Abstract
High-throughput technologies for multiomics or molecular phenomics profiling have been extensively adopted in biomedical research and clinical applications, offering a more comprehensive understanding of biological processes and diseases. Omics reference materials play a pivotal role in ensuring the accuracy, reliability, and comparability of laboratory measurements and analyses. However, the current application of omics reference materials has revealed several issues, including inappropriate selection and underutilization, leading to inconsistencies across laboratories. This review aims to address these concerns by emphasizing the importance of well-characterized reference materials at each level of omics, encompassing (epi-)genomics, transcriptomics, proteomics, and metabolomics. By summarizing their characteristics, advantages, and limitations along with appropriate performance metrics pertinent to study purposes, we provide an overview of how omics reference materials can enhance data quality and data integration, thus fostering robust scientific investigations with omics technologies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, the adoption of multiomics approaches in biomedical research and clinical application has increased significantly (Hasin et al. 2017; Hoadley et al. 2018). The integration of multiomics or molecular phenomics data (including genomics, epigenomics, transcriptomics, proteomics, and metabolomics) along with deep phenotypic data enables the discovery of correlations between the diverse levels of genetic and regulatory information and distinct phenotypic traits, fostering a more comprehensive understanding of biological processes and facilitating the identification of disease mechanisms, potential therapeutic targets, and disease biomarkers (Jiang et al. 2023b). In 2015, NIST released the primary human genome DNA reference material, RM 8398, derived from HG001/NA12878, a healthy female of European ancestry. To improve the representation of human genetic diversity, NIST further developed DNA reference materials from different ethnic populations, including an Ashkenazi Jewish family trio (RM8392) and a Han Chinese son (RM8393) (Zook et al. 2018). The Quartet Project, led by Fudan University in close collaboration with the National Institute of Metrology of China and other organizations, established four immortalized lymphoblastoid cell lines from a Chinese Quartet family, including a father, mother and two monozygotic daughters (Ren et al. 2019). The GeT-RM has characterized DNA RMs for a wide range of genetic disorders, such as cystic fibrosis (Pratt et al. 2009), Duchenne and Becker muscular dystrophy (Kalman et al. 2011), fragile X syndrome (Amos Wilson et al. 2008), Huntington disease (Kalman et al. 2007), and many others, including 11 human leukocyte antigen loci (Bettinotti et al. 2018) and pharmacogenetic loci (Gaedigk et al. 2019; Pratt et al. 2016). These reference materials represent specific mutations associated with diseases and are available for research, clinical test development, quality assurance and control, and proficiency testing to ensure the accuracy of clinical testing.
Somatic variants are genetic mutations that occur in non-germline cells. They are typically detected in tumors from sequencing datasets of paired tumor and normal samples, with normal samples used to remove germline variants. Accurate and reliable detection of somatic variants is crucial for gaining insights into cancer biology, guiding targeted therapies and improving patient outcomes in cancer treatment. DNA RMs used to benchmark somatic variants usually consist of matched tumor and normal genomes.
The MicroArray and Sequencing Quality Control (MAQC-IV/SEQC2) consortium recently completed its fourth project, which aimed to develop standard analysis protocols and quality control metrics for the use of high-throughput DNA sequencing data in regulatory science research and precision medicine (MAQC Consortium 2021). The Somatic Mutation Working Group (WG1) of SEQC2 established paired tumor-normal DNA RMs and corresponding whole-genome reference datasets for small variants and structural variants (Fang et al. 2016) created DNA RMs from a metastatic melanoma (COLO829) and its paired B-lymphoblastoid normal cell line (COLO829BL).
While WGS and WES provide a more comprehensive view of the entire genome, targeted sequencing, also known as oncopanel sequencing, offers a more cost-effective and efficient approach by focusing on a limited number of cancer hotspot variants. It can detect variants with a variant allele frequency (VAF) as low as 0.5%. The Oncopanel Sequencing Working Group (WG2) of SEQC2 established two DNA RMs for oncopanel benchmarking (Jones et al. 2006, 2014), covering as many clinically related variants as possible to increase variant density in coding regions. Sample B is derived from a non-cancer male cell line (Agilent OneSeq Human Reference DNA, PN 5190-8848). To emulate the range of VAFs typically encountered in targeted sequencing and ctDNA sequencing, tumor Sample A was diluted by normal Sample B at different ratios to create a series of tumor DNA reference materials with even lower VAFs of variants. The SEQC2 WG2 employed these DNA RMs to conduct cross-platform multi-laboratory evaluations of commercially available oncopanels, and developed actionable guidelines to improve the performance and consistency of oncopanel sequencing across different laboratories and platforms (Deveson et al. 2023). This set of RNA reference materials enables the evaluation of the performance of isoform-specific RNA-seq workflows, and thus provides a more comprehensive evaluation of RNA-seq performance.
RNA-seq can be used to sequence long RNAs, such as messenger RNAs, as well as short RNAs, such as microRNAs (miRNAs), that differ in length. The Extracellular RNA Communication Consortium led a benchmark study for miRNA quantification across multiple protocols and laboratories using small RNA-seq (Giraldez et al. 2018). They used diverse combinations of synthetic RNAs to evaluate sequence-specific biases and accuracy. An equimolar pool consisted of over 1000 chemically synthesized RNA oligonucleotides (15–90 nt) mixed at equal concentration was used to assess reproducibility of absolute RNA sequences abundance at counts per million (CPM) level. Two synthetic small RNA pools with RNAs varied in defined relative amount were used to assess the concordance for relative quantification. Synthetic pools with unedited and edited miRNA variants in different ratios were used to determine the accuracy of quantifying miRNA editing.
Protein Reference Materials
The proteome refers to the entire set of proteins expressed by a cell, tissue or organism at a particular time. Proteomics is the systematic, high-throughput study of the composition, functions, and interactions of all proteins. In proteomics research, where the sheer multitude of proteins presents a formidable challenge, mass spectrometry (MS) is commonly used for both qualitative and quantitative protein analysis. This process involves comparing detected peptide maps with protein sequences sourced from databases. However, the complexity of MS-based proteomics experiments and their potential for considerable variability can hinder the achievement of accurate and reproducible results. To enhance the reliability and reproducibility of proteomics, numerous initiatives have been actively working for decades to establish community standards and guidelines. These efforts aim to ensure consistency, promote rigorous experimental practices, and facilitate the generation of reliable and comparable proteomic data across different laboratories and studies. For example, the Proteomics Standards Initiative (PSI) of the Human Proteome Organization (HUPO) standardized practices and guidelines for data reporting formats (Deutsch et al. 2017), data quality control framework (Bittremieux et al. 2017) and data interpretation (Omenn 2021). CPTAC, launched by the US National Cancer Institute (NCI), intends to improve MS-based proteomics measurement quality for biomarker discovery in cancer research (Tabb et al. 2016; Zhou et al. 2017). The Proteomics Standards Research Group (sPRG) of the Association of Biomolecular Resource Facilities (ABRF) develops and implements standards to reflect the accuracy and consistency of proteomics (Tabb et al. 2010).
The limitations of traditional methods have driven the development of new technologies tailored for highly sensitive protein biomarker discovery while demanding minimal quantities of biological materials (Eldjarn et al. 2023; Sun et al. 2023). Examples of this innovation include Olink's Proximity Extension Assay (PEA) (Petrera et al. 2021; Wik et al. 2021), SomaLogic's SomaScan Assay (Candia et al. 2017, 2022), and Seer's Proteograph (Blume et al. 2023).
Synthetic Protein Reference Materials
Synthetic protein reference materials have been extensively used in benchmark studies of proteomic measurements to determine experimental and analytical variations by big consortia (Paulovich et al. 2010; Tabb et al. 2010). Notable examples of standard protein mixtures include: the Universal Proteomics Standards (UPS1 and UPS2), a mixture of 48 human recombinant proteins jointly developed by ABRF's sPRG and Sigma-Aldrich (Andrews et al. 2006); the HUPO Gold MS Protein Standard, a mixture of 20 human proteins, developed by the joint efforts of HUPO and Invitrogen (Bell et al. 2009); a mixture of 20 purified human proteins (NCI-20), produced by NIST and employed by CPTAC for intra- and inter-laboratory studies aiming at evaluating repeatability and comparability of qualitative proteomics (Tabb et al. 2010; Wang et al. 2014).
Chemical synthetic or modified peptide mixtures are also utilized as RMs. In comparison to protein mixtures, peptide mixtures have a simpler composition. However, it is important to note that they cannot fully capture the variability introduced during enzymatic digestion, as different laboratories may employ diverse proteolytic enzymes, chemicals, and conditions for digestion. Several synthetic peptide reference materials are commercially available, such as a mixture of 1000 heavy-label proteotypic peptides for conserved proteins across three species (human, mouse and rat), established by ABRF and JPT Peptide Technologies (2023). Synthetic peptides are especially important to evaluate the performance of targeted quantitative proteomic measurement, such as multiple reaction monitoring (MRM) and parallel reaction monitoring (PRM). They are often used to predict retention times (RTs) for large-scale scheduled liquid chromatography multiple reaction monitoring (LC-MRM) measurements with a single calibration run before the analytical runs. Biognosys has developed a mixture of 11 artificial synthetic peptides (iRT) to determine peptide retention time (RT) values and calibrate chromatographic systems for increasing the throughput (Escher et al. 2012). Additionally, well-defined synthetic protein reference materials can be added into biological protein reference materials or test samples to provide additional information of qualitative accuracy. An important consideration when spiking synthetic peptides into other samples is that these peptides should not overlap with the original sample content.
Metabolite Reference Materials
Metabolomics encompasses the extensive investigation of small molecules, known as metabolites, within cells, biological fluids, tissues, or organisms. It integrates the influences of factors from genomics, transcriptomics, proteomics, as well as environmental elements like diet and lifestyle. Since metabolites serve as indicators of the downstream effects of these factors on cellular functions, they closely represent the actual phenotypes of cells, tissues, or organisms, offering novel insights into metabolism and its regulation in physiological and pathological processes, including health, aging, and diseases. Metabolomics involves the simultaneous identification and quantification of various small molecule types, including amino acids, fatty acids, carbohydrates, and other products of cellular metabolic functions. In comparison to genomics, transcriptomics, and proteomics, the reliable identification and quantification of the metabolome are significantly more complex due to the chemical complexity and the presence of isomers—compounds with the same molecular formula but different structural arrangements—introducing challenges for precise identification and quantification.
To promote the advancement of metabolomics toward higher quality, several large research consortia have emerged in the field, aiming to enhance the reproducibility of metabolomics research results through comprehensive quality assurance and quality control measures. These consortia have undertaken various efforts, including the establishment of best practices, promotion of communication and education, and the advancement of the field toward higher-quality standards. The mQACC, consisting of experts in quality assurance and quality control, is focused on develo** universal best practices and reporting standards to ensure the robustness and reproducibility of untargeted metabolomics research (Beger et al. 2019; Evans et al. 2020). The Metabolomics Society Data Quality Task Group (DQTG) aims to enhance the robustness of quality assurance and quality control in the metabolomics community through communication, advocacy, education, and the promotion of best practices (Kirwan et al. 2022). The Standard Metabolic Reporting Structures (SMRS) group is dedicated to standardizing metabolomics analysis and provides comprehensive reports and summaries on relevant key issues (Beckonert et al. 2007; Lindon et al. 2005). The ABRF Metabolomics Research Group aims to study the reproducibility of metabolomics research and propose best data analysis strategies by comparing analysis groups using the same dataset (Turck et al. 2020). Additionally, the ABRF plays a role in improving the core competencies of biotechnology laboratories through research, communication, and education (Cheema et al. 2015; Turck et al. 2020). The Metabolomics Consortium has proposed guidelines for achieving high-quality reporting of LC–MS-derived metabolomics data, including the identification and prioritization of test materials, assessment of useful indicators of data quality, and descriptions of common practices and variations in quality assurance and quality control workflows (Broadhurst et al. 2018).
Quality control samples can be categorized into three primary types based on their intended purposes. System suitability test samples serve as a quality assurance measure applied before data acquisition to instill confidence in the eventual high-quality results (Broadhurst et al. 2018; Kirwan et al. 2022). Typically, these samples consist of solutions containing a small number of authentic chemical standards, typically ranging from five to 10 analytes, with known concentrations. They play a critical role in instrument calibration and assessment of critical system parameters, including mass-to-charge (m/z) ratio and chromatographic characteristics such as retention time, peak area, and peak shape.
Blank quality control samples and matrix-matched quality control samples are essential components of quality control measures to ensure that the quality management process is fulfilled. Blank quality control samples consist of samples devoid of metabolites, serving to identify potential sample contamination or instrument-related background signals, thereby eliminating interference from external contaminants or instrument-related background signals, thereby eliminating interference from external contaminants or the instrument itself (Kirwan et al. 2022). By comparing data from the actual samples to that from the blank samples, researchers can distinguish genuine metabolite signals from potential interferences or background noise. Within the category of matrix-matched quality control samples, the most commonly used are pooled samples. These samples are created by pooling a small amount of each analyzed biological sample within a study, representing both the sample matrix and metabolite composition. Pooled QC samples play a multifaceted role, conditioning the analytical platform, conducting intra-study reproducibility measurements, and mathematically correcting for systematic changes in parameter values (Broadhurst et al. 2018). A specific type of pooled QC sample can be used to assess data quality across different studies within the same laboratory, termed long-term reference (LTR) QC samples (Broadhurst et al. 2018). These samples are obtained either through the commercial purchase of the required sample types or by collecting representative samples from various studies within the laboratory. In this review, we focus on the use of external RMs for assessing performance across different laboratories, which are created and sold by a certified group.
