Abstract
Prickly pear (Opuntia ficus-indica L. Miller) is a natural source of antioxidant compounds that have gained significant attention due to their potential health benefits. This study aims to investigate the effectiveness of pressurized liquid extraction (PLE) and Ultrasound-Assisted Extraction (UAE), combined with water–ethanol mixtures from 0 to 60%, at moderate temperatures between 50 °C to 70 °C to improve the recovery of antioxidant compounds from red peel prickly pear. The recovery of these compounds was affected by solvent composition and temperature for both extraction techniques. Interestingly, the use of pure water at 70 °C during the PLE process allowed the recovery of high concentrations of antioxidant compounds (12.96 mg GAE/g dw, IC5O: 2.03 mg/mL, ORAC: 625 µmol TE/g dw). On the contrary, when the UAE process was combined with 30% ethanol at 50 °C, the extractability of these compounds (10.52 mg GAE/g dw, IC5O: 3.09 mg/mL, ORAC: 561.26 µmol TE/g dw) was improved. In addition, different solvent compositions were identified to improve the recovery of specific polyphenols. For PLE, pure water at 70 °C was effective in recovering phenolic acids, flavanols, and flavonols, while the highest concentrations of stilbenes were obtained with 60% ethanol at 70 °C. For UAE, the use of 30% ethanol at 50 °C was more effective in extracting phenolic acids and stilbenes, whereas pure water at 50 °C provided high concentrations of flavanols and flavonols. These results contribute to the development of sustainable and efficient extraction strategies for obtaining antioxidant-rich extracts from prickly pear peel with important applications in functional foods, nutraceuticals, and pharmaceutical industries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The prickly pear is a fruit extensively cultivated in Latin America, with a global cultivation area estimated at 500,000 ha, where Mexico (50,000 ha), Colombia (40,000 ha), and Peru (35,000 ha) are countries that stand out as the leading in terms of production [1]. The consumption of this fruit has increased considerably due to its particular flavour, colour, and aroma, which present different ecotypes such as red, purple, and orange [2]. However, the consumption and processing of this product generate agro-industrial wastes (peel and seeds), which need to be utilized by the industry and represent an environmental management problem [3, 4].
Several studies have shown that both wastes (skin and seed) present significant concentrations of bioactive compounds. In particular, the peel of this fruit contains substantial concentrations of polyphenols, ranging from 8 to 49 mg of gallic acid equivalents (GAE) per gram of dry weight (g dw) [5, 6]. Polyphenols are secondary metabolites from plants, characterized by at least one aromatic group, which must be hydroxylated [7]. In particular, red peel prickly pear presents distinct families of these compounds like phenolic acids, flavonols, flavanols, stilbenes, and flavones, which, due to their bioactive properties, can be used for the formulation of nutraceutical foods and the treatment of degenerative diseases associated with oxidative stress [5, 8]. For example, phenolic acids like caffeic acid have demonstrated bioactivity in combating skin cancer [9]. Flavonols like kaempferol present significant anti-proliferative effects against various carcinoma cells, including those associated with the breast and stomach [10]. In addition, isorhamnetin and other flavonols exhibit a range of pharmacological effects on cardiovascular diseases and rheumatism [11]. Flavanols as catechin present a high antimicrobial and anti-inflammatory activity [12]. Interestingly, the red and purple varieties of prickly pears contain betanin. In contrast, the yellow and orange varieties are characterized by high concentrations of indicaxanthin, piscidic acid and isorhamnetin glycosides [13]. These compounds present important bioactive properties. For example, betanin is a betacyanin that can act as a potent scavenger of inflammatory factors like hypochlorous acid [14]. Indicaxanthin’s amphiphilic nature and reduced potential enable it to interact with various lipoperoxyl radicals [15]. Thus, extracting these compounds would allow their use in develo** products with significant bioactive properties for treating and preventing degenerative diseases.
Traditional solid–liquid extraction techniques under atmospheric conditions like maceration, magnetic stirring, and soxhlet extraction have been employed to recover bioactive compounds from prickly pear [16]. However, these techniques use long process times (> 6 h) and high quantities of solvents like acetone, methanol, and hexane, which are potentially hazardous and impede their applicability in the formulation of nutraceutical products [17]. Thus, in response to these challenges, alternative technologies like pressurized liquid extraction (PLE) and ultrasound-assisted extraction (UAE) have emerged as promising extraction techniques. These technologies can be combined with environmentally friendly solvents like pure water and water–ethanol mixtures [18]. In addition, the high yields and the short process times allow reducing process costs compared to conventional extraction methods [19, 20].
Pressurized liquid extraction (PLE) is an alternative technology that uses high temperatures (50–200 °C), high pressures (10 MPa), and short extraction times (5–20 min) [21]. These specific operating conditions allow the solvent to remain in a liquid state and induce significant changes in its properties, such as polarity, dielectric constant, and viscosity, improving the efficiency and selectivity of the extraction of polyphenols from different plant matrices [22,23,24]. Compared to traditional extraction techniques, PLE is more efficient in performance and economically profitable [25]. For example, Gómez-López et al. [26] found that increasing the ethanol concentration from 0 to 50% during pressurized liquid extraction (PLE) at 25 °C improved by 53%, 96% and 20% the total flavonoids, phenolic acids, and total betalains content from Opuntia stricta var. Dillenii, respectively. Specifically, isorhamnetin glucoxyl-rhamnosyl-pentoside (0.25 mg/g dw), piscidic acid (2.08 mg/g dw), and protocatechuic acid (3.91 mg/g dw) were the most abundant polyphenols. In comparison, betanin (2.34 mg/g dw) and neobetanin (2.32 mg/g dw) were the betalains with the highest concentrations.
