Abstract
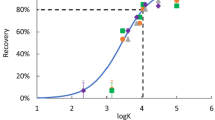
Chemical characterization studies for medical devices rely on extract preparation techniques such as liquid–liquid extraction to render the extract more amenable to instrumental analysis. Considering these studies are generally non-targeted, the effect of extract preparation on the full range of potential analytes must be considered. However, there is currently no workflow for evaluating the impact of extract preparation on non-targeted extractables. Herein, we present a framework for approaching this problem by defining the applicable chemical space, selecting a model that appropriately predicts recovery from the extract preparation method, selecting example chemicals with a range of expected recoveries, and experimentally measuring the recoveries to verify the model performance under laboratory conditions. The framework is demonstrated for liquid–liquid extraction, with recovery demonstrably dependent on extractable pKa and distribution coefficient. Method parameters including pH, volume ratios, and extraction iterations were also considered critical factors in determining recovery. The analytical method was subsequently verified by incorporating deliberate deviations in the critical parameters as part of robustness determination for the method using a central composite design. Overall, the method parameters selected in this work resulted in coverage of approximately 85% of the selected chemical space. Further, for the various permutations evaluated, the model was found to predict recovery with a root-mean-square error of 19%. A result of this approach is increased clarity in the use of surrogate standards for evaluation of extract preparation methods, indicating that they should be chosen based on the variables included in the predictive model. Additionally, the verified model can be applied to the selected chemical space so that estimations can be made about which relevant analytes are likely to be poorly recovered during extract preparation. This framework is generally applicable to understand the effect of extract preparation on non-targeted analysis and how these considerations can be integrated into method development and validation.




Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
ISO, ISO 10993-18 Biological Evaluation of Medical Devices - Part 18: Chemical Characterization of Medical Device Materials Within a Risk Management Process (International Organization for Standardization (ISO), 2020)
AAMI, Biological evaluation of medical devices—Part 1: Evaluation and testing within a risk management process, in ANSI/AAMI/ISO 10993-1:2018; Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing Within a Risk Management Process (AAMI, Melbourne, 2020). https://doi.org/10.2345/9781570207556.ch1
E.M. Sussman et al., Chemical characterization and non-targeted analysis of medical device extracts: a review of current approaches, gaps, and emerging practices. ACS Biomater. Sci. Eng. 8(3), 939–963 (2022). https://doi.org/10.1021/acsbiomaterials.1c01119
C.I.C. Silvestre, J.L.M. Santos, J.L.F.C. Lima, E.A.G. Zagatto, Liquid–liquid extraction in flow analysis: a critical review. Anal. Chim. Acta 652(1–2), 54–65 (2009). https://doi.org/10.1016/j.aca.2009.05.042
P.G. Mazzola et al., Liquid–liquid extraction of biomolecules: an overview and update of the main techniques. J. Chem. Technol. Biotechnol. 83(2), 143–157 (2008). https://doi.org/10.1002/jctb.1794
K. Levsen, Sample preparation for water analysis, in Sampling and Sample Preparation for Field and Laboratory, ed. by J. Pawliszyn ((Elsevier Science B.V., Amsterdam, 2002), pp. 721–778
C.W. Coley, Defining and exploring chemical spaces. Trends Chem. 3(2), 133–145 (2021). https://doi.org/10.1016/j.trechm.2020.11.004
B.L. Milman, I.K. Zhurkovich, The chemical space for non-target analysis. TrAC Trends Anal. Chem. 97, 179–187 (2017). https://doi.org/10.1016/j.trac.2017.09.013
G. Black et al., Exploring chemical space in non-targeted analysis: a proposed ChemSpace tool. Anal. Bioanal. Chem. (2022). https://doi.org/10.1007/s00216-022-04434-4