Biological Metabolite Reference Materials
SRM 1950 released by NIST is one of the first developed metabolite reference materials, which is intended for quality control of identifying and quantifying metabolites in human plasma, such as fatty acids, electrolytes, vitamins, hormones, and amino acids (Phinney et al. 2013). It is a mixture of human plasma samples from 100 individuals reflecting a racial distribution in the US population at the time of implementation (77% white, 12% African-American or black, 2% American Indian or Askan Native, 4% Asian, 5% other, with about 15% Hispanic origin). A total of 90 metabolites are assigned with high confidence values of absolute concentrations by integrating several different analytical methods. SRM 1950 was initially designed for targeted metabolomics, and has been extensively used to benchmark platforms, protocols and workflows (McGaw et al. 2010; Misra and Olivier 2020; Siskos et al. 2017; Thompson et al. 2019). Recently, it has also been used in benchmark studies of untargeted metabolomics and lipidomics (Azab et al. 2019; Bowden et al. 2017; Cajka et al. 2017). NIST also released other standalone natural-matrix reference materials for organic contaminants from an assortment of biological materials, including frozen non-fortified human milk (SRM 1953), fortified human milk (SRM 1954), non-fortified human serum (SRM 1957), fortified human serum (SRM 1958) (Schantz et al. 2013), lyophilized human serum (SRM 909b and SRM 909c) (Aristizabal-Henao et al. 2021), smokers' human urine (SRM 3672), and non-smokers' urine (SRM 3673).
Like other quantitative omics, such as transcriptomics and proteomics, identifying differentially expressed metabolites between sample groups is one of the main purposes for metabolomics-based biomarker researches. RMs consisting of two or more sample groups can be used to assess the performance of distinguishing sample groups. The NIST Metabolomics Quality Assurance and Quality Control Materials (MetQual) Program released a suite of pooled plasma materials (RM 8231) comprising four different metabolic health states, including type 2 diabetes plasma, hypertriglyceridemia plasma, normal African-American plasma and normal human plasma (SRM 1950) (Met Qual Program Coordinators 2023). The MetQual Program is planning to conduct an inter-laboratory study to obtain consensus characterization of RM 8231 and assess measurement variability within the metabolomics community. NIST also developed several multi-sample metabolite reference materials from other biological resources. RM 971a consists of two serum mixtures: one from a pool of healthy, premenopausal adult females, and the other one from a pool of healthy adult males. It is intended to evaluate the accuracy of identify and quantify hormones in human serum (Aristizabal-Henao et al. 2021). SRM 1949 Frozen Human Prenatal Serum is a four-level material that was pooled from non-pregnant women and women during each trimester of pregnancy, aiming at quality control for the measurement of hormones and nutritional elements throughout pregnancy (Boggs et al. 2021; Sempos et al. 2022). A suite of human urine reference materials (RM 8232) is under development. The suite will consist of four pooled urine samples from female non-smokers, female smokers, male non-smokers and male smokers. Relative metabolite fold changes, percent differences for the top 20 metabolites and the identified top 30 abundant metabolites of the urine samples will be characterized by both LC–MS and nuclear magnetic resonance. RM 8462 Frozen Human Liver Suite mentioned in the protein reference materials section can be also used for metabolomics (Lippa et al. 2022).
The Quartet Project also developed a multi-sample metabolite RM suite by extracting metabolites from the four immortalized lymphoblastoid cell lines. Aiming at assessing the performance of detecting biological differences between different sample groups, reference datasets for fold changes of absolute abundance values between samples groups were constructed, by consensus across platforms, laboratories and replicates. The performance of quantitative metabolomics can be assessed not only by the consistency between fold changes of differentially expressed metabolites in query datasets and reference datasets, but also by SNR by measuring the ability to discriminate the intrinsic biological differences between the four sample groups.
Synthetic Metabolite Reference Materials
Synthetic metabolite reference materials are artificial substances that have identical chemical properties to naturally occurring metabolites in biological systems. They play an important role as calibration standards for analytical methods to allow accurate identification and quantification of metabolites. Synthetic metabolite RMs contain known concentrations of chemical components, which can be run separately or used as internal standards to perform system suitability tests, calibration, and metabolite quantification. These RMs can be prepared in individual laboratories to fit specific purposes for each study or can be purchased from vendors. They can be produced using chemical synthesis or enzymatic reactions, and they can be used for a range of applications, including targeted and untargeted metabolomics, and in the development and validation of new analytical methods. Synthetic metabolite RMs can also be used to assess the accuracy and precision of different analytical platforms and to facilitate inter-laboratory comparisons.
One example of a synthetic metabolite RM is the deuterated internal standards that are frequently used in MS-based metabolomics. These internal standards are made by incorporating deuterium into the metabolite of interest, allowing for accurate quantification of the metabolite in biological samples. Commercially available synthetic metabolite reference materials are typically mixtures of isotopically labeled or U-13C labeled metabolites that span a broad range of molecular weights, possess varied ionization propensities, and cover a distribution in class and retention time. Examples of commercially available synthetic metabolite reference materials include the QReSS kit from Cambridge Isotope Laboratories (CIL) (Cambridge Isotope Laboratories, Inc. 2023), the IROA-Long-Term Reference Standard (IROA-LTRS) from IROA Technologies (Evans et al. 2020), the Lipidyzer Platform kits from SCIEX (Lippa et al. 2022), and quantitative metabolic profiling kits from Biocrates (Biocrates 2023).
Multiomics Reference Materials
Multiomics integrates diverse omics data to better cluster and classify sample (sub)groups, and more comprehensively understand the mechanisms underlying biological processes by investigating molecular interaction across omics layers (Karczewski and Snyder 2018; Price et al. 2017; Schussler-Fiorenza Rose et al. 2019). Multiomics analysis inherits challenges from the single omics datasets and confronts new challenges in data harmonization and integration across different omics layers with varying numbers of features and statistical properties (Athieniti and Spyrou 2023; Sonia Tarazona 2021). Multiomics RMs derived from the same source that incorporate multiple omics types and provide unbiased ground truth serve as crucial tools for assessing the performance of methods for normalizing and integrating multiomics datasets, conducting cross-omics validation, and imputing missing data (Krassowski et al. 2020; Zheng et al. 2021). For example, the uniformity of MS1 intensity distribution reflects the consistency of chromatographic spray and mass spectrometry sensitivity, while the uniformity of MS2 intensity distribution reflects the consistency of fragment ion detection sensitivity. There is no universally accepted standard for pre-analytical performance metrics. Appropriate thresholds depend on specific library preparation protocols, sequencers or instruments, and algorithms. While these metrics can be calculated for samples of interest, the use of widely adopted reference materials facilitates better understanding of performance across different assays and laboratories.
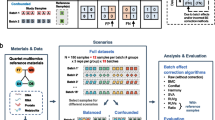
Schematic overview of multiomics profiling and quality control workflows showing the use of reference materials. Omics reference materials can evaluate all steps of sequencing workflow, including sample or library preparation, sequencing, raw reads, and profiling. Illustrated is the workflow of sequencing and key performance metrics for each step
In cases where reference datasets are either unavailable or do not contain the features of interest, alternative methods can be employed to assess the performance. One such method involves evaluating the reproducibility of replicates, which compares the results of multiple measurements conducted on the same sample. Another approach is to utilize built-in truth from multi-sample reference materials, whereby a known standard is employed to evaluate the accuracy of the experiment.
The performance of variants calling results can be assessed by the repeatability and reproducibility of technical replicates or the Mendelian consistent ratio of family members. Technical replicates share the same variant calls and de novo mutations are rare; therefore, the majority of discordant variants is likely to represent genoty** errors (Veltman and Brunner 2012). The advantage of those reference datasets independent metrics is that they can evaluate the precision of variant calling on the whole genome without being restricted to the benchmark regions. However, these metrics cannot indicate how many true variants should be identified, or what the recall rate is.
To assess the accuracy of quantitative omics, three levels of reference dataset-independent metrics can be employed based on the number of available reference materials (Fig. 2). If a single reference material is available, the reproducibility between technical replicates is used to assess the performance of profiling results. However, a high correlation between two replicates of the same sample is not enough to ensure to accuracy in detecting differences between sample groups, because the replicates may share the same technical biases. If a pair of RMs is available, fold changes of features between sample pairs are expected to be the same as the designed expression signal ratios. If three or more RMs are available in a suite, PCA-based metrics can be used to assess the performance of distinguishing the intrinsic biological differences between sample groups.
Utilization of Reference Materials
Identifying reliable biomarkers that can accurately predict disease risk or response to treatment is a critical goal of omics-based cohort studies. Large cohort studies that involve collecting samples over a long period of time and profiling the samples with multiple platforms at multiple labs may suffer from issues related to data incomparability and batch effects, which add difficulties for biomarker discovery. In this section, we discuss how omics RMs can be integrated in large cohort studies to enhance the rigor and reproducibility of biomarker discovery (Fig. 4).
To ensure accurate and reliable results from large-scale analysis of precious cohort samples, it is important to assess the suitability of experimental and analytical pipelines using reference materials prior to initiating the data generation process. The first important step is to choose the suitable RMs based on study design and instruments available. Points of consideration include the availability of RMs, their comparability to the test material, and whether the assigned property values and their confidence levels include the features of interest. The matrix composition of RMs is a critical consideration in the QC process of LC–MS. The performance indicators, such as calibration effectiveness, extraction efficiency, column performance, and ion suppression level, are directly influenced by the composition of the sample matrix. To ensure accurate and reliable performance assessment, it is recommended to employ RMs with a matrix composition as similar as possible to that of the study samples.
At each omics level, a variety of sample preparation methods, data generation platforms, and bioinformatic tools are available. By utilizing RMs in benchmark studies and proficiency test, researchers can gain insights into the strengths and limitations of various methods and technologies. This knowledge facilitates the selection of appropriate experimental and analytical procedures tailored to the specific goals, samples types, and available resources.
RMs can also be effectively used to optimize protocols and parameters by identifying and troubleshooting potential issues. For example, in genomics and transcriptomics by NGS, sequencing performance is influenced by the insert fragment size, which is associated with DNA shearing time. Longer shearing time produces shorter DNA fragments, and the insert fragment sizes must be measured to ensure that they fall within the expected molecular weight range (Fang et al. High-throughput profiling technologies have revolutionized omics studies by enabling the generation of vast amounts of data in a relatively short period of time, allowing researchers to comprehensively study complex biological systems at an unprecedented level of resolution. However, performing high-throughput profiling is a highly complex and challenging process, and there are many potential sources of variability that can impact the results and reproducibility. Therefore, rigorous QA/QC is crucial to ensure confidence in the resulting data and biological discoveries. The use of RMs is an important aspect of QA/QC in high-throughput technologies to ensure accurate and reliable results. In this review, we aim to offer a comprehensive overview of the significance of utilizing well-characterized RMs across different levels of omics research, including genomics, transcriptomics, proteomics, and metabolomics. We provide insights into the characteristics, advantages, and limitations of RMs in each omics field, which are summarized in Table 2. Our goal is to assist researchers in making informed decisions when selecting suitable RMs for their specific research questions and analytical methods. Ultimately, the utilization of appropriate RMs can greatly enhance the accuracy and reliability of omics research outcomes. By incorporating well-characterized RMs into omics research, researchers can overcome various challenges and limitations. RMs provide a standardized reference point that enables calibration and quality control throughout the experimental workflow. They serve as valuable tools for method optimization, validation, and troubleshooting, allowing researchers to assess the performance of their analytical methods and identify any potential biases or errors. Furthermore, the use of RMs facilitates inter-laboratory comparisons and promotes data harmonization, enabling the integration and comparison of results across different studies and platforms. Although the profiling of RMs may entail additional costs, implementing a thorough QA/QC methodology is important for evaluating and monitoring the performance of data generation processes. This upfront investment contributes to the long-term reliability and accuracy of the results, minimizing potential errors and ensuring the accuracy and reliability of the omics research. The careful selection of RMs is crucial to ensure their relevance and applicability to the study at hand. Researchers should consider the intended use of the study and choose RMs that closely resemble the properties of the samples being investigated. Additionally, the selected RMs should be qualitatively and quantitatively representative of the entire collection of samples included in the study. This ensures that the RMs effectively mimic the characteristics of the biological samples, enabling accurate and meaningful comparisons and interpretations. When studying specific genetic or phenotypical features that vary among different ethnic groups, it is important to choose RMs that match the ethnicity of the study samples. This approach ensures that the RMs accurately reflect the characteristics of the study population, enabling the assessment of the detection performance of those specific genetic or phenotypical features (Hardwick et al. 2017). As profiling methods continue to advance and new technologies emerge, the reference datasets for existing RMs will undergo continuous updates and refinements. One example of this is the utilization of long reads in genomic sequencing. Long reads are particularly valuable for profiling repetitive and complex regions, which are challenging to be mapped by short reads (Wenger et al. 2019). By incorporating long reads, benchmark variants in these regions can be better characterized (Wagner et al. 2022). Additionally, long-read technologies enable precise transcript detection and RNA modifications (Leger et al. 2021; Soneson et al. 2019). In proteomics, MS techniques are extensively used to study post-translational modification (PTMs) of proteins (Zecha et al. 2022). The reference materials will expand to encompass more omics types along with the development of technologies. For example, reference datasets of DNA epigenomics for DNA RMs can be developed, RNA RMs can include small RNA profiling and RNA modification reference datasets, and protein RMs can incorporate PTM reference datasets. Challenges persist in the global promotion and adoption of reference materials and reference datasets. First, regulatory challenges, especially across different regions of the world, can pose additional obstacles in adopting a universal RM (Guerrier et al. 2012; Krogstad et al. 2010). Biological RMs, especially those intended for human genomics and transcriptomics, which are frequently derived from human specimens, require stricter adherence to informed consent principles and governmental controls. Currently, there is no single, comprehensive international model for governing human genetic resources. The distinct nature of informed consent across different countries, influenced by diverse cultures and social traditions, necessitated addressing legal, ethical, and logistical aspects related to genetic materials and data utilization while respecting each nation's sovereignty and cultural norms. International collaboration and agreements are imperative in addressing these challenges and ensuring the conscientious and equitable utilization of human genetic resources worldwide (Gainotti et al. 2016; van Belle et al. 2015). Second, we strongly recommend that QC data should be made available alongside the study samples in databases or repositories that adhere to the FAIR principles (Findable, Accessible, Interoperable, and Reusable), which is crucial for enhancing data management and sharing (Conesa and Beck 2019; Wilkinson et al. 2016). Currently, QC information is often omitted from scientific publications, leading to uncertainty about the performance methodology used. In the future, guidelines may be developed to mandate the inclusion of QC metrics in data submissions to public repositories, similar to existing guidelines for other aspects of data reporting. Coupling comprehensive QC information to the experimental data will allow for quick assessment of the reliability of an experiment, which is crucial in light of recent reports of the general reproducibility crisis in various scientific fields (Anonymous 2021; Baker 2016; Shi et al. 2017). It is essential to prioritize and formalize QC practices to ensure the quality and reproducibility of high-throughput multiomics profiling results by fully utilizing well-characterized RMs and appropriate QC metrics. In this review, we summarized reference materials across all levels of omics, including (epi-)genomics, transcriptomics, proteomics, and metabolomics. We have offered a comprehensive overview of leveraging omics reference materials to enhance data quality. This initiative is geared toward promoting robust scientific research and advancing our understanding of complex biological systems through the thoughtful application of omics technologies.Challenges and Future Directions
Conclusion
Data Availability
Not applicable.