On the other hand, ultrasound-assisted extraction (UAE) operates on the principle of cavitation, a phenomenon wherein bubbles are generated and collapse on the surface of plant tissue, which induces fissures and ruptures, facilitating the extraction of bioactive compounds by the solvent [27, 28]. For instance, Gómez-López et al. [29] reported that using UAE combined with ethanol concentrations ranging from 0 to 100% at 25 °C significantly decreased the recovery of betalains, phenolic acids, and flavonoids by 92%, 42%, and 46% from Opuntia stricta var. Dillenii, respectively. Under these conditions, piscidic acid (2.32 mg/g dw) was identified as the most notable phenolic compound.
Although PLE and UAE have demonstrated favorable performances in recovering bioactive compounds, it is imperative to conduct a comparative analysis of both methods to choose the best method combined with alternative solvents. Thus, this study investigated the impact of both alternative methods (PLE and UAE) in conjunction with water–ethanol mixtures (ranging from 0 to 60%) at moderate temperatures (50 and 70 °C) on the content of total polyphenols, antioxidant capacity, reducing sugars, and polyphenol profile in the red prickly pear peel.
2 Materials and methods
2.1 Sample
The sample does not present any risk of extinction according to the guidelines of the General Directorate of Biological Diversity of Peru [30]. Thus, 12 kg of red prickly pear fruits were purchased from the local supermarket of the San Cristóbal district, Mariscal Nieto province, Moquegua department, Peru (Latitude: − 16.740336 and Longitude: − 70.682376). After careful selection, 5 kg of undamaged fruits were obtained. The pulp, peel, and seeds were separated manually and made up 36%, 42%, and 22% of the whole fruit, respectively. The peel was frozen at − 20 °C before the extraction process. Subsequently, the sample was reduced to a particle size of approximately 1 mm using a universal chopper (Ergomixx Bosch MS6CA4120-Italia).
2.2 Reagents
Reagents such as Folin Ciocalteu (2N), sodium carbonate (Na2CO3), DPPH (2,2-diphenyl-1-picrylhydrazyl), AAPH (2,2′-azobis (2-methyl-propanimidamide) dihydrochloride), and Trolox (Acid 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic) were purchased from Sigma Aldrich Chemical Co. (St. Louis, MO, USA). Solvents like ethanol (≥ 99%), methanol (99.9) and acetone (≥ 98%) were obtained from PanReac AppliChem ITW Reagents (Barcelona, Spain). For the preparation of PBS buffer, potassium dihydrogen phosphate (KH2PO4) and potassium monobasic phosphate (K2HPO4) salts were purchased from JT Baker Chemical Co. (Temixco, Mexico). Polyphenol standards such as caffeic acid (98%), vanillic acid (≥ 97%), catechin (≥ 95%), epicatechin (≥ 98%), procyanidin A2 and B2 (≥ 98%), quercitrin (≥ 90%), kaempferol (≥ 97%), rutin (≥ 95%), resveratrol (≥ 99%), and apigenin (≥ 97%), as well as glucose (> 99%) and fructose (> 99%) were supplied by Sigma Aldrich Chemical Co. (St. Louis, MO, USA).
2.3 Pressurized liquid extraction
Huaman-Castilla et al. [31] proposed a method under subcritical conditions for extracting antioxidant compounds. PLE was carried out using a DIONEX ASE 150 system (Thermofisher, San Jose, CA, USA). The sample (3 g) was mixed with quartz sand (30 g) and placed in an extraction cell with 100 mL. Afterwards, water–ethanol mixtures (0%, 30%, and 60%) were then combined with moderate temperatures (50 and 70 °C), whose fixed parameters were the pressure of 10 MPa, a single extraction cycle, 20% of wash volume, 250 s nitrogen purge, and 5 min of static extraction time. The extract obtained was transferred to amber vials and stored at − 20 °C for subsequent chemical analysis.
2.4 Ultrasound assisted extraction
The methodology proposed by Bhagya Raj and Dash [32] was used with some modifications. In brief, 3 g of sample were sonicated at different temperatures (50 and 70 °C) combined with water–ethanol mixtures (0, 30, and 60%) for 20 min in an ultrasound system equipped with a probe tip (ø: 4 mm) (model CY-500 probe, Optic Ivymen Systems™). The extracts obtained were centrifuged at 4000 rpm for 10 min. Then, the supernatant was filtered with standard filter paper. Finally, these collected extracts were transferred to amber vials and stored at − 20 °C for subsequent chemical analysis.
2.5 Total polyphenols content
The Folin-Ciocalteu colourimetric method, proposed by Singleton and Rossi [33] with some modifications, was used to calculate the total polyphenol content. Briefly, 20 µL of diluted extract, 150 µL of distilled water, 10 µL of Folin-Ciocalteu reagent (1 N), and 20 µL of 10% sodium carbonate were mixed. After allowing a one-hour reaction period, a Microplate Reader (SynergyTM HTX Multimodal) was used to measure absorbance at 765 nm. A standard curve of gallic acid (0.02 to 0.085 mg/mL) was prepared simultaneously. The results were expressed in mg GAE/g dw.