Y. Liu, X. Yu, J. Chen, Quantitative structure–property relationship of distribution coefficients of organic compounds. SAR QSAR Environ. Res. 31(8), 585–596 (2020). https://doi.org/10.1080/1062936X.2020.1782468
K.L. Dionisio et al., The chemical and products database, a resource for exposure-relevant data on chemicals in consumer products. Sci. Data 5(1), 180125 (2018). https://doi.org/10.1038/sdata.2018.125
Nagao et al. The ELSIE Extractable and Leachable Database [Internet]. Pharm. Outsourcing 2011. https://www.pharmoutsourcing.com/Featured-Articles/37828-The-ELSIE-Extractables-and-Leachables-Database/. Accessed on 28 Apr 2023
H. Wiesinger, Z. Wang, S. Hellweg, Deep dive into plastic monomers, additives, and processing aids. Environ. Sci. Technol. 55(13), 9339–9351 (2021). https://doi.org/10.1021/acs.est.1c00976
California Office of Environmental Health Hazard Assessment. Proposition 65 LIst of Chemicals. [Internet] 2022. https://oehhca.gov/proposition-65/proposition-65-list. Accessed 28 Apr 2023
U.S. Environmental Protection Agency. TSCA Chemical Substance Inventory. [Internet]; (2022), https://www.epa.gov/tsca-inventory. Accessed 28 Apr 2023
K. Mansouri et al., Open-source QSAR models for pKa prediction using multiple machine learning approaches. J. Cheminform. 11(1), 60 (2019). https://doi.org/10.1186/s13321-019-0384-1
T.N. Brown, QSPRs for predicting equilibrium partitioning in solvent-air systems from the chemical structures of solutes and solvents. J. Solut. Chem. 51(9), 1101–1132 (2022). https://doi.org/10.1007/s10953-022-01162-2
M.H. Abraham, R.E. Smith, R. Luchtefeld, A.J. Boorem, R. Luo, W.E. Acree, Prediction of solubility of drugs and other compounds in organic solvents. J. Pharm. Sci. 99(3), 1500–1515 (2010). https://doi.org/10.1002/jps.21922
N. Ulrich et al., “UFZ-LSER database,” UFZ-LSER database v 3.2.1. http://www.ufz.de/lserd
Q 2 (R1) Validation of Analytical Procedures: Text and Methodology (2006)
R.S. Bohacek, C. McMartin, W.C. Guida, The art and practice of structure-based drug design: a molecular modeling perspective. Med. Res. Rev. 16(1), 3–50 (1996)
J.-L. Reymond, The chemical space project. Acc. Chem. Res. 48(3), 722–730 (2015). https://doi.org/10.1021/ar500432k
T. Hoffmann, M. Gastreich, The next level in chemical space navigation: going far beyond enumerable compound libraries. Drug Discov. Today 24(5), 1148–1156 (2019). https://doi.org/10.1016/j.drudis.2019.02.013
BioByte Corporation, BioByte (2019), http://www.biobyte.com/. Accessed 28 April 2023
Advanced Chemistry Development ACDLabs, Chemistry software for analytical and chemical knowledge management. (2019), https://www.acdlabs.com/. Accessed 28 Apr 2023
Simulations Plus, Simulations plus: model-based drug development to make better data-driven decisions (2019), https://www.simulations-plus.com/. Accessed 28 Apr 2023
ChemAxon Ltd. Chemicalize (2019), https://chemaxon.com/products/chemicalize. Accessed 28 Apr 2023
W. Horwitz, Evaluation of analytical methods used for regulation of foods and drugs. Anal. Chem. (1982). https://doi.org/10.1021/ac00238a002
B. Schulze et al., An assessment of quality assurance/quality control efforts in high resolution mass spectrometry non-target workflows for analysis of environmental samples. TrAC Trends Anal. Chem. 133, 116063 (2020). https://doi.org/10.1016/j.trac.2020.116063
Acknowledgements
This research was funded in part by an appointment to the Research Participation Program administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the US Department of Energy and the US Food and Drug Administration.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors have no competing interests to disclose.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Duelge, K.J., Young, J.A. Estimating Recovery in the Liquid–Liquid Extraction Chemical Space. Biomedical Materials & Devices 2, 557–565 (2024). https://doi.org/10.1007/s44174-023-00123-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44174-023-00123-7