Code Availability
Not applicable.
Abbreviations
- ABRF:
-
Association of Biomolecular Resource Facilities
- ATCC:
-
American Type Culture Collection
- CDC:
-
Centers for Disease Control and Prevention
- CNA:
-
Copy number alteration
- CPTAC:
-
Clinical Proteomic Tumor Analysis Consortium
- CRM:
-
Certified reference material
- ctDNA:
-
Circulating tumor DNA
- EBV:
-
Epstein–Barr virus
- ERCC:
-
External RNA Control Consortium
- FFPE:
-
Formalin-Fixed Paraffin-Embedded
- gDNA:
-
Genomic DNA
- GeT-RM:
-
Genetic Testing Reference Materials Coordination Program
- GIAB:
-
Genome in a Bottle Consortium
- HPRC:
-
Human Pangenome Reference Consortium
- HUPO:
-
Human Proteome Organization
- IMMSA:
-
International Microbiome and Multi-Omics Standards Alliance
- JRC:
-
Joint Research Centre
- LDT:
-
Laboratory developed tests
- LOD:
-
Limit of detect
- LTR:
-
Long-term reference
- MAQC:
-
MicroArray Quality Control Consortium
- MCC:
-
Matthews correlation coefficient
- MetQual:
-
Metabolomics Quality Assurance and Quality Control Program
- MMR:
-
Mismatch repair
- mQACC:
-
Metabolomics Quality Assurance and Quality Control Consortium
- MRM:
-
Multiple reaction monitoring
- MS:
-
Mass spectrometry
- NCI:
-
National Cancer Institute
- NIM:
-
National Institute of Metrology
- NIST:
-
National Institute of Standards and Technology
- NMR:
-
Nuclear magnetic resonance
- PCA:
-
Principal component analysis
- PEA:
-
Proximity Extension Assay
- PRM:
-
Parallel reaction monitoring
- PSI:
-
Proteomics Standards Initiative
- PTM:
-
Post-translational modification
- QA:
-
Quality assurance
- QC:
-
Quality control
- RM:
-
Reference material
- RMSE:
-
Root mean squared error
- RT:
-
Retention time
- SEQC:
-
Sequencing Quality Control Consortium
- SNR:
-
Signal-to-noise ratio
- SNV:
-
Single-nucleotide variations
- SRM:
-
Standard reference materials
- SV:
-
Structural variants
- T2T:
-
Telomere-to-Telomere
- TCGA:
-
The Cancer Genome Atlas
- TMB:
-
Tumor mutation burden
- TNBC:
-
Triple-negative breast cancer
- UHRR:
-
Agilent Universal Human Reference RNA material
- VAF:
-
Variant allele frequency
- WES:
-
Whole-exome sequencing
- WGS:
-
Whole-genome sequencing
References
Amos Wilson J, Pratt VM, Phansalkar A, Muralidharan K, Highsmith WE Jr, Beck JC, Bridgeman S, Courtney EM, Epp L, Ferreira-Gonzalez A, Hjelm NL, Holtegaard LM, Jama MA, Jakupciak JP, Johnson MA, Labrousse P, Lyon E, Prior TW, Richards CS, Richie KL, Roa BB, Rohlfs EM, Sellers T, Sherman SL, Siegrist KA, Silverman LM, Wiszniewska J, Kalman LV, Fragile Xperts Working Group of the Association for Molecular Pathology Clinical Practice C (2008) Consensus characterization of 16 FMR1 reference materials: a consortium study. J Mol Diagn 10(1):2–12. https://doi.org/10.2353/jmoldx.2008.070105
Andrews PC, Arnott DP, Gawinowicz MA, Kowalak JA, Lane WS, Lilley KS, Martin LT, Stein S (2006) ABRF-sPRG 2006 study: a proteomics standard. ABRF 2006: Long Beach, CA, 2006
Anonymous (2021) Replicating scientific results is tough—but essential. Nature 600(7889):359–360. https://doi.org/10.1038/d41586-021-03736-4
Anwaier A, Zhu SX, Tian X, Xu WH, Wang Y, Palihati M, Wang WY, Shi GH, Qu YY, Zhang HL, Ye DW (2022) Large-scale proteomics data reveal integrated prognosis-related protein signatures and role of SMAD4 and RAD50 in prognosis and immune infiltrations of prostate cancer microenvironment. Phenomics 2(6):404–418. https://doi.org/10.1007/s43657-022-00070-1
Aristizabal-Henao JJ, Jones CM, Lippa KA, Bowden JA (2020) Nontargeted lipidomics of novel human plasma reference materials: hypertriglyceridemic, diabetic, and African-American. Anal Bioanal Chem 412(27):7373–7380. https://doi.org/10.1007/s00216-020-02910-3
Aristizabal-Henao JJ, Lemas DJ, Griffin EK, Costa KA, Camacho C, Bowden JA (2021) Metabolomic profiling of biological reference materials using a multiplatform high-resolution mass spectrometric approach. J Am Soc Mass Spectrom 32(9):2481–2489. https://doi.org/10.1021/jasms.1c00194
Athieniti E, Spyrou GM (2023) A guide to multi-omics data collection and integration for translational medicine. Comput Struct Biotechnol J 21:134–149. https://doi.org/10.1016/j.csbj.2022.11.050
Azab S, Ly R, Britz-McKibbin P (2019) Robust method for high-throughput screening of fatty acids by multisegment injection-nonaqueous capillary electrophoresis-mass spectrometry with stringent quality control. Anal Chem 91(3):2329–2336. https://doi.org/10.1021/acs.analchem.8b05054
Baker M (2016) 1500 scientists lift the lid on reproducibility. Nature 533(7604):452–454. https://doi.org/10.1038/533452a
Baker SC, Bauer SR, Beyer RP, Brenton JD, Bromley B, Burrill J, Causton H, Conley MP, Elespuru R, Fero M, Foy C, Fuscoe J, Gao X, Gerhold DL, Gilles P, Goodsaid F, Guo X, Hackett J, Hockett RD, Ikonomi P, Irizarry RA, Kawasaki ES, Kaysser-Kranich T, Kerr K, Kiser G, Koch WH, Lee KY, Liu C, Liu ZL, Lucas A, Manohar CF, Miyada G, Modrusan Z, Parkes H, Puri RK, Reid L, Ryder TB, Salit M, Samaha RR, Scherf U, Sendera TJ, Setterquist RA, Shi L, Shippy R, Soriano JV, Wagar EA, Warrington JA, Williams M, Wilmer F, Wilson M, Wolber PK, Wu X, Zadro R, External RNACC (2005) The external RNA controls consortium: a progress report. Nat Methods 2(10):731–734. https://doi.org/10.1038/nmeth1005-731
Beasley-Green A, Bunk D, Rudnick P, Kilpatrick L, Phinney K (2012) A proteomics performance standard to support measurement quality in proteomics. Proteomics 12(7):923–931. https://doi.org/10.1002/pmic.201100522
Beckonert O, Keun HC, Ebbels TM, Bundy J, Holmes E, Lindon JC, Nicholson JK (2007) Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat Protoc 2(11):2692–2703. https://doi.org/10.1038/nprot.2007.376
Beger RD, Dunn WB, Bandukwala A, Bethan B, Broadhurst D, Clish CB, Dasari S, Derr L, Evans A, Fischer S, Flynn T, Hartung T, Herrington D, Higashi R, Hsu PC, Jones C, Kachman M, Karuso H, Kruppa G, Lippa K, Maruvada P, Mosley J, Ntai I, O’Donovan C, Playdon M, Raftery D, Shaughnessy D, Souza A, Spaeder T, Spalholz B, Tayyari F, Ubhi B, Verma M, Walk T, Wilson I, Witkin K, Bearden DW, Zanetti KA (2019) Towards quality assurance and quality control in untargeted metabolomics studies. Metabolomics 15(1):4. https://doi.org/10.1007/s11306-018-1460-7
Begley CG, Ioannidis JP (2015) Reproducibility in science: improving the standard for basic and preclinical research. Circ Res 116(1):116–126. https://doi.org/10.1161/CIRCRESAHA.114.303819
Bell AW, Deutsch EW, Au CE, Kearney RE, Beavis R, Sechi S, Nilsson T, Bergeron JJ, Group HTSW (2009) A HUPO test sample study reveals common problems in mass spectrometry-based proteomics. Nat Methods 6(6):423–430. https://doi.org/10.1038/nmeth.1333
Bettinotti MP, Ferriola D, Duke JL, Mosbruger TL, Tairis N, Jennings L, Kalman LV, Monos D (2018) Characterization of 108 genomic DNA reference materials for 11 human leukocyte antigen loci: a GeT-RM collaborative project. J Mol Diagn 20(5):703–715. https://doi.org/10.1016/j.jmoldx.2018.05.009
Biocrates (2023) Biocrates metabolomics technology. https://biocrates.com/
Bittremieux W, Walzer M, Tenzer S, Zhu W, Salek RM, Eisenacher M, Tabb DL (2017) The human proteome organization-proteomics standards initiative quality control working group: making quality control more accessible for biological mass spectrometry. Anal Chem 89(8):4474–4479. https://doi.org/10.1021/acs.analchem.6b04310
Bittremieux W, Tabb DL, Impens F, Staes A, Timmerman E, Martens L, Laukens K (2018) Quality control in mass spectrometry-based proteomics. Mass Spectrom Rev 37(5):697–711. https://doi.org/10.1002/mas.21544
Blackburn J, Wong T, Madala BS, Barker C, Hardwick SA, Reis ALM, Deveson IW, Mercer TR (2019) Use of synthetic DNA spike-in controls (sequins) for human genome sequencing. Nat Protoc 14(7):2119–2151. https://doi.org/10.1038/s41596-019-0175-1
Blume JE, Manning WC, Troiano G, Hornburg D, Figa M, Hesterberg L, Platt TL, Zhao X, Cuaresma RA, Everley PA, Ko M, Liou H, Mahoney M, Ferdosi S, Elgierari EM, Stolarczyk C, Tangeysh B, **a H, Benz R, Siddiqui A, Carr SA, Ma P, Langer R, Farias V, Farokhzad OC (2020) Rapid, deep and precise profiling of the plasma proteome with multi-nanoparticle protein corona. Nat Commun 11(1):3662. https://doi.org/10.1038/s41467-020-17033-7
Boggs ASP, Kilpatrick LE, Burdette CQ, Tevis DS, Fultz ZA, Nelson MA, Jarrett JM, Kemp JV, Singh RJ, Grebe SKG, Wise SA, Kassim BL, Long SE (2021) Development of a pregnancy-specific reference material for thyroid biomarkers, vitamin D, and nutritional trace elements in serum. Clin Chem Lab Med 59(4):671–679. https://doi.org/10.1515/cclm-2020-0977
Bowden JA, Heckert A, Ulmer CZ, Jones CM, Koelmel JP, Abdullah L, Ahonen L, Alnouti Y, Armando AM, Asara JM, Bamba T, Barr JR, Bergquist J, Borchers CH, Brandsma J, Breitkopf SB, Cajka T, Cazenave-Gassiot A, Checa A, Cinel MA, Colas RA, Cremers S, Dennis EA, Evans JE, Fauland A, Fiehn O, Gardner MS, Garrett TJ, Gotlinger KH, Han J, Huang Y, Neo AH, Hyotylainen T, Izumi Y, Jiang H, Jiang H, Jiang J, Kachman M, Kiyonami R, Klavins K, Klose C, Kofeler HC, Kolmert J, Koal T, Koster G, Kuklenyik Z, Kurland IJ, Leadley M, Lin K, Maddipati KR, McDougall D, Meikle PJ, Mellett NA, Monnin C, Moseley MA, Nandakumar R, Oresic M, Patterson R, Peake D, Pierce JS, Post M, Postle AD, Pugh R, Qiu Y, Quehenberger O, Ramrup P, Rees J, Rembiesa B, Reynaud D, Roth MR, Sales S, Schuhmann K, Schwartzman ML, Serhan CN, Shevchenko A, Somerville SE, St John-Williams L, Surma MA, Takeda H, Thakare R, Thompson JW, Torta F, Triebl A, Trotzmuller M, Ubhayasekera SJK, Vuckovic D, Weir JM, Welti R, Wenk MR, Wheelock CE, Yao L, Yuan M, Zhao XH, Zhou S (2017) Harmonizing lipidomics: NIST interlaboratory comparison exercise for lipidomics using SRM 1950-Metabolites in Frozen Human Plasma. J Lipid Res 58(12):2275–2288. https://doi.org/10.1194/jlr.M079012
Bowden JA, Ulmer CZ, Jones CM, Koelmel JP, Yost RA (2018) NIST lipidomics workflow questionnaire: an assessment of community-wide methodologies and perspectives. Metabolomics 14(5):53. https://doi.org/10.1007/s11306-018-1340-1
Broadhurst D, Goodacre R, Reinke SN, Kuligowski J, Wilson ID, Lewis MR, Dunn WB (2018) Guidelines and considerations for the use of system suitability and quality control samples in mass spectrometry assays applied in untargeted clinical metabolomic studies. Metabolomics 14(6):72. https://doi.org/10.1007/s11306-018-1367-3
Bunk DM (2010) Design considerations for proteomic reference materials. Proteomics 10(23):4220–4225. https://doi.org/10.1002/pmic.201000242