2.6 Antioxidant capacity by DPPH method
The antioxidant capacity evaluated by the DPPH method was developed according to the methodology proposed by Saravanakumar et al. [34] with some modifications. In brief, 100 µL of diluted extract was mixed with 100 µL of DPPH methanolic solution (0.3 mM) in a 96-well plate. Simultaneously, 200 µL of methanol was used as a blank, while 100 µL of DPPH solution and 100 µL of solvent extraction were mixed and used as a control. These mixtures were incubated at room temperature under dark for 45 min. Then, a Microplate Reader (Synergy™ HTX Multi-Modal Microplate Reader) was allowed to measure the absorbance at 517 nm. Finally, The IC50 value was determined to express the amount of extract necessary to inhibit the activity of the DPPH radical, which was calculated from the following equation.
where IC is the Inhibitory Concentration, As is the absorbance of samples, and Ac is the absorbance of control.
2.7 Antioxidant capacity by ORAC method
The assessment of antioxidant capacity was evaluated using the ORAC method, based on the methodology proposed by Chirinos et al. [35]. Previously, a PBS buffer solution with a concentration of 75 nM and a pH of 7.4 was prepared. This buffer served multiple purposes, including diluting AAPH (153 nM), constructing the Trolox curve, preparing fluorescein (55 nM), and diluting the extracts under evaluation. Then, diluted extract and Trolox standard solution (at concentrations of 8, 16, 24, 32, and 40 µM) were placed in a black 96-well plate. After, this plate was incubated for 10 min at 37 °C under darkness. Then, fluorescein and diluted AAPH were injected into the microplate reader (Synergy™ HTX Multi-Modal Microplate Reader), configured at 485 nm excitation and 520 nm emission. The results were expressed as Trolox equivalent (TE) micromoles per gram of dried weight (µmol TE/g dw).
2.8 Sugar content
The methodology proposed by Mariotti-Celis et al. [36] was used to determine sucrose, glucose, and fructose content with some modifications. First, 3 mL of water was mixed with 2 mL of the extract obtained. Then, a nylon filter (0.22 μm) was used to filter 3 mL of this mixture, which was combined with acetonitrile (3:7, v/v)). Afterwards, 20 μL of this mixture was injected into an HPLC-IR system (Ultimate 3000, Dionex Thermo Scientific, Sunnyvale, CA, USA), coupled to a refractive index detector. Previously, standard solutions (glucose and fructose) were prepared under the same conditions to establish a calibration curve. Finally, the reducing sugar concentrations were determined by their chromatographic peak areas. The results were expressed in milligrams of specific reducing sugar per gram of dry weight.
2.9 Polyphenol profile
The polyphenol profile was determined using the method suggested by Huamán-Castilla et al. [37] with some modifications. First, a C18 cartridge (Thermo Scientific, USA) was used to purify 5 mL of extract. Then, 2 µL of this sample was injected into an HPLC system (Agilent 1290 II, USA) coupled with a reversed-phase C18 column (2.1 × 150 mm × 1.9 μm) and a photodiode array detector to measure specific polyphenols. The process conditions were established with a 0.3 mL/min flow rate at 30 °C. Then, two mobile phases, A and B, were prepared with acetonitrile and formic acid (0.1%) and water and formic acid (0.1%), respectively. For elution, A (95%) and B (5%) were used for 15 min; after, a new mixture of A (60%) and B (40%) were used for 18 min. Meanwhile, 95% A and 5% B were used for 20 min to regenerate the resin. Previously, the calibration curves were created by graphing peak areas versus concentrations of standard solutions of specific polyphenols from 0.05 to 15 µg/L, with a detection limit of 0.03 µg/L and a quantification limit of 0.1 µg/L. The results were expressed as µg of polyphenol per gram dry weight.
2.10 Statistical analysis
This work employed a factorial experiment to evaluate the impact of different factors such as ethanol composition (0, 30 and 60%) and moderate temperature (50 and 70 °C) on antioxidant compounds and sugar-reducing content. An Analysis of Variance (One-way ANOVA) allowed us to identify significant differences among the observed groups, followed by Tukey’s Honest Significant Difference (HSD) test for further analysis. Finally, the data was analyzed using Statgraphics Plus for Windows 4.0 (Statpoint Technologies, Inc., Warrenton, VA, USA).
3 Results and discussion
3.1 Polyphenol content
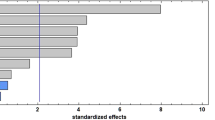
During the PLE process (subcritical conditions), high temperatures and solvent composition positively affected the polyphenol's recovery (Fig. 1). In this sense, the use of pure water, 30% ethanol, and 60% ethanol at high temperatures (70 °C) allowed the recovery of 12.96, 11.39, and 8.26 mg GAE/g dw, respectively (Fig. 1a). This positive impact of pure water was also documented in other studies under atmospheric conditions. For example, Aguirre Joya et al. [38] reported that the use of pure water at 70 °C significantly enhanced the recovery of polyphenols by 36% compared to water–ethanol mixtures (82%, v/v) from prickly pear peel. Similar behavior was also reported by Iftikhar et al. [39], who reported that pure water at 25 °C allowed to recover 2.5 times more content of phenolic compounds compared to the use of 100% ethanol from prickly pear cladodes. Ethanol can denature proteins and cellulosic material in prickly pear peels, forming complex networks that hinder the solubility of polyphenols [40, 41]. In addition, when the temperature increases during the PLE process, the solvent's kinetic energy increases, facilitating the disruption of hydrogen bonds between polyphenols and other compounds like proteins, polysaccharides, and fibers; consequently, their solubility is favoured [24, 37].
