundation of China (42377305), the “20 New Items of Universities” funding project of **an (No. 202228043), and Guangdong Provincial Key Laboratory of Environmental Health and Land Resource (2020B121201014).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Kang Zhao. The first draft of the manuscript was written by Kang Zhao, and the submitted version of the manuscript was finalized by Jianying Shang. The funding acquisition of this publication was supported by Jianying Shang. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Jianying Shang is an editorial board member of Carbon Research and was not involved in the editorial review, or the decision to publish, this article. All authors declare that there are no competing interests.
Additional information
Handling editor: Fengchang Wu.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, K., Shang, J. Optical and molecular insights into dissolved organic matter release in soils induced by downward migration of biochar colloids. Carbon Res. 3, 51 (2024). https://doi.org/10.1007/s44246-024-00136-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44246-024-00136-x