Buttner R, Longshore JW, Lopez-Rios F, Merkelbach-Bruse S, Normanno N, Rouleau E, Penault-Llorca F (2019) Implementing TMB measurement in clinical practice: considerations on assay requirements. ESMO Open 4(1):e000442. https://doi.org/10.1136/esmoopen-2018-000442
Cajka T, Smilowitz JT, Fiehn O (2017) Validating quantitative untargeted lipidomics across nine liquid chromatography-high-resolution mass spectrometry platforms. Anal Chem 89(22):12360–12368. https://doi.org/10.1021/acs.analchem.7b03404
Cambridge Isotope Laboratories, Inc. (2023) Metabolomics QReSSTM Kit. https://isotope.com/en-us/metabolomics-mixes-and-kits/metabolomics-qress-kit-msk-qress-kit
Candia J, Cheung F, Kotliarov Y, Fantoni G, Sellers B, Griesman T, Huang J, Stuccio S, Zingone A, Ryan BM, Tsang JS, Biancotto A (2017) Assessment of variability in the SOMAscan assay. Sci Rep 7(1):14248. https://doi.org/10.1038/s41598-017-14755-5
Candia J, Daya GN, Tanaka T, Ferrucci L, Walker KA (2022) Assessment of variability in the plasma 7k SomaScan proteomics assay. Sci Rep 12(1):17147. https://doi.org/10.1038/s41598-022-22116-0
Centers for Disease Control and Prevention (2019) Genetic testing reference materials coordination program. https://www.cdc.gov/labquality/get-rm/index.html
Cheema AK, Asara JM, Wang Y, Neubert TA, Tolstikov V, Turck CW (2015) The ABRF metabolomics research group 2013 study: investigation of spiked compound differences in a human plasma matrix. J Biomol Tech 26(3):83–89. https://doi.org/10.7171/jbt.15-2603-001
Chen W, Zhao Y, Chen X, Yang Z, Xu X, Bi Y, Chen V, Li J, Choi H, Ernest B, Tran B, Mehta M, Kumar P, Farmer A, Mir A, Mehra UA, Li JL, Moos M Jr, **ao W, Wang C (2021) A multicenter study benchmarking single-cell RNA sequencing technologies using reference samples. Nat Biotechnol 39(9):1103–1114. https://doi.org/10.1038/s41587-020-00748-9
Chicco D, Jurman G (2020) The advantages of the Matthews correlation coefficient (MCC) over F1 score and accuracy in binary classification evaluation. BMC Genomics 21(1):6. https://doi.org/10.1186/s12864-019-6413-7
Chin CS, Wagner J, Zeng Q, Garrison E, Garg S, Fungtammasan A, Rautiainen M, Aganezov S, Kirsche M, Zarate S, Schatz MC, **ao C, Rowell WJ, Markello C, Farek J, Sedlazeck FJ, Bansal V, Yoo B, Miller N, Zhou X, Carroll A, Barrio AM, Salit M, Marschall T, Dilthey AT, Zook JM (2020) A diploid assembly-based benchmark for variants in the major histocompatibility complex. Nat Commun 11(1):4794. https://doi.org/10.1038/s41467-020-18564-9
Chiva C, Mendes Maia T, Panse C, Stejskal K, Douche T, Matondo M, Loew D, Helm D, Rettel M, Mechtler K, Impens F, Nanni P, Shevchenko A, Sabido E (2021) Quality standards in proteomics research facilities: Common standards and quality procedures are essential for proteomics facilities and their users. EMBO Rep 22(6):e52626. https://doi.org/10.15252/embr.202152626
Clark DJ, Hu Y, Bocik W, Chen L, Schnaubelt M, Roberts R, Shah P, Whiteley G, Zhang H (2018) Evaluation of NCI-7 cell line panel as a reference material for clinical proteomics. J Proteome Res 17(6):2205–2215. https://doi.org/10.1021/acs.jproteome.8b00165
Collins BC, Hunter CL, Liu Y, Schilling B, Rosenberger G, Bader SL, Chan DW, Gibson BW, Gingras AC, Held JM, Hirayama-Kurogi M, Hou G, Krisp C, Larsen B, Lin L, Liu S, Molloy MP, Moritz RL, Ohtsuki S, Schlapbach R, Selevsek N, Thomas SN, Tzeng SC, Zhang H, Aebersold R (2017) Multi-laboratory assessment of reproducibility, qualitative and quantitative performance of SWATH-mass spectrometry. Nat Commun 8(1):291. https://doi.org/10.1038/s41467-017-00249-5
Conesa A, Beck S (2019) Making multi-omics data accessible to researchers. Sci Data 6(1):251. https://doi.org/10.1038/s41597-019-0258-4
Conrad DF, Keebler JE, DePristo MA, Lindsay SJ, Zhang Y, Casals F, Idaghdour Y, Hartl CL, Torroja C, Garimella KV, Zilversmit M, Cartwright R, Rouleau GA, Daly M, Stone EA, Hurles ME, Awadalla P, Genomes P (2011) Variation in genome-wide mutation rates within and between human families. Nat Genet 43(7):712–714. https://doi.org/10.1038/ng.862
Coriell Institute (2023) Available samples for genetic testing reference materials coordination program. https://www.coriell.org/1/NIGMS/Additional-Resources/Multiply-Confirmed-Mutations-GeT-RM
Craig DW, Nasser S, Corbett R, Chan SK, Murray L, Legendre C, Tembe W, Adkins J, Kim N, Wong S, Baker A, Enriquez D, Pond S, Pleasance E, Mungall AJ, Moore RA, McDaniel T, Ma Y, Jones SJ, Marra MA, Carpten JD, Liang WS (2016) A somatic reference standard for cancer genome sequencing. Sci Rep 6:24607. https://doi.org/10.1038/srep24607
Davis WC, Kilpatrick LE, Ellisor DL, Neely BA (2019) Characterization of a human liver reference material fit for proteomics applications. Sci Data 6(1):324. https://doi.org/10.1038/s41597-019-0336-7
Deutsch EW, Orchard S, Binz PA, Bittremieux W, Eisenacher M, Hermjakob H, Kawano S, Lam H, Mayer G, Menschaert G, Perez-Riverol Y, Salek RM, Tabb DL, Tenzer S, Vizcaino JA, Walzer M, Jones AR (2017) Proteomics standards initiative: fifteen years of progress and future work. J Proteome Res 16(12):4288–4298. https://doi.org/10.1021/acs.jproteome.7b00370
Deveson IW, Chen WY, Wong T, Hardwick SA, Andersen SB, Nielsen LK, Mattick JS, Mercer TR (2016) Representing genetic variation with synthetic DNA standards. Nat Methods 13(9):784–791. https://doi.org/10.1038/nmeth.3957
Deveson IW, Madala BS, Blackburn J, Barker C, Wong T, Barton KM, Smith MA, Watkins DN, Mercer TR (2019) Chiral DNA sequences as commutable controls for clinical genomics. Nat Commun 10(1):1342. https://doi.org/10.1038/s41467-019-09272-0
Deveson IW, Gong B, Lai K, LoCoco JS, Richmond TA, Schageman J, Zhang Z, Novoradovskaya N, Willey JC, Jones W, Kusko R, Chen G, Madala BS, Blackburn J, Stevanovski I, Bhandari A, Close D, Conroy J, Hubank M, Marella N, Mieczkowski PA, Qiu F, Sebra R, Stetson D, Sun L, Szankasi P, Tan H, Tang LY, Arib H, Best H, Burgher B, Bushel PR, Casey F, Cawley S, Chang CJ, Choi J, Dinis J, Duncan D, Eterovic AK, Feng L, Ghosal A, Giorda K, Glenn S, Happe S, Haseley N, Horvath K, Hung LY, Jarosz M, Kushwaha G, Li D, Li QZ, Li Z, Liu LC, Liu Z, Ma C, Mason CE, Megherbi DB, Morrison T, Pabon-Pena C, Pirooznia M, Proszek PZ, Raymond A, Rindler P, Ringler R, Scherer A, Shaknovich R, Shi T, Smith M, Song P, Strahl M, Thodima VJ, Tom N, Verma S, Wang J, Wu L, **ao W, Xu C, Yang M, Zhang G, Zhang S, Zhang Y, Shi L, Tong W, Johann DJ Jr, Mercer TR, Xu J, Group SOSW (2021a) Evaluating the analytical validity of circulating tumor DNA sequencing assays for precision oncology. Nat Biotechnol 39(9):1115–1128. https://doi.org/10.1038/s41587-021-00857-z
Deveson IW, Gong B, Lai K, LoCoco JS, Richmond TA, Schageman J, Zhang Z, Novoradovskaya N, Willey JC, Jones W, Kusko R, Chen G, Madala BS, Blackburn J, Stevanovski I, Bhandari A, Close D, Conroy J, Hubank M, Marella N, Mieczkowski PA, Qiu F, Sebra R, Stetson D, Sun L, Szankasi P, Tan H, Tang LY, Arib H, Best H, Burgher B, Bushel PR, Casey F, Cawley S, Chang CJ, Choi J, Dinis J, Duncan D, Eterovic AK, Feng L, Ghosal A, Giorda K, Glenn S, Happe S, Haseley N, Horvath K, Hung LY, Jarosz M, Kushwaha G, Li D, Li QZ, Li Z, Liu LC, Liu Z, Ma C, Mason CE, Megherbi DB, Morrison T, Pabon-Pena C, Pirooznia M, Proszek PZ, Raymond A, Rindler P, Ringler R, Scherer A, Shaknovich R, Shi T, Smith M, Song P, Strahl M, Thodima VJ, Tom N, Verma S, Wang J, Wu L, **ao W, Xu C, Yang M, Zhang G, Zhang S, Zhang Y, Shi L, Tong W, Johann DJ Jr, Mercer TR, Xu J, Group SOSW (2021b) Evaluating the analytical validity of circulating tumor DNA sequencing assays for precision oncology. Nat Biotechnol. https://doi.org/10.1038/s41587-021-00857-z
Eberle MA, Fritzilas E, Krusche P, Kallberg M, Moore BL, Bekritsky MA, Iqbal Z, Chuang HY, Humphray SJ, Halpern AL, Kruglyak S, Margulies EH, McVean G, Bentley DR (2017) A reference data set of 5.4 million phased human variants validated by genetic inheritance from sequencing a three-generation 17-member pedigree. Genome Res 27(1):157–164. https://doi.org/10.1101/gr.210500.116
Eldjarn GH, Ferkingstad E, Lund SH, Helgason H, Magnusson OT, Gunnarsdottir K, Olafsdottir TA, Halldorsson BV, Olason PI, Zink F, Gudjonsson SA, Sveinbjornsson G, Magnusson MI, Helgason A, Oddsson A, Halldorsson GH, Magnusson MK, Saevarsdottir S, Eiriksdottir T, Masson G, Stefansson H, Jonsdottir I, Holm H, Rafnar T, Melsted P, Saemundsdottir J, Norddahl GL, Thorleifsson G, Ulfarsson MO, Gudbjartsson DF, Thorsteinsdottir U, Sulem P, Stefansson K (2023) Large-scale plasma proteomics comparisons through genetics and disease associations. Nature 622(7982):348–358. https://doi.org/10.1038/s41586-023-06563-x
Escher C, Reiter L, MacLean B, Ossola R, Herzog F, Chilton J, MacCoss MJ, Rinner O (2012) Using iRT, a normalized retention time for more targeted measurement of peptides. Proteomics 12(8):1111–1121. https://doi.org/10.1002/pmic.201100463
Evans AM, O’Donovan C, Playdon M, Beecher C, Beger RD, Bowden JA, Broadhurst D, Clish CB, Dasari S, Dunn WB, Griffin JL, Hartung T, Hsu PC, Huan T, Jans J, Jones CM, Kachman M, Kleensang A, Lewis MR, Monge ME, Mosley JD, Taylor E, Tayyari F, Theodoridis G, Torta F, Ubhi BK, Vuckovic D, Metabolomics Quality Assurance QCC (2020) Dissemination and analysis of the quality assurance (QA) and quality control (QC) practices of LC-MS based untargeted metabolomics practitioners. Metabolomics 16(10):113. https://doi.org/10.1007/s11306-020-01728-5
Fang LT, Zhu B, Zhao Y, Chen W, Yang Z, Kerrigan L, Langenbach K, de Mars M, Lu C, Idler K, Jacob H, Zheng Y, Ren L, Yu Y, Jaeger E, Schroth GP, Abaan OD, Talsania K, Lack J, Shen TW, Chen Z, Stanbouly S, Tran B, Shetty J, Kriga Y, Meerzaman D, Nguyen C, Petitjean V, Sultan M, Cam M, Mehta M, Hung T, Peters E, Kalamegham R, Sahraeian SME, Mohiyuddin M, Guo Y, Yao L, Song L, Lam HYK, Drabek J, Vojta P, Maestro R, Gasparotto D, Koks S, Reimann E, Scherer A, Nordlund J, Liljedahl U, Jensen RV, Pirooznia M, Li Z, **ao C, Sherry ST, Kusko R, Moos M, Donaldson E, Tezak Z, Ning B, Tong W, Li J, Duerken-Hughes P, Catalanotti C, Maheshwari S, Shuga J, Liang WS, Keats J, Adkins J, Tassone E, Zismann V, McDaniel T, Trent J, Foox J, Butler D, Mason CE, Hong H, Shi L, Wang C, **ao W, Somatic Mutation Working Group of Sequencing Quality Control Phase IIC (2021) Establishing community reference samples, data and call sets for benchmarking cancer mutation detection using whole-genome sequencing. Nat Biotechnol 39(9):1151–1160. https://doi.org/10.1038/s41587-021-00993-6