Contrary to the PLE process, when ultrasound-assisted extraction (UAE) was used, lower temperatures of 50 °C and intermediate ethanol concentrations were more effective in recovering polyphenols (Fig. 1b). In this context, the use of 30% ethanol at 50 °C was 26% and 33% more effective in recovering polyphenols compared to pure water and 60% ethanol, respectively (Fig. 1b). This negative effect of temperature was reported by Karunanithi & Venkatachalam [17], who observed that an increase from 40 to 45 °C reduces the recovery of polyphenols by 14% from prickly pear peel. Tabio-Garcia et al. [42] reported that an increase from 45 to 55 °C reduced the extractability of polyphenols by 18% during the UAE process. Probably during the UAE process, higher temperatures diminish the cavitation phenomenon (the formation, expansion, and collapse of bubbles) due to the heightened vapour pressure of the solvent, thereby reducing the implosion capacity of the bubbles and consequently lowering the release of polyphenols [43, 44]. The ethanol impact was also reported by Waterloo and Bouska [45], who found that lower ethanol concentrations (13%) at 30 °C recovered 12% more total polyphenol content than 50% ethanol during the UAE process. Similarly, Galvan et al. [46] reported that 50% ethanol at 60 °C extracted 2 and 4 times more total polyphenol content than 80% and 95% ethanol under the same conditions, respectively. Under atmospheric conditions, the use of high concentrations of ethanol reduces the boiling point of the extraction solvent [47]. This affects the solvent's ability to interact with polyphenols, and consequently, the extraction yield decreases.
3.2 Antioxidant capacity
The antioxidant capacity of polyphenols can be determined by analyzing their ability to transfer hydrogen atoms to reactive radicals. However, due to their complex chemical composition, a single test cannot identify their antioxidant capability [48]. Thus, the ORAC and DPPH techniques are used to evaluate their antioxidant capability [49]. Polyphenols typically reduce the DPPH radical by giving up hydrogen atoms. On the other hand, the ORAC method evaluates the potential of an antioxidant compound to donate or transfer hydrogen atoms to highly reactive peroxyl radicals. These radicals are similar to those generated by the human body, which can cause damage to cells and tissues if they are not neutralized [50].
For the PLE process, an increase in temperature improved the extract's ability to inhibit the DPPH radical. For instance, when the temperature was increased from 50 to 70 °C, the inhibition capacity increased by 71%, 23%, and 5% with pure water, 30% ethanol, and 60% ethanol, respectively (Fig. 2a). On the contrary, when UAE was used, the increase in temperature had a negative impact on the IC50 value (Fig. 2b). Specifically, an increase in temperature from 50 to 70 °C reduced the IC50 value by 27%, 96%, and 62% with pure water, 30% ethanol, and 60% ethanol, respectively.
On the other hand, the use of PLE and UAE allowed the obtain extracts with a higher ability to reduce the peroxyl radical, whose ORAC values vary from 169.14 to 625.68 μmol ET/g dw (Fig. 2c and d). For PLE, high temperatures (70 °C) and pure water were 63% more effective in inhibiting the peroxyl radical compared to 30% and 60% ethanol (Fig. 2c). On the other hand, the use of UAE, combined with an increase from 50 to 70 °C and employing 30% ethanol, diminishes the extracts' capability to inhibit the peroxyl radical by 24% (Fig. 2d).
3.3 Sugar reducing
Regardless of the extraction method, the use of temperatures and solvent composition should allow for reducing the presence of sugars in the extracts to propose their use in future food and pharmaceutical applications. According to our results, both methods (PLE and UAE) combined with high ethanol concentration at lower temperatures (50 °C) allowed to reduce the presence of reducing sugars. However, the application of the PLE process with 60% ethanol at 50 °C allowed a reduction of 88% and 90% in the recovery of glucose and fructose compared to the UAE process, respectively (Table 1). Mariotti et al. [36] reported that increasing the ethanol content from 0 to 15% at 90 °C reduced the fructose and glucose content by 17 and 24% in grape pomace extracts. Huaman et al. [31] observed that the utilization of high ethanol concentrations from 15 to 50% at 90 °C decreased by 60% in polyphenolic extracts under subcritical conditions. The addition of ethanol reduces the polarity of the solvent (non-polar medium), reducing the ability of the solvent to interact through hydrogen bonds with reducing sugars; simultaneously, lower temperatures during extraction result in reduced kinetic energy, thereby decreasing the recovery of these compounds [24, 37]. On the other hand, during the PLE process, the elevated pressures (10 atm) cause a decrease in solvent polarity, thereby reducing polar interactions between water molecules and sugar reducing [31]. This phenomenon elucidates why the PLE process was more effective in reducing sugar content than the UAE process conducted under atmospheric conditions.