Foox J, Tighe SW, Nicolet CM, Zook JM, Byrska-Bishop M, Clarke WE, Khayat MM, Mahmoud M, Laaguiby PK, Herbert ZT, Warner D, Grills GS, Jen J, Levy S, **ang J, Alonso A, Zhao X, Zhang W, Teng F, Zhao Y, Lu H, Schroth GP, Narzisi G, Farmerie W, Sedlazeck FJ, Baldwin DA, Mason CE (2021) Performance assessment of DNA sequencing platforms in the ABRF next-generation sequencing study. Nat Biotechnol 39(9):1129–1140. https://doi.org/10.1038/s41587-021-01049-5
Gaedigk A, Turner A, Everts RE, Scott SA, Aggarwal P, Broeckel U, McMillin GA, Melis R, Boone EC, Pratt VM, Kalman LV (2019) Characterization of reference materials for genetic testing of CYP2D6 alleles: a GeT-RM collaborative project. J Mol Diagn 21(6):1034–1052. https://doi.org/10.1016/j.jmoldx.2019.06.007
Gainotti S, Turner C, Woods S, Kole A, McCormack P, Lochmuller H, Riess O, Straub V, Posada M, Taruscio D, Mascalzoni D (2016) Improving the informed consent process in international collaborative rare disease research: effective consent for effective research. Eur J Hum Genet 24(9):1248–1254. https://doi.org/10.1038/ejhg.2016.2
Giraldez MD, Spengler RM, Etheridge A, Godoy PM, Barczak AJ, Srinivasan S, De Hoff PL, Tanriverdi K, Courtright A, Lu S, Khoory J, Rubio R, Baxter D, Driedonks TAP, Buermans HPJ, Nolte-’t Hoen ENM, Jiang H, Wang K, Ghiran I, Wang YE, Van Keuren-Jensen K, Freedman JE, Woodruff PG, Laurent LC, Erle DJ, Galas DJ, Tewari M (2018) Comprehensive multi-center assessment of small RNA-seq methods for quantitative miRNA profiling. Nat Biotechnol 36(8):746–757. https://doi.org/10.1038/nbt.4183
Gong B, Li D, Kusko R, Novoradovskaya N, Zhang Y, Wang S, Pabon-Pena C, Zhang Z, Lai K, Cai W, LoCoco JS, Lader E, Richmond TA, Mittal VK, Liu LC, Johann DJ Jr, Willey JC, Bushel PR, Yu Y, Xu C, Chen G, Burgess D, Cawley S, Giorda K, Haseley N, Qiu F, Wilkins K, Arib H, Attwooll C, Babson K, Bao L, Bao W, Lucas AB, Best H, Bhandari A, Bisgin H, Blackburn J, Blomquist TM, Boardman L, Burgher B, Butler DJ, Chang CJ, Chaubey A, Chen T, Chierici M, Chin CR, Close D, Conroy J, Cooley Coleman J, Craig DJ, Crawford E, Del Pozo A, Deveson IW, Duncan D, Eterovic AK, Fan X, Foox J, Furlanello C, Ghosal A, Glenn S, Guan M, Haag C, Hang X, Happe S, Hennigan B, Hipp J, Hong H, Horvath K, Hu J, Hung LY, Jarosz M, Kerkhof J, Kipp B, Kreil DP, Labaj P, Lapunzina P, Li P, Li QZ, Li W, Li Z, Liang Y, Liu S, Liu Z, Ma C, Marella N, Martin-Arenas R, Megherbi DB, Meng Q, Mieczkowski PA, Morrison T, Muzny D, Ning B, Parsons BL, Paweletz CP, Pirooznia M, Qu W, Raymond A, Rindler P, Ringler R, Sadikovic B, Scherer A, Schulze E, Sebra R, Shaknovich R, Shi Q, Shi T, Silla-Castro JC, Smith M, Lopez MS, Song P, Stetson D, Strahl M, Stuart A, Supplee J, Szankasi P, Tan H, Tang LY, Tao Y, Thakkar S, Thierry-Mieg D, Thierry-Mieg J, Thodima VJ, Thomas D, Tichy B, Tom N, Garcia EV, Verma S, Walker K, Wang C, Wang J, Wang Y, Wen Z, Wirta V, Wu L, **ao C, **ao W, Xu S, Yang M, Ying J, Yip SH, Zhang G, Zhang S, Zhao M, Zheng Y, Zhou X, Mason CE, Mercer T, Tong W, Shi L, Jones W, Xu J (2021) Cross-oncopanel study reveals high sensitivity and accuracy with overall analytical performance depending on genomic regions. Genome Biol 22(1):109. https://doi.org/10.1186/s13059-021-02315-0
Guerrier G, Sicard D, Brey PT (2012) Informed consent: cultural differences. Nature 483(7387):36. https://doi.org/10.1038/483036a
Hardwick SA, Chen WY, Wong T, Deveson IW, Blackburn J, Andersen SB, Nielsen LK, Mattick JS, Mercer TR (2016) Spliced synthetic genes as internal controls in RNA sequencing experiments. Nat Methods 13(9):792–798. https://doi.org/10.1038/nmeth.3958
Hardwick SA, Deveson IW, Mercer TR (2017) Reference standards for next-generation sequencing. Nat Rev Genet 18(8):473–484. https://doi.org/10.1038/nrg.2017.44
Hasin Y, Seldin M, Lusis A (2017) Multi-omics approaches to disease. Genome Biol 18(1):83. https://doi.org/10.1186/s13059-017-1215-1
Hoadley KA, Yau C, Hinoue T, Wolf DM, Lazar AJ, Drill E, Shen R, Taylor AM, Cherniack AD, Thorsson V, Akbani R, Bowlby R, Wong CK, Wiznerowicz M, Sanchez-Vega F, Robertson AG, Schneider BG, Lawrence MS, Noushmehr H, Malta TM, Cancer Genome Atlas N, Stuart JM, Benz CC, Laird PW (2018) Cell-of-origin patterns dominate the molecular classification of 10,000 tumors from 33 types of cancer. Cell 173(2):291-304 e296. https://doi.org/10.1016/j.cell.2018.03.022
Horizon Discovery (2023) Multiplex I cfDNA reference standard set. https://horizondiscovery.com/en/reference-standards/products/multiplex-i-cfdna-reference-standard-set
International Organization for Standardization, ISO Guide 30:2015—reference materials—selected terms and definitions. https://webstore.ansi.org/standards/iso/isoguide302015
International Organization for Standardization, ISO 9000:2015—Quality management systems. https://www.iso.org/standard/45481.html
Jennings LJ, Arcila ME, Corless C, Kamel-Reid S, Lubin IM, Pfeifer J, Temple-Smolkin RL, Voelkerding KV, Nikiforova MN (2017) Guidelines for validation of next-generation sequencing-based oncology panels: a joint consensus recommendation of the association for molecular pathology and college of American pathologists. J Mol Diagn 19(3):341–365. https://doi.org/10.1016/j.jmoldx.2017.01.011
Jia S, Zhang R, Lin G, Peng R, Gao P, Han Y, Fu Y, Ding J, Wu Q, Zhang K, **e J, Li J (2018) A novel cell line generated using the CRISPR/Cas9 technology as universal quality control material for KRAS G12V mutation testing. J Clin Lab Anal 32(5):e22391. https://doi.org/10.1002/jcla.22391
Jia P, Dong L, Yang X, Wang B, Bush SJ, Wang T, Lin J, Wang S, Zhao X, Xu T, Che Y, Dang N, Ren L, Zhang Y, Wang X, Liang F, Wang Y, Ruan J, **a H, Zheng Y, Shi L, Lv Y, Wang J, Ye K (2023) Haplotype-resolved assemblies and variant benchmark of a Chinese Quartet. Genome Biol 24(1):277. https://doi.org/10.1186/s13059-023-03116-3
Jiang L, Schlesinger F, Davis CA, Zhang Y, Li R, Salit M, Gingeras TR, Oliver B (2011) Synthetic spike-in standards for RNA-seq experiments. Genome Res 21(9):1543–1551. https://doi.org/10.1101/gr.121095.111
Jiang YZ, Ma D, Suo C, Shi J, Xue M, Hu X, **ao Y, Yu KD, Liu YR, Yu Y, Zheng Y, Li X, Zhang C, Hu P, Zhang J, Hua Q, Zhang J, Hou W, Ren L, Bao D, Li B, Yang J, Yao L, Zuo WJ, Zhao S, Gong Y, Ren YX, Zhao YX, Yang YS, Niu Z, Cao ZG, Stover DG, Verschraegen C, Kaklamani V, Daemen A, Benson JR, Takabe K, Bai F, Li DQ, Wang P, Shi L, Huang W, Shao ZM (2019) Genomic and transcriptomic landscape of triple-negative breast cancers: subtypes and treatment strategies. Cancer Cell 35(3):428-440 e425. https://doi.org/10.1016/j.ccell.2019.02.001
Jones W, Gong B, Novoradovskaya N, Li D, Kusko R, Richmond TA, Johann DJ Jr, Bisgin H, Sahraeian SME, Bushel PR, Pirooznia M, Wilkins K, Chierici M, Bao W, Basehore LS, Lucas AB, Burgess D, Butler DJ, Cawley S, Chang CJ, Chen G, Chen T, Chen YC, Craig DJ, Del Pozo A, Foox J, Francescatto M, Fu Y, Furlanello C, Giorda K, Grist KP, Guan M, Hao Y, Happe S, Hariani G, Haseley N, Jasper J, Jurman G, Kreil DP, Labaj P, Lai K, Li J, Li QZ, Li Y, Li Z, Liu Z, Lopez MS, Miclaus K, Miller R, Mittal VK, Mohiyuddin M, Pabon-Pena C, Parsons BL, Qiu F, Scherer A, Shi T, Stiegelmeyer S, Suo C, Tom N, Wang D, Wen Z, Wu L, **ao W, Xu C, Yu Y, Zhang J, Zhang Y, Zhang Z, Zheng Y, Mason CE, Willey JC, Tong W, Shi L, Xu J (2021) A verified genomic reference sample for assessing performance of cancer panels detecting small variants of low allele frequency. Genome Biol 22(1):111. https://doi.org/10.1186/s13059-021-02316-z
JPT Peptide Technologies (2023) SpikeMix for targeted proteomics. https://www.jpt.com/products-services/peptide-pools/spikemix-targeted-proteomics/
Kalman L, Johnson MA, Beck J, Berry-Kravis E, Buller A, Casey B, Feldman GL, Handsfield J, Jakupciak JP, Maragh S, Matteson K, Muralidharan K, Richie KL, Rohlfs EM, Schaefer F, Sellers T, Spector E, Richards CS (2007) Development of genomic reference materials for Huntington disease genetic testing. Genet Med 9(10):719–723. https://doi.org/10.1097/gim.0b013e318156e8c1
Kalman L, Leonard J, Gerry N, Tarleton J, Bridges C, Gastier-Foster JM, Pyatt RE, Stonerock E, Johnson MA, Richards CS, Schrijver I, Ma T, Miller VR, Adadevoh Y, Furlong P, Beiswanger C, Toji L (2011) Quality assurance for Duchenne and Becker muscular dystrophy genetic testing: development of a genomic DNA reference material panel. J Mol Diagn 13(2):167–174. https://doi.org/10.1016/j.jmoldx.2010.11.018
Karczewski KJ, Snyder MP (2018) Integrative omics for health and disease. Nat Rev Genet 19(5):299–310. https://doi.org/10.1038/nrg.2018.4
Khayat MM, Sahraeian SME, Zarate S, Carroll A, Hong H, Pan B, Shi L, Gibbs RA, Mohiyuddin M, Zheng Y, Sedlazeck FJ (2021) Hidden biases in germline structural variant detection. Genome Biol 22(1):347. https://doi.org/10.1186/s13059-021-02558-x
Kirwan JA, Gika H, Beger RD, Bearden D, Dunn WB, Goodacre R, Theodoridis G, Witting M, Yu LR, Wilson ID, Metabolomics Quality A, Quality Control C (2022) Quality assurance and quality control reporting in untargeted metabolic phenoty**: mQACC recommendations for analytical quality management. Metabolomics 18(9):70. https://doi.org/10.1007/s11306-022-01926-3
Kocher T, Pichler P, Swart R, Mechtler K (2011) Quality control in LC-MS/MS. Proteomics 11(6):1026–1030. https://doi.org/10.1002/pmic.201000578
Krassowski M, Das V, Sahu SK, Misra BB (2020) State of the field in multi-omics research: from computational needs to data mining and sharing. Front Genet 11:610798. https://doi.org/10.3389/fgene.2020.610798
Krogstad DJ, Diop S, Diallo A, Mzayek F, Keating J, Koita OA, Toure YT (2010) Informed consent in international research: the rationale for different approaches. Am J Trop Med Hyg 83(4):743–747. https://doi.org/10.4269/ajtmh.2010.10-0014
Ku X, Wang J, Li H, Meng C, Yu F, Yu W, Li Z, Zhou Z, Zhang C, Hua Y, Yan W, ** J (2023) Proteomic portrait of human lymphoma reveals protein molecular fingerprint of disease specific subtypes and progression. Phenomics 3(2):148–166. https://doi.org/10.1007/s43657-022-00075-w
Kumaran M, Subramanian U, Devarajan B (2019) Performance assessment of variant calling pipelines using human whole exome sequencing and simulated data. BMC Bioinformatics 20(1):342. https://doi.org/10.1186/s12859-019-2928-9