3.4 Impact of study factors on polyphenol profile
3.4.1 Phenolic acids
Although the solvent composition was an important factor in recovering phenolic acids for both techniques (PLE and UAE), the PLE process was more effective in recovering these compounds. During the PLE process, pure water at 70 °C recovered 11 times more caffeic acid than ethanol under the same conditions (Table 2). Contrary to our results, other works have reported that the use of ethanol during the PLE Process is more effective in recovering phenolic acids than pure water from agroindustrial wastes [24, 31, 37]. However, prickly pear peel contains high concentrations of mucilage, which can interact with ethanol molecules to form a highly viscous solution that could reduce the release of certain specific polyphenols like phenolic acids [51]. In addition, Phenolic acid molecules contain hydroxyl and carbonyl groups in their chemical structure, allowing them to form a greater number of hydrogen bonds with water molecules compared to ethanol molecules, which only have a single hydroxyl group.
3.4.2 Stilbenes
In contrast to the behavior observed with phenolic acids, the use of the PLE process combined with 60% ethanol at 70 °C was 23% more effective in recovering stilbenes compared to pure water under the same conditions (Table 2). Similar behavior was reported by Huaman-Castilla et al. [31] who found that an increase from 15 to 50% ethanol at 150 °C improved by 83% the recovery of resveratrol during the PLE process. Meanwhile, Karacabey et al. [52] reported that the use of 25% ethanol at 105 °C allowed for the recovery of 14% more trans-resveratrol compared to pure water under the same conditions. This could be attributed to resveratrol's intermediate polarity, which makes it more soluble in non-polar solvents, such as ethanol [53].
3.4.3 Flavanols
Interestingly, the UAE process combined with intermediate ethanol concentration (30%) at lower temperatures (50 °C) was 24 times more effective in recovering monomers of flavanols like catechin compared to the PLE process (Table 2). During the UAE process, cavitation helps break the cellular matrix, releasing polyphenols, and the presence of ethanol improves interactions with the polyphenols, improving the extractability of these compounds [54]. Garcia-Castello et al. [55] also reported this positive effect of low ethanol concentration, finding that 20% ethanol at 61 °C was 14% more effective in recovering flavanols than 80% ethanol under the same conditions.
3.4.4 Flavonols
Although the use of pure water was an important factor in recovering flavonols for both techniques (PLE and UAE), the UAE process was more effective in recovering these compounds. Under atmospheric conditions, the use of pure water at 50° allowed to improve the extractability of quercetin (95.35 μg/g dw) compared to the use of 60% ethanol (72.03 μg/g dw) (Table 2). Huaman-Castilla et al. [31] reported that the presence of ketone-type carbonyl groups in the structure of flavonols increases their solubility in water. At the same time, a higher ethanol concentration will decrease the extractability of these compounds.
4 Conclusions
Although both PLE and UAE techniques allowed the extraction of polyphenolic compounds, the solvent composition and temperature were critical factors affecting their recovery. Higher temperatures have showed to be more effective for PLE, while lower temperatures have facilitated higher recovery of these compounds for UAE. Depending on the extraction method, solvent composition, and temperature, it is possible to optimize antioxidant compound recovery and enhance the efficiency of specific polyphenols. In the PLE process at 70 °C, pure water was more effective in recovering phenolic acids, flavanols, and flavonols; while 60% ethanol recovered high concentrations of stilbenes. For UAE process at 50 °C, 30% ethanol was more effective in recovering phenolic acids and stilbenes. Meanwhile, pure water at 50 °C improved the recovering of flavanols and flavonols. Finally, the antioxidant-rich extracts obtained through these alternative extraction techniques hold promise for various applications in the food, nutraceutical, and pharmaceutical industries.
References
Albuquerque TG, Pereira P, Silva MA, Vicente F, Ramalho R, Costa HS. Prickly Pear. In Nutritional composition and antioxidant properties of fruits and vegetables; 2020; pp. 709–728 ISBN 9780128127803.
Yahia EM, Saenz C. Cactus pear fruit and cladodes. Fruit Veg Phytochem Chem Hum Heal. 2017;2:941–56. https://doi.org/10.1002/9781119158042.ch44.
Kadda S. Temperature effects on yields, fatty acids and tocopherols of prickly pear (Opuntia ficus indica L.) seed oil of oriental region of Morocco. Environ Sci Pollut Res Int. 2021;29:1–16.
Ahmed SAA, AbdEl-Rahman GI, Behairy A, Beheiry RR, Hendam BM, Alsubaie FM, Khalil SR. Influence of feeding quinoa (Chenopodium quinoa) seeds and prickly pear fruit (Opuntia ficus indica) peel on the immune response and resistance to Aeromonas Sobria infection in Nile Tilapia (Oreochromis niloticus). Animals. 2020;10:1–31. https://doi.org/10.3390/ani10122266.
Amaya-Cruz DM, Pérez-Ramírez IF, Delgado-García J, Mondragón-Jacobo C, Dector-Espinoza A, Reynoso-Camacho R. An integral profile of bioactive compounds and functional properties of prickly pear (Opuntia ficus indica L.) peel with different tonalities. Food Chem. 2019;278:568–78. https://doi.org/10.1016/j.foodchem.2018.11.031.
Aruwa CE, Amoo S, Kudanga T. Phenolic compound profile and biological activities of southern African Opuntia ficus-indica fruit pulp and peels. Lwt. 2019;111:337–44. https://doi.org/10.1016/j.lwt.2019.05.028.