Lazzarotto CR, Malinin NL, Li Y, Zhang R, Yang Y, Lee G, Cowley E, He Y, Lan X, Jividen K, Katta V, Kolmakova NG, Petersen CT, Qi Q, Strelcov E, Maragh S, Krenciute G, Ma J, Cheng Y, Tsai SQ (2020) CHANGE-seq reveals genetic and epigenetic effects on CRISPR-Cas9 genome-wide activity. Nat Biotechnol 38(11):1317–1327. https://doi.org/10.1038/s41587-020-0555-7
Leger A, Amaral PP, Pandolfini L, Capitanchik C, Capraro F, Miano V, Migliori V, Toolan-Kerr P, Sideri T, Enright AJ, Tzelepis K, van Werven FJ, Luscombe NM, Barbieri I, Ule J, Fitzgerald T, Birney E, Leonardi T, Kouzarides T (2021) RNA modifications detection by comparative Nanopore direct RNA sequencing. Nat Commun 12(1):7198. https://doi.org/10.1038/s41467-021-27393-3
Lexogen (2023) Spike-in RNA variants (SIRV). https://www.lexogen.com/sirvs/
Li H, Bloom JM, Farjoun Y, Fleharty M, Gauthier L, Neale B, MacArthur D (2018) A synthetic-diploid benchmark for accurate variant-calling evaluation. Nat Methods 15(8):595–597. https://doi.org/10.1038/s41592-018-0054-7
Li J, Zhang L, Li L, Li X, Zhang X, Zhai S, Gao H, Li Y, Wu G, Wu Y (2020) Development of genomic DNA certified reference materials for genetically modified rice Kefeng 6. ACS Omega 5(34):21602–21609. https://doi.org/10.1021/acsomega.0c02274
Lin G, Zhang K, Han Y, Peng R, Li J (2022) Preparation of multiplexed control materials for cancer mutation analysis by genome editing in GM12878 cells. J Clin Lab Anal 36(1):e24139. https://doi.org/10.1002/jcla.24139
Lindon JC, Nicholson JK, Holmes E, Keun HC, Craig A, Pearce JT, Bruce SJ, Hardy N, Sansone SA, Antti H, Jonsson P, Daykin C, Navarange M, Beger RD, Verheij ER, Amberg A, Baunsgaard D, Cantor GH, Lehman-McKeeman L, Earll M, Wold S, Johansson E, Haselden JN, Kramer K, Thomas C, Lindberg J, Schuppe-Koistinen I, Wilson ID, Reily MD, Robertson DG, Senn H, Krotzky A, Kochhar S, Powell J, van der Ouderaa F, Plumb R, Schaefer H, Spraul M, Standard Metabolic Reporting Structures Working G (2005) Summary recommendations for standardization and reporting of metabolic analyses. Nat Biotechnol 23(7):833–838. https://doi.org/10.1038/nbt0705-833
Lippa KA, Aristizabal-Henao JJ, Beger RD, Bowden JA, Broeckling C, Beecher C, Clay Davis W, Dunn WB, Flores R, Goodacre R, Gouveia GJ, Harms AC, Hartung T, Jones CM, Lewis MR, Ntai I, Percy AJ, Raftery D, Schock TB, Sun J, Theodoridis G, Tayyari F, Torta F, Ulmer CZ, Wilson I, Ubhi BK (2022) Reference materials for MS-based untargeted metabolomics and lipidomics: a review by the metabolomics quality assurance and quality control consortium (mQACC). Metabolomics 18(4):24. https://doi.org/10.1007/s11306-021-01848-6
Mangiante L, Alcala N, Sexton-Oates A, Di Genova A, Gonzalez-Perez A, Khandekar A, Bergstrom EN, Kim J, Liu X, Blazquez-Encinas R, Giacobi C, Le Stang N, Boyault S, Cuenin C, Tabone-Eglinger S, Damiola F, Voegele C, Ardin M, Michallet MC, Soudade L, Delhomme TM, Poret A, Brevet M, Copin MC, Giusiano-Courcambeck S, Damotte D, Girard C, Hofman V, Hofman P, Mouroux J, Cohen C, Lacomme S, Mazieres J, de Montpreville VT, Perrin C, Planchard G, Rousseau N, Rouquette I, Sagan C, Scherpereel A, Thivolet F, Vignaud JM, Jean D, Ilg AGS, Olaso R, Meyer V, Boland-Auge A, Deleuze JF, Altmuller J, Nuernberg P, Ibanez-Costa A, Castano JP, Lantuejoul S, Ghantous A, Maussion C, Courtiol P, Hernandez-Vargas H, Caux C, Girard N, Lopez-Bigas N, Alexandrov LB, Galateau-Salle F, Foll M, Fernandez-Cuesta L (2023) Multiomic analysis of malignant pleural mesothelioma identifies molecular axes and specialized tumor profiles driving intertumor heterogeneity. Nat Genet 55(4):607–618. https://doi.org/10.1038/s41588-023-01321-1
MAQC Consortium (2006) The MicroArray Quality Control (MAQC) project shows inter- and intraplatform reproducibility of gene expression measurements. Nat Biotechnol 24(9):1151–1161. https://doi.org/10.1038/nbt1239
MAQC Consortium (2014) A comprehensive assessment of RNA-seq accuracy, reproducibility and information content by the Sequencing Quality Control Consortium. Nat Biotechnol 32(9):903–914. https://doi.org/10.1038/nbt.2957
MAQC Consortium (2021) Sequencing quality control 2 nature collection. https://www.nature.com/collections/fjhdjcdefg
Martinez-Ruiz C, Black JRM, Puttick C, Hill MS, Demeulemeester J, Larose Cadieux E, Thol K, Jones TP, Veeriah S, Naceur-Lombardelli C, Toncheva A, Prymas P, Rowan A, Ward S, Cubitt L, Athanasopoulou F, Pich O, Karasaki T, Moore DA, Salgado R, Colliver E, Castignani C, Dietzen M, Huebner A, Al Bakir M, Tanic M, Watkins TBK, Lim EL, Al-Rashed AM, Lang D, Clements J, Cook DE, Rosenthal R, Wilson GA, Frankell AM, de Carne TS, East P, Kanu N, Litchfield K, Birkbak NJ, Hackshaw A, Beck S, Van Loo P, Jamal-Hanjani M, Consortium TR, Swanton C, McGranahan N (2023) Genomic-transcriptomic evolution in lung cancer and metastasis. Nature 616(7957):543–552. https://doi.org/10.1038/s41586-023-05706-4
McGaw EA, Phinney KW, Lowenthal MS (2010) Comparison of orthogonal liquid and gas chromatography–mass spectrometry platforms for the determination of amino acid concentrations in human plasma. J Chromatogr A 1217(37):5822–5831. https://doi.org/10.1016/j.chroma.2010.07.025
Medical Device Innovation Consortium (2019) MDIC SRS report: somatic variant reference samples for NGS. https://mdic.org/wp-content/uploads/2019/03/MDIC-SRS-Landscape-Analysis-Report-20190306.pdf
Merino DM, McShane LM, Fabrizio D, Funari V, Chen SJ, White JR, Wenz P, Baden J, Barrett JC, Chaudhary R, Chen L, Chen WS, Cheng JH, Cyanam D, Dickey JS, Gupta V, Hellmann M, Helman E, Li Y, Maas J, Papin A, Patidar R, Quinn KJ, Rizvi N, Tae H, Ward C, **e M, Zehir A, Zhao C, Dietel M, Stenzinger A, Stewart M, Allen J, Consortium TMBH (2020) Establishing guidelines to harmonize tumor mutational burden (TMB): in silico assessment of variation in TMB quantification across diagnostic platforms: phase I of the Friends of Cancer Research TMB Harmonization Project. J Immunother Cancer. https://doi.org/10.1136/jitc-2019-000147
Met Qual Program Coordinators (2023) The NIST metabolomics quality assurance and quality control materials (MetQual) program. https://www.nist.gov/programs-projects/metabolomics-quality-assurance-and-quality-control-materials-metqual-program
Misra BB, Olivier M (2020) High resolution GC-orbitrap-MS metabolomics using both electron ionization and chemical ionization for analysis of human plasma. J Proteome Res 19(7):2717–2731. https://doi.org/10.1021/acs.jproteome.9b00774
Morgenstern D, Barzilay R, Levin Y (2021) RawBeans: a simple, vendor-independent, raw-data quality-control tool. J Proteome Res 20(4):2098–2104. https://doi.org/10.1021/acs.jproteome.0c00956
Munro SA, Lund SP, Pine PS, Binder H, Clevert DA, Conesa A, Dopazo J, Fasold M, Hochreiter S, Hong H, Jafari N, Kreil DP, Labaj PP, Li S, Liao Y, Lin SM, Meehan J, Mason CE, Santoyo-Lopez J, Setterquist RA, Shi L, Shi W, Smyth GK, Stralis-Pavese N, Su Z, Tong W, Wang C, Wang J, Xu J, Ye Z, Yang Y, Yu Y, Salit M (2014) Assessing technical performance in differential gene expression experiments with external spike-in RNA control ratio mixtures. Nat Commun 5:5125. https://doi.org/10.1038/ncomms6125
Nakayasu ES, Gritsenko M, Piehowski PD, Gao Y, Orton DJ, Schepmoes AA, Fillmore TL, Frohnert BI, Rewers M, Krischer JP, Ansong C, Suchy-Dicey AM, Evans-Molina C, Qian WJ, Webb-Robertson BM, Metz TO (2021) Tutorial: best practices and considerations for mass-spectrometry-based protein biomarker discovery and validation. Nat Protoc 16(8):3737–3760. https://doi.org/10.1038/s41596-021-00566-6
National Institute of Standards and Technology (2022) The 2022 NIST-hosted workshop on standards for microbiome and multi-omics measurements. https://www.nist.gov/news-events/events/2022/08/2022-nist-hosted-workshop-standards-microbiome-and-multiomics
National Institute of Standards and Technology (2023a) NIST SRM definitions. https://www.nist.gov/srm/srm-definitions
National Institute of Standards and Technology (2023b) Genome in a bottle. https://www.nist.gov/programs-projects/genome-bottle
Nurk S, Koren S, Rhie A, Rautiainen M, Bzikadze AV, Mikheenko A, Vollger MR, Altemose N, Uralsky L, Gershman A, Aganezov S, Hoyt SJ, Diekhans M, Logsdon GA, Alonge M, Antonarakis SE, Borchers M, Bouffard GG, Brooks SY, Caldas GV, Chen NC, Cheng H, Chin CS, Chow W, de Lima LG, Dishuck PC, Durbin R, Dvorkina T, Fiddes IT, Formenti G, Fulton RS, Fungtammasan A, Garrison E, Grady PGS, Graves-Lindsay TA, Hall IM, Hansen NF, Hartley GA, Haukness M, Howe K, Hunkapiller MW, Jain C, Jain M, Jarvis ED, Kerpedjiev P, Kirsche M, Kolmogorov M, Korlach J, Kremitzki M, Li H, Maduro VV, Marschall T, McCartney AM, McDaniel J, Miller DE, Mullikin JC, Myers EW, Olson ND, Paten B, Peluso P, Pevzner PA, Porubsky D, Potapova T, Rogaev EI, Rosenfeld JA, Salzberg SL, Schneider VA, Sedlazeck FJ, Shafin K, Shew CJ, Shumate A, Sims Y, Smit AFA, Soto DC, Sovic I, Storer JM, Streets A, Sullivan BA, Thibaud-Nissen F, Torrance J, Wagner J, Walenz BP, Wenger A, Wood JMD, **ao C, Yan SM, Young AC, Zarate S, Surti U, McCoy RC, Dennis MY, Alexandrov IA, Gerton JL, O’Neill RJ, Timp W, Zook JM, Schatz MC, Eichler EE, Miga KH, Phillippy AM (2022) The complete sequence of a human genome. Science 376(6588):44–53. https://doi.org/10.1126/science.abj6987
Olink (2023) Olink data generation and QC. https://olink.com/our-platform/our-pea-technology/data-generation-and-qc/
Omenn GS (2021) Reflections on the HUPO human proteome project, the flagship project of the human proteome organization, at 10 years. Mol Cell Proteomics 20:100062. https://doi.org/10.1016/j.mcpro.2021.100062
Pan B, Ren L, Onuchic V, Guan M, Kusko R, Bruinsma S, Trigg L, Scherer A, Ning B, Zhang C, Glidewell-Kenney C, **ao C, Donaldson E, Sedlazeck FJ, Schroth G, Yavas G, Grunenwald H, Chen H, Meinholz H, Meehan J, Wang J, Yang J, Foox J, Shang J, Miclaus K, Dong L, Shi L, Mohiyuddin M, Pirooznia M, Gong P, Golshani R, Wolfinger R, Lababidi S, Sahraeian SME, Sherry S, Han T, Chen T, Shi T, Hou W, Ge W, Zou W, Guo W, Bao W, **ao W, Fan X, Gondo Y, Yu Y, Zhao Y, Su Z, Liu Z, Tong W, **ao W, Zook JM, Zheng Y, Hong H (2022) Assessing reproducibility of inherited variants detected with short-read whole genome sequencing. Genome Biol 23(1):2. https://doi.org/10.1186/s13059-021-02569-8
Patel RK, Jain M (2012) NGS QC toolkit: a toolkit for quality control of next generation sequencing data. PLoS ONE 7(2):e30619. https://doi.org/10.1371/journal.pone.0030619