Gülçin I. Antioxidant activity of food constituents: an overview. Arch Toxicol. 2012;86:345–91. https://doi.org/10.1007/s00204-011-0774-2.
El-Mostafa K, El Kharrassi Y, Badreddine A, Andreoletti P, Vamecq J, El Kebbaj MS, Latruffe N, Lizard G, Nasser B, Cherkaoui-Malki M. Nopal Cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition. Health Dis Mol. 2014;19:14879–901. https://doi.org/10.3390/molecules190914879.
Khatabi O, Hanine H, Elothmani D, Hasib A. Extraction and determination of polyphenols and betalain pigments in the moroccan prickly pear fruits (Opuntia ficus indica). Arab J Chem. 2016;9:S278–81. https://doi.org/10.1016/j.arabjc.2011.04.001.
Liao W, Chen L, Ma X, Jiao R, Li X, Wang Y. Protective effects of kaempferol against reactive oxygen species-induced hemolysis and its antiproliferative activity on human cancer cells. Eur J Med Chem. 2016;114:24–32. https://doi.org/10.1016/j.ejmech.2016.02.045.
Zhai T, Zhang X, Hei Z, ** L, Han C, Ko AT, Yu X, Wang J. Isorhamnetin inhibits human gallbladder cancer cell proliferation and metastasis via PI3K/AKT signaling pathway inactivation. Front Pharmacol. 2021;12:1–12. https://doi.org/10.3389/fphar.2021.628621.
Fan FY, Sang LX, Jiang M, McPhee DJ. Catechins and their therapeutic benefits to inflammatory bowel disease. Molecules. 2017. https://doi.org/10.3390/molecules22030484.
Gómez-Maqueo A, Antunes-Ricardo M, Welti-Chanes J, Cano MP. Digestive stability and bioaccessibility of antioxidants in prickly pear fruits from the Canary Islands: healthy foods and ingredients. Antioxidants. 2020. https://doi.org/10.3390/antiox9020164.
Starzak K, Sutor K, Świergosz T, Nemzer B, Pietrzkowski Z, Popenda Ł, Liu SR, Wu SP, Wybraniec S. The responses of bioactive betanin pigment and its derivatives from a red beetroot (Beta vulgaris l.) betalain-rich extract to hypochlorous acid. Int J Mol Sci. 2021;22:1–18. https://doi.org/10.3390/ijms22031155.
Allegra M, Tutone M, Tesoriere L, Almerico AM, Culletta G, Livrea MA, Attanzio A. Indicaxanthin, a multi-target natural compound from Opuntia ficus-indica fruit: from its poly-pharmacological effects to biochemical mechanisms and molecular modelling studies. Eur J Med Chem. 2019;179:753–64. https://doi.org/10.1016/j.ejmech.2019.07.006.
Chougui N, Djerroud N, Naraoui F, Hadjal S, Aliane K, Zeroual B, Larbat R. Physicochemical properties and storage stability of margarine containing Opuntia ficus-indica peel extract as antioxidant. Food Chem. 2015;173:382–90. https://doi.org/10.1016/j.foodchem.2014.10.025.
Karunanithi A, Venkatachalam S. Ultrasonic-assisted solvent extraction of phenolic compounds from Opuntia ficus-indica peel: phytochemical identification and comparison with soxhlet extraction. J Food Process Eng. 2019;42:1–10. https://doi.org/10.1111/jfpe.13126.
Ameer K, Shahbaz HM, Kwon JH. Green extraction methods for polyphenols from plant matrices and their byproducts: a review. Compr Rev Food Sci Food Saf. 2017;16:295–315. https://doi.org/10.1111/1541-4337.12253.
Panja P. Green extraction methods of food polyphenols from vegetable materials. Curr Opin Food Sci. 2018;23:173–82. https://doi.org/10.1016/j.cofs.2017.11.012.
Soquetta MB, de Terra LM, Bastos CP. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CyTA J Food. 2018;16:400–12. https://doi.org/10.1080/19476337.2017.1411978.
Pagano I, Campone L, Celano R, Piccinelli AL, Rastrelli L. Green non-conventional techniques for the extraction of polyphenols from agricultural food by-products: a review. J Chromatogr A. 2021;1651: 462295. https://doi.org/10.1016/j.chroma.2021.462295.
da Silva LC, Viganó J, de Souza Mesquita LM, Dias ALB, de Souza MC, Sanches VL, Chaves JO, Pizani RS, Contieri LS, Rostagno MA. Recent advances and trends in extraction techniques to recover polyphenols compounds from apple by-products. Food Chem X. 2021. https://doi.org/10.1016/j.fochx.2021.100133.
Huamán-Castilla NL, Gajardo-Parra N, Pérez-Correa JR, Canales RI, Martínez-Cifuentes M, Contreras-Contreras G, Mariotti-Celis MS. Enhanced polyphenols recovery from grape pomace: a comparison of pressurized and atmospheric extractions with deep eutectic solvent aqueous mixtures. Antioxidants. 2023. https://doi.org/10.3390/antiox12071446.