Paulovich AG, Billheimer D, Ham AJ, Vega-Montoto L, Rudnick PA, Tabb DL, Wang P, Blackman RK, Bunk DM, Cardasis HL, Clauser KR, Kinsinger CR, Schilling B, Tegeler TJ, Variyath AM, Wang M, Whiteaker JR, Zimmerman LJ, Fenyo D, Carr SA, Fisher SJ, Gibson BW, Mesri M, Neubert TA, Regnier FE, Rodriguez H, Spiegelman C, Stein SE, Tempst P, Liebler DC (2010) Interlaboratory study characterizing a yeast performance standard for benchmarking LC–MS platform performance. Mol Cell Proteomics 9(2):242–254. https://doi.org/10.1074/mcp.M900222-MCP200
Petrera A, von Toerne C, Behler J, Huth C, Thorand B, Hilgendorff A, Hauck SM (2021) Multiplatform approach for plasma proteomics: complementarity of olink proximity extension assay technology to mass spectrometry-based protein profiling. J Proteome Res 20(1):751–762. https://doi.org/10.1021/acs.jproteome.0c00641
Pfeifer JD, Loberg R, Lofton-Day C, Zehnbauer BA (2022) Reference samples to compare next-generation sequencing test performance for oncology therapeutics and diagnostics. Am J Clin Pathol 157(4):628–638. https://doi.org/10.1093/ajcp/aqab164
Phinney KW, Ballihaut G, Bedner M, Benford BS, Camara JE, Christopher SJ, Davis WC, Dodder NG, Eppe G, Lang BE, Long SE, Lowenthal MS, McGaw EA, Murphy KE, Nelson BC, Prendergast JL, Reiner JL, Rimmer CA, Sander LC, Schantz MM, Sharpless KE, Sniegoski LT, Tai SS, Thomas JB, Vetter TW, Welch MJ, Wise SA, Wood LJ, Guthrie WF, Hagwood CR, Leigh SD, Yen JH, Zhang NF, Chaudhary-Webb M, Chen H, Fazili Z, LaVoie DJ, McCoy LF, Momin SS, Paladugula N, Pendergrast EC, Pfeiffer CM, Powers CD, Rabinowitz D, Rybak ME, Schleicher RL, Toombs BM, Xu M, Zhang M, Castle AL (2013) Development of a standard reference material for metabolomics research. Anal Chem 85(24):11732–11738. https://doi.org/10.1021/ac402689t
Pratt VM, Caggana M, Bridges C, Buller AM, DiAntonio L, Highsmith WE, Holtegaard LM, Muralidharan K, Rohlfs EM, Tarleton J, Toji L, Barker SD, Kalman LV (2009) Development of genomic reference materials for cystic fibrosis genetic testing. J Mol Diagn 11(3):186–193. https://doi.org/10.2353/jmoldx.2009.080149
Pratt VM, Everts RE, Aggarwal P, Beyer BN, Broeckel U, Epstein-Baak R, Hujsak P, Kornreich R, Liao J, Lorier R, Scott SA, Smith CH, Toji LH, Turner A, Kalman LV (2016) Characterization of 137 genomic DNA reference materials for 28 pharmacogenetic genes: a GeT-RM collaborative project. J Mol Diagn 18(1):109–123. https://doi.org/10.1016/j.jmoldx.2015.08.005
Price ND, Magis AT, Earls JC, Glusman G, Levy R, Lausted C, McDonald DT, Kusebauch U, Moss CL, Zhou Y, Qin S, Moritz RL, Brogaard K, Omenn GS, Lovejoy JC, Hood L (2017) A wellness study of 108 individuals using personal, dense, dynamic data clouds. Nat Biotechnol 35(8):747–756. https://doi.org/10.1038/nbt.3870
Reis ALM, Deveson IW, Wong T, Madala BS, Barker C, Blackburn J, Marcellin E, Mercer TR (2020) A universal and independent synthetic DNA ladder for the quantitative measurement of genomic features. Nat Commun 11(1):3609. https://doi.org/10.1038/s41467-020-17445-5
Ren L, Duan X, Dong L, Zhang R, Yang J, Gao Y, Peng R, Hou W, Liu Y, Li J, Yu Y, Zhang N, Shang J, Liang F, Wang D, The Quartet Project Team, Scherer A, Nordlund J, **ao W, Xu J, Tong W, Hu X, Li J, ** L, Shi L, Hong H, Wang J, Fan S, Fang X, Zheng Y (2023) Quartet DNA reference materials and datasets for comprehensively evaluating germline variants calling performance. Genome Biol 24:270. https://doi.org/10.1186/s13059-023-03109-2
Robasky K, Lewis NE, Church GM (2014) The role of replicates for error mitigation in next-generation sequencing. Nat Rev Genet 15(1):56–62. https://doi.org/10.1038/nrg3655
Rudnick PA, Markey SP, Roth J, Mirokhin Y, Yan X, Tchekhovskoi DV, Edwards NJ, Thangudu RR, Ketchum KA, Kinsinger CR, Mesri M, Rodriguez H, Stein SE (2016) A description of the clinical proteomic tumor analysis consortium (CPTAC) common data analysis pipeline. J Proteome Res 15(3):1023–1032. https://doi.org/10.1021/acs.jproteome.5b01091
Sahraeian SME, Fang LT, Karagiannis K, Moos M, Smith S, Santana-Quintero L, **ao C, Colgan M, Hong H, Mohiyuddin M, **ao W (2022) Achieving robust somatic mutation detection with deep learning models derived from reference data sets of a cancer sample. Genome Biol 23(1):12. https://doi.org/10.1186/s13059-021-02592-9
Sammut SJ, Crispin-Ortuzar M, Chin SF, Provenzano E, Bardwell HA, Ma W, Cope W, Dariush A, Dawson SJ, Abraham JE, Dunn J, Hiller L, Thomas J, Cameron DA, Bartlett JMS, Hayward L, Pharoah PD, Markowetz F, Rueda OM, Earl HM, Caldas C (2022) Multi-omic machine learning predictor of breast cancer therapy response. Nature 601(7894):623–629. https://doi.org/10.1038/s41586-021-04278-5
Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, Barron DA, Zehir A, Jordan EJ, Omuro A, Kaley TJ, Kendall SM, Motzer RJ, Hakimi AA, Voss MH, Russo P, Rosenberg J, Iyer G, Bochner BH, Bajorin DF, Al-Ahmadie HA, Chaft JE, Rudin CM, Riely GJ, Baxi S, Ho AL, Wong RJ, Pfister DG, Wolchok JD, Barker CA, Gutin PH, Brennan CW, Tabar V, Mellinghoff IK, DeAngelis LM, Ariyan CE, Lee N, Tap WD, Gounder MM, D’Angelo SP, Saltz L, Stadler ZK, Scher HI, Baselga J, Razavi P, Klebanoff CA, Yaeger R, Segal NH, Ku GY, DeMatteo RP, Ladanyi M, Rizvi NA, Berger MF, Riaz N, Solit DB, Chan TA, Morris LGT (2019) Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 51(2):202–206. https://doi.org/10.1038/s41588-018-0312-8
Schantz MM, Eppe G, Focant JF, Hamilton C, Heckert NA, Heltsley RM, Hoover D, Keller JM, Leigh SD, Patterson DG Jr, Pintar AL, Sharpless KE, Sjodin A, Turner WE, Vander Pol SS, Wise SA (2013) Milk and serum standard reference materials for monitoring organic contaminants in human samples. Anal Bioanal Chem 405(4):1203–1211. https://doi.org/10.1007/s00216-012-6524-3
Schussler-Fiorenza Rose SM, Contrepois K, Moneghetti KJ, Zhou W, Mishra T, Mataraso S, Dagan-Rosenfeld O, Ganz AB, Dunn J, Hornburg D, Rego S, Perelman D, Ahadi S, Sailani MR, Zhou Y, Leopold SR, Chen J, Ashland M, Christle JW, Avina M, Limcaoco P, Ruiz C, Tan M, Butte AJ, Weinstock GM, Slavich GM, Sodergren E, McLaughlin TL, Haddad F, Snyder MP (2019) A longitudinal big data approach for precision health. Nat Med 25(5):792–804. https://doi.org/10.1038/s41591-019-0414-6
Sempos CT, Lindhout E, Heureux N, Hars M, Parkington DA, Dennison E, Durazo-Arvizu R, Jones KS, Wise SA (2022) Towards harmonization of directly measured free 25-hydroxyvitamin D using an enzyme-linked immunosorbent assay. Anal Bioanal Chem 414(27):7793–7803. https://doi.org/10.1007/s00216-022-04313-y
Seracare (2023a) Seraseq gDNA TMB reference panel mix. https://www.seracare.com/Seraseq-gDNA-TMB-Reference-Panel-Mix-0710-2463/
Seracare (2023b) Seraseq ctDNA reference materials. https://www.seracare.com/Seraseq-ctDNA-Complete-Reference-Material-AF05-0710-0672/
Shi L, Kusko R, Wolfinger RD, Haibe-Kains B, Fischer M, Sansone SA, Mason CE, Furlanello C, Jones WD, Ning B, Tong W (2017) The international MAQC society launches to enhance reproducibility of high-throughput technologies. Nat Biotechnol 35(12):1127–1128. https://doi.org/10.1038/nbt.4029
Simon-Manso Y, Lowenthal MS, Kilpatrick LE, Sampson ML, Telu KH, Rudnick PA, Mallard WG, Bearden DW, Schock TB, Tchekhovskoi DV, Blonder N, Yan X, Liang Y, Zheng Y, Wallace WE, Neta P, Phinney KW, Remaley AT, Stein SE (2013) Metabolite profiling of a NIST Standard Reference Material for human plasma (SRM 1950): GC–MS, LC–MS, NMR, and clinical laboratory analyses, libraries, and web-based resources. Anal Chem 85(24):11725–11731. https://doi.org/10.1021/ac402503m
Siskos AP, Jain P, Romisch-Margl W, Bennett M, Achaintre D, Asad Y, Marney L, Richardson L, Koulman A, Griffin JL, Raynaud F, Scalbert A, Adamski J, Prehn C, Keun HC (2017) Interlaboratory reproducibility of a targeted metabolomics platform for analysis of human serum and plasma. Anal Chem 89(1):656–665. https://doi.org/10.1021/acs.analchem.6b02930
Soneson C, Yao Y, Bratus-Neuenschwander A, Patrignani A, Robinson MD, Hussain S (2019) A comprehensive examination of Nanopore native RNA sequencing for characterization of complex transcriptomes. Nat Commun 10(1):3359. https://doi.org/10.1038/s41467-019-11272-z
Sonia Tarazona AA-LAC (2021) Undisclosed, unmet and neglected challenges in multi-omics studies. Nat Comput Sci 1:395–402. https://doi.org/10.1038/s43588-021-00086-z
Stenzinger A, Allen JD, Maas J, Stewart MD, Merino DM, Wempe MM, Dietel M (2019) Tumor mutational burden standardization initiatives: recommendations for consistent tumor mutational burden assessment in clinical samples to guide immunotherapy treatment decisions. Genes Chromosomes Cancer 58(8):578–588. https://doi.org/10.1002/gcc.22733
Stephens PJ, McBride DJ, Lin ML, Varela I, Pleasance ED, Simpson JT, Stebbings LA, Leroy C, Edkins S, Mudie LJ, Greenman CD, Jia M, Latimer C, Teague JW, Lau KW, Burton J, Quail MA, Swerdlow H, Churcher C, Natrajan R, Sieuwerts AM, Martens JW, Silver DP, Langerod A, Russnes HE, Foekens JA, Reis-Filho JS, Van’t Veer L, Richardson AL, Borresen-Dale AL, Campbell PJ, Futreal PA, Stratton MR (2009) Complex landscapes of somatic rearrangement in human breast cancer genomes. Nature 462(7276):1005–1010. https://doi.org/10.1038/nature08645
Sun BB, Chiou J, Traylor M, Benner C, Hsu YH, Richardson TG, Surendran P, Mahajan A, Robins C, Vasquez-Grinnell SG, Hou L, Kvikstad EM, Burren OS, Davitte J, Ferber KL, Gillies CE, Hedman AK, Hu S, Lin T, Mikkilineni R, Pendergrass RK, Pickering C, Prins B, Baird D, Chen CY, Ward LD, Deaton AM, Welsh S, Willis CM, Lehner N, Arnold M, Worheide MA, Suhre K, Kastenmuller G, Sethi A, Cule M, Raj A, Alnylam Human G, AstraZeneca Genomics I, Biogen Biobank T, Bristol Myers S, Genentech Human G, GlaxoSmithKline Genomic S, Pfizer Integrative B, Population Analytics of Janssen Data S, Regeneron Genetics C, Burkitt-Gray L, Melamud E, Black MH, Fauman EB, Howson JMM, Kang HM, McCarthy MI, Nioi P, Petrovski S, Scott RA, Smith EN, Szalma S, Waterworth DM, Mitnaul LJ, Szustakowski JD, Gibson BW, Miller MR, Whelan CD (2023) Plasma proteomic associations with genetics and health in the UK Biobank. Nature 622(7982):329–338. https://doi.org/10.1038/s41586-023-06592-6
Suzuki T, Tsukumo Y, Furihata C, Naito M, Kohara A (2020) Preparation of the standard cell lines for reference mutations in cancer gene-panels by genome editing in HEK 293 T/17 cells. Genes Environ 42:8. https://doi.org/10.1186/s41021-020-0147-2