Huamán-Castilla NL, Copa-Chipana C, Mamani-Apaza LO, Luque-Vilca OM, Campos-Quiróz CN, Zirena-Vilca F, Mariotti-Celis MS. Selective recovery of polyphenols from discarded blueberries (Vaccinium corymbosum L.) using hot pressurized liquid extraction combined with isopropanol as an environmentally friendly solvent. Foods. 2023;12:1–11. https://doi.org/10.3390/foods12193694.
Erdogan S, Ates B, Durmaz G, Yilmaz I, Seckin T. Pressurized liquid extraction of phenolic compounds from Anatolia propolis and their radical scavenging capacities. Food Chem Toxicol. 2011;49:1592–7. https://doi.org/10.1016/j.fct.2011.04.006.
Gómez-López I, Mendiola JA, Portillo MP, Cano MP. Pressurized green liquid extraction of betalains and phenolic compounds from Opuntia Stricta Var. Dillenii whole fruit: process optimization and biological activities of green extracts. Innov Food Sci Emerg Technol. 2022. https://doi.org/10.1016/j.ifset.2022.103066.
Kumar K, Srivastav S, Sharanagat VS. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: a review. Ultrason Sonochem. 2021;70: 105325. https://doi.org/10.1016/j.ultsonch.2020.105325.
Roselló-Soto E, Galanakis CM, Brnčić M, Orlien V, Trujillo FJ, Mawson R, Knoerzer K, Tiwari BK, Barba FJ. Clean recovery of antioxidant compounds from plant foods, by-products and algae assisted by ultrasounds processing. Modeling approaches to optimize processing conditions. Trends Food Sci Technol. 2015;42:134–49. https://doi.org/10.1016/j.tifs.2015.01.002.
Gómez-López I, Lobo-Rodrigo G, Portillo MP, Cano MP. Ultrasound-assisted “Green” extraction (Uae) of antioxidant compounds (betalains and phenolics) from Opuntia stricta Var. Dilenii’s fruits: optimization and biological activities. Antioxidants. 2021. https://doi.org/10.3390/antiox10111786.
Ministerio del Ambiente Especies CITES Peruanas. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://cdn.www.gob.pe/uploads/document/file/300623/d155091_opt.pdf.
Huaman-Castilla NL, Martínez-Cifuentes M, Camilo C, Pedreschi F, Mariotti-Celis M, Pérez-Correa JR. The impact of temperature and ethanol concentration on the global recovery of specific polyphenols in an integrated HPLE/RP process on Carménère Pomace extracts. Molecules. 2019;24:1–16. https://doi.org/10.3390/molecules24173145.
Bhagya Raj GVS, Dash KK. Ultrasound-assisted extraction of phytocompounds from dragon fruit peel: optimization, kinetics and thermodynamic studies. Ultrason Sonochem. 2020;68: 105180. https://doi.org/10.1016/j.ultsonch.2020.105180.
Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–58.
Saravanakumar A, Ganesh M, Peng MM, Aziz AS, Jang HT. Comparative antioxidant and antimycobacterial activities of Opuntia ficus-indica fruit extracts from summer and rainy seasons. Front Life Sci. 2015;8:182–91. https://doi.org/10.1080/21553769.2015.1028655.
Chirinos R, Pedreschi R, Rogez H, Larondelle Y, Campos D. Phenolic compound contents and antioxidant activity in plants with nutritional and/or medicinal properties from the Peruvian Andean region. Ind Crops Prod. 2013;47:145–52. https://doi.org/10.1016/j.indcrop.2013.02.025.
Mariotti-Celis MS, Martínez-Cifuentes M, Huamán-Castilla N, Pedreschi F, Iglesias-Rebolledo N, Pérez-Correa JR. Impact of an integrated process of hot pressurised liquid extraction-macroporous resin purification over the polyphenols, hydroxymethylfurfural and reducing sugars content of Vitis vinifera ‘Carménère’ Pomace extracts. Int J Food Sci Technol. 2018;53:1072–8. https://doi.org/10.1111/ijfs.13684.
Huamán-Castilla NL, Díaz Huamaní KS, Palomino Villegas YC, Allcca-Alca EE, León-Calvo NC, Colque Ayma EJ, Zirena Vilca F, Mariotti-Celis MS. Exploring a sustainable process for polyphenol extraction from olive leaves. Foods. 2024;13:1–13. https://doi.org/10.3390/foods13020265.
Aguirre Joya J, De La Garza TH, Zugasti Cruz A, Belmares Cerda R, Aguilar Cristóbal N. The Optimization of phenolic compounds extraction from cactus pear (Opuntia ficus-indica) skin in a reflux system using response surface methodology. Asian Pac J Trop Biomed. 2013;3:436–42. https://doi.org/10.1016/S2221-1691(13)60093-3.
Iftikhar K, Siddique F, Ameer K, Arshad M, Kharal S, Mohamed Ahmed IA, Yasmin Z, Aziz N. Phytochemical profiling, antimicrobial, and antioxidant activities of hydroethanolic extracts of prickly pear (Opuntia ficus indica ) fruit and pulp. Food Sci Nutr. 2023. https://doi.org/10.1002/fsn3.3226.
Yang L, Jiang JG, Li WF, Chen J, Wang DY, Zhu L. Optimum extraction process of polyphenols from the bark of Phyllanthus emblica L. Based on the response surface methodology. J Sep Sci. 2009;32:1437–44. https://doi.org/10.1002/jssc.200800744.
Doulabi M, Golmakani MT, Ansari S. Evaluation and optimization of microwave-assisted extraction of bioactive compounds from eggplant peel by-product. J Food Process Preserv. 2020;44:1–13. https://doi.org/10.1111/jfpp.14853.
Tabio-García D, Paraguay-Delgado F, Lardizabal Gutiérrez D, Quintero-Ramos A, Meléndez-Pizarro CO, Ochoa-Martínez LA, Sánchez-Madrigal M, Ruiz-Gutiérrez MG, Espinoza-Hicks JC. Effectiveness of Opuntia ficus-indica mucilage as a carrier agent in microencapsulation of bioactive compounds of Amaranthus hypochondriacus Var. Nutrisol. Food Biosci. 2023. https://doi.org/10.1016/j.fbio.2023.102368.
Chemat F, Rombaut N, Sicaire AG, Meullemiestre A, Fabiano-Tixier AS, Abert-Vian M. Ultrasound assisted extraction of food and natural products. mechanisms, techniques, combinations, protocols and applications. A review. Ultrason Sonochem. 2017;34:540–60. https://doi.org/10.1016/j.ultsonch.2016.06.035.
Abi-Khattar AM, Boussetta N, Rajha HN, Abdel-Massih RM, Louka N, Maroun RG, Vorobiev E, Debs E. Mechanical damage and thermal effect induced by ultrasonic treatment in olive leaf tissue, impact on polyphenols recovery. Ultrason Sonochem. 2022. https://doi.org/10.1016/j.ultsonch.2021.105895.
Watrelot AA, Bouska L. Optimization of the ultrasound-assisted extraction of polyphenols from Aronia and Grapes. Food Chem. 2022;386: 132703. https://doi.org/10.1016/j.foodchem.2022.132703.
Galvan D’Alessandro L, Kriaa K, Nikov I, Dimitrov K. Ultrasound assisted extraction of polyphenols from black chokeberry. Sep Purif Technol. 2012;93:42–7. https://doi.org/10.1016/j.seppur.2012.03.024.
Oman E, Gunnelius A. Calculation of points in the ethyl alcohol-water distillation curve. Ind Eng Chem. 1925;17:964. https://doi.org/10.1021/ie50189a034.
Prior RL. Oxygen radical absorbance capacity (ORAC): new horizons in relating dietary antioxidants/bioactives and health benefits. J Funct Foods. 2015;18:797–810. https://doi.org/10.1016/j.jff.2014.12.018.
Liang N, Kitts DD. Antioxidant property of coffee components: assessment of methods that define mechanism of action. Molecules. 2014;19:19180–208. https://doi.org/10.3390/molecules191119180.
Huang D, Boxin OU, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–56. https://doi.org/10.1021/jf030723c.
Mannai F, Elhleli H, Yılmaz M, Khiari R, Belgacem MN, Moussaoui Y. Precipitation solvents effect on the extraction of mucilaginous polysaccharides from Opuntia ficus-indica (Cactaceae): structural, functional and rheological properties. Ind Crops Prod. 2023;202: 117072. https://doi.org/10.1016/j.indcrop.2023.117072.
Karacabey E, Mazza G. Optimization of Solid-liquid extraction of resveratrol and other phenolic compounds from milled grape canes (Vitis vinifera). J Agric Food Chem. 2008;56:6318–25. https://doi.org/10.1021/jf800687b.
Camont L, Cottart CH, Rhayem Y, Nivet-Antoine V, Djelidi R, Collin F, Beaudeux JL, Bonnefont-Rousselot D. Simple spectrophotometric assessment of the trans-/cis-resveratrol ratio in aqueous solutions. Anal Chim Acta. 2009;634:121–8. https://doi.org/10.1016/j.aca.2008.12.003.
Beaudor M, Vauchel P, Pradal D, Aljawish A, Phalip V. Comparing the efficiency of extracting antioxidant polyphenols from spent coffee grounds using an innovative ultrasound-assisted extraction equipment versus conventional method. Chem Eng Process - Process Intensif. 2023;188: 109358. https://doi.org/10.1016/j.cep.2023.109358.
Garcia-Castello EM, Rodriguez-Lopez AD, Mayor L, Ballesteros R, Conidi C, Cassano A. Optimization of conventional and ultrasound assisted extraction of flavonoids from grapefruit (Citrus paradisi L.) solid wastes. Lwt. 2015;64:1114–22. https://doi.org/10.1016/j.lwt.2015.07.024.
Funding
The authors are grateful to the National University of Moquegua for funding this project (Resolución de Comisión Organizadora N° 0262-2021-UNAM and N° 428-2021-UNAM).
Author information
Authors and Affiliations
Contributions
N.L.H.C., project administration; N.L.H.C. and E.E.A.A., writing—original draft preparation; S.M.P. and ML.M.J., investigation, extraction process, and analysis for TPC, DPPH, and ORAC; F.Z.V. and O.M.L.V., validation and formal analysis for polyphenol profile and reducing sugar; E.E.P., statistical analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Parí, S.M., Juárez, M.L.M., Vilca, F.Z. et al. Alternative green extraction techniques to enhance recovery of antioxidant compounds from red peel prickly pear (Opuntia ficus-indica L. Miller). Discov Food 4, 58 (2024). https://doi.org/10.1007/s44187-024-00140-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44187-024-00140-5