Tabb DL, Vega-Montoto L, Rudnick PA, Variyath AM, Ham AJ, Bunk DM, Kilpatrick LE, Billheimer DD, Blackman RK, Cardasis HL, Carr SA, Clauser KR, Jaffe JD, Kowalski KA, Neubert TA, Regnier FE, Schilling B, Tegeler TJ, Wang M, Wang P, Whiteaker JR, Zimmerman LJ, Fisher SJ, Gibson BW, Kinsinger CR, Mesri M, Rodriguez H, Stein SE, Tempst P, Paulovich AG, Liebler DC, Spiegelman C (2010) Repeatability and reproducibility in proteomic identifications by liquid chromatography-tandem mass spectrometry. J Proteome Res 9(2):761–776. https://doi.org/10.1021/pr9006365
Tabb DL, Wang X, Carr SA, Clauser KR, Mertins P, Chambers MC, Holman JD, Wang J, Zhang B, Zimmerman LJ, Chen X, Gunawardena HP, Davies SR, Ellis MJ, Li S, Townsend RR, Boja ES, Ketchum KA, Kinsinger CR, Mesri M, Rodriguez H, Liu T, Kim S, McDermott JE, Payne SH, Petyuk VA, Rodland KD, Smith RD, Yang F, Chan DW, Zhang B, Zhang H, Zhang Z, Zhou JY, Liebler DC (2016) Reproducibility of differential proteomic technologies in CPTAC fractionated xenografts. J Proteome Res 15(3):691–706. https://doi.org/10.1021/acs.jproteome.5b00859
Talsania K, Shen TW, Chen X, Jaeger E, Li Z, Chen Z, Chen W, Tran B, Kusko R, Wang L, Pang AWC, Yang Z, Choudhari S, Colgan M, Fang LT, Carroll A, Shetty J, Kriga Y, German O, Smirnova T, Liu T, Li J, Kellman B, Hong K, Hastie AR, Natarajan A, Moshrefi A, Granat A, Truong T, Bombardi R, Mankinen V, Meerzaman D, Mason CE, Collins J, Stahlberg E, **ao C, Wang C, **ao W, Zhao Y (2022) Structural variant analysis of a cancer reference cell line sample using multiple sequencing technologies. Genome Biol 23(1):255. https://doi.org/10.1186/s13059-022-02816-6
Thermo Scientific (2020) AcroMetrixTM oncology hotspot control. https://assets.thermofisher.com/TFS-Assets/CDD/manuals/MAN0010820-AMX-Oncology-Hotspot-Ctrl-EN.pdf
Thompson JW, Adams KJ, Adamski J, Asad Y, Borts D, Bowden JA, Byram G, Dang V, Dunn WB, Fernandez F, Fiehn O, Gaul DA, Huhmer AF, Kalli A, Koal T, Koeniger S, Mandal R, Meier F, Naser FJ, O’Neil D, Pal A, Patti GJ, Pham-Tuan H, Prehn C, Raynaud FI, Shen T, Southam AD, St John-Williams L, Sulek K, Vasilopoulou CG, Viant M, Winder CL, Wishart D, Zhang L, Zheng J, Moseley MA (2019) International ring trial of a high resolution targeted metabolomics and lipidomics platform for serum and plasma analysis. Anal Chem 91(22):14407–14416. https://doi.org/10.1021/acs.analchem.9b02908
Tian S, Zhan D, Yu Y, Liu M, Wang Y, Song L, Qin Z, Li X, Liu Y, Li Y, Ji S, Li Y, Li L, Wang S, Analysis PM, Control Q, Zheng Y, He F, Qin J, Ding C (2023) Quartet protein reference materials and datasets for multi-platform assessment of label-free proteomics. Genome Biol 24:202. https://doi.org/10.1186/s13059-023-03048-y
Turck CW, Mak TD, Goudarzi M, Salek RM, Cheema AK (2020) The ABRF metabolomics research group 2016 exploratory study: investigation of data analysis methods for untargeted metabolomics. Metabolites. https://doi.org/10.3390/metabo10040128
van Belle G, Mentzelopoulos SD, Aufderheide T, May S, Nichol G (2015) International variation in policies and practices related to informed consent in acute cardiovascular research: results from a 44 country survey. Resuscitation 91:76–83. https://doi.org/10.1016/j.resuscitation.2014.11.029
Vega DM, Yee LM, McShane LM, Williams PM, Chen L, Vilimas T, Fabrizio D, Funari V, Newberg J, Bruce LK, Chen SJ, Baden J, Carl Barrett J, Beer P, Butler M, Cheng JH, Conroy J, Cyanam D, Eyring K, Garcia E, Green G, Gregersen VR, Hellmann MD, Keefer LA, Lasiter L, Lazar AJ, Li MC, MacConaill LE, Meier K, Mellert H, Pabla S, Pallavajjalla A, Pestano G, Salgado R, Samara R, Sokol ES, Stafford P, Budczies J, Stenzinger A, Tom W, Valkenburg KC, Wang XZ, Weigman V, **e M, **e Q, Zehir A, Zhao C, Zhao Y, Stewart MD, Allen J, Consortium TMB (2021) Aligning tumor mutational burden (TMB) quantification across diagnostic platforms: phase II of the Friends of Cancer Research TMB Harmonization Project. Ann Oncol 32(12):1626–1636. https://doi.org/10.1016/j.annonc.2021.09.016
Veltman JA, Brunner HG (2012) De novo mutations in human genetic disease. Nat Rev Genet 13(8):565–575. https://doi.org/10.1038/nrg3241
Wagner J, Olson ND, Harris L, Khan Z, Farek J, Mahmoud M, Stankovic A, Kovacevic V, Yoo B, Miller N, Rosenfeld JA, Ni B, Zarate S, Kirsche M, Aganezov S, Schatz MC, Narzisi G, Byrska-Bishop M, Clarke W, Evani US, Markello C, Shafin K, Zhou X, Sidow A, Bansal V, Ebert P, Marschall T, Lansdorp P, Hanlon V, Mattsson CA, Barrio AM, Fiddes IT, **ao C, Fungtammasan A, Chin CS, Wenger AM, Rowell WJ, Sedlazeck FJ, Carroll A, Salit M, Zook JM (2022) Benchmarking challenging small variants with linked and long reads. Cell Genom. https://doi.org/10.1016/j.xgen.2022.100128
Wang X, Lu M, Qian J, Yang Y, Li S, Lu D, Yu S, Meng W, Ye W, ** L (2009) Rationales, design and recruitment of the Taizhou Longitudinal Study. BMC Public Health 9:223. https://doi.org/10.1186/1471-2458-9-223
Wang X, Chambers MC, Vega-Montoto LJ, Bunk DM, Stein SE, Tabb DL (2014) QC metrics from CPTAC raw LC–MS/MS data interpreted through multivariate statistics. Anal Chem 86(5):2497–2509. https://doi.org/10.1021/ac4034455
Wang D, Zhang Y, Li R, Li J, Zhang R (2023) Consistency and reproducibility of large panel next-generation sequencing: Multi-laboratory assessment of somatic mutation detection on reference materials with mismatch repair and proofreading deficiency. J Adv Res 44:161–172. https://doi.org/10.1016/j.jare.2022.03.016
Wenger AM, Peluso P, Rowell WJ, Chang PC, Hall RJ, Concepcion GT, Ebler J, Fungtammasan A, Kolesnikov A, Olson ND, Topfer A, Alonge M, Mahmoud M, Qian Y, Chin CS, Phillippy AM, Schatz MC, Myers G, DePristo MA, Ruan J, Marschall T, Sedlazeck FJ, Zook JM, Li H, Koren S, Carroll A, Rank DR, Hunkapiller MW (2019) Accurate circular consensus long-read sequencing improves variant detection and assembly of a human genome. Nat Biotechnol 37(10):1155–1162. https://doi.org/10.1038/s41587-019-0217-9
Wik L, Nordberg N, Broberg J, Bjorkesten J, Assarsson E, Henriksson S, Grundberg I, Pettersson E, Westerberg C, Liljeroth E, Falck A, Lundberg M (2021) Proximity extension assay in combination with next-generation sequencing for high-throughput proteome-wide analysis. Mol Cell Proteomics 20:100168. https://doi.org/10.1016/j.mcpro.2021.100168
Wilkinson MD, Dumontier M, Aalbersberg IJ, Appleton G, Axton M, Baak A, Blomberg N, Boiten JW, da Silva Santos LB, Bourne PE, Bouwman J, Brookes AJ, Clark T, Crosas M, Dillo I, Dumon O, Edmunds S, Evelo CT, Finkers R, Gonzalez-Beltran A, Gray AJ, Groth P, Goble C, Grethe JS, Heringa J, t Hoen PA, Hooft R, Kuhn T, Kok R, Kok J, Lusher SJ, Martone ME, Mons A, Packer AL, Persson B, Rocca-Serra P, Roos M, van Schaik R, Sansone SA, Schultes E, Sengstag T, Slater T, Strawn G, Swertz MA, Thompson M, van der Lei J, van Mulligen E, Velterop J, Waagmeester A, Wittenburg P, Wolstencroft K, Zhao J, Mons B (2016) The FAIR Guiding Principles for scientific data management and stewardship. Sci Data 3:160018. https://doi.org/10.1038/sdata.2016.18
**ao W, Ren L, Chen Z, Fang LT, Zhao Y, Lack J, Guan M, Zhu B, Jaeger E, Kerrigan L, Blomquist TM, Hung T, Sultan M, Idler K, Lu C, Scherer A, Kusko R, Moos M, **ao C, Sherry ST, Abaan OD, Chen W, Chen X, Nordlund J, Liljedahl U, Maestro R, Polano M, Drabek J, Vojta P, Koks S, Reimann E, Madala BS, Mercer T, Miller C, Jacob H, Truong T, Moshrefi A, Natarajan A, Granat A, Schroth GP, Kalamegham R, Peters E, Petitjean V, Walton A, Shen TW, Talsania K, Vera CJ, Langenbach K, de Mars M, Hipp JA, Willey JC, Wang J, Shetty J, Kriga Y, Raziuddin A, Tran B, Zheng Y, Yu Y, Cam M, Jailwala P, Nguyen C, Meerzaman D, Chen Q, Yan C, Ernest B, Mehra U, Jensen RV, Jones W, Li JL, Papas BN, Pirooznia M, Chen YC, Seifuddin F, Li Z, Liu X, Resch W, Wang J, Wu L, Yavas G, Miles C, Ning B, Tong W, Mason CE, Donaldson E, Lababidi S, Staudt LM, Tezak Z, Hong H, Wang C, Shi L (2021) Toward best practice in cancer mutation detection with whole-genome and whole-exome sequencing. Nat Biotechnol 39(9):1141–1150. https://doi.org/10.1038/s41587-021-00994-5
Yang J, Liu Y, Shang J, Chen Q, Chen Q, Ren L, Zhang N, Yu Y, Li Z, Song Y, Scherer A, Niehues A, Tong W, Hong H, Shi L, **ao W, Zheng Y (2023) The Quartet Data Portal: integration of community-wide resources for multiomics quality control. Genome Biol 24:245. https://doi.org/10.1186/s13059-023-03091-9
Yarchoan M, Hopkins A, Jaffee EM (2017) Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med 377(25):2500–2501. https://doi.org/10.1056/NEJMc1713444
Yu Y, Hou W, Wang H, Dong L, Liu Y, Sun S, Yang J, Cao Z, Zhang P, Zi Y, Li Z, Liu R, Gao J, Chen Q, Zhang N, Li J, Ren L, Jiang H, Shang J, Zhu S, Wang X, Qing T, Bao D, Li B, Li B, Suo C, Pi Y, The Quartet Project Team, Wang X, Dai F, Scherer A, Mattila P, Han J, Zhang L, Jiang H, Thierry-Mieg D, Thierry-Mieg J, **ao W, Hong H, Tong W, Wang J, Li J, Fang X, ** L, Shi L, Xu J, Qian F, Zhang R, Zheng Y (2023) Quartet RNA reference materials and ratio-based reference datasets for reliable transcriptomic profiling. Nat Biotechnol. https://doi.org/10.1038/s41587-023-01867-9
Zecha J, Gabriel W, Spallek R, Chang YC, Mergner J, Wilhelm M, Bassermann F, Kuster B (2022) Linking post-translational modifications and protein turnover by site-resolved protein turnover profiling. Nat Commun 13(1):165. https://doi.org/10.1038/s41467-021-27639-0
Zehnbauer B, Lofton-Day C, Pfeifer J, Shaughnessy E, Goh L (2017) Diagnostic quality assurance pilot: a model to demonstrate comparative laboratory test performance with an oncology companion diagnostic assay. J Mol Diagn 19(1):1–3. https://doi.org/10.1016/j.jmoldx.2016.10.001
Zhang XH, Tee LY, Wang XG, Huang QS, Yang SH (2015) Off-target effects in CRISPR/Cas9-mediated genome engineering. Mol Ther Nucl Acids 4:e264. https://doi.org/10.1038/mtna.2015.37
Zhang R, Peng R, Li Z, Gao P, Jia S, Yang X, Ding J, Han Y, **e J, Li J (2017) Synthetic circulating cell-free DNA as quality control materials for somatic mutation detection in liquid biopsy for cancer. Clin Chem 63(9):1465–1475. https://doi.org/10.1373/clinchem.2017.272559
Zhang K, Lin G, Han D, Han Y, Wang J, Shen Y, Li J (2020) An initial survey of the performances of exome variant analysis and clinical reporting among diagnostic laboratories in China. Front Genet 11:582637. https://doi.org/10.3389/fgene.2020.582637
Zhang W, Wang R, Fang H, Ma X, Li D, Liu T, Chen Z, Wang K, Hao S, Yu Z, Chang Z, Na C, Wang Y, Bai J, Zhang Y, Chen F, Li M, Chen C, Wei L, Li J, Chang X, Qu S, Yang L, Huang J (2021) Influence of low tumor content on tumor mutational burden estimation by whole-exome sequencing and targeted panel sequencing. Clin Transl Med 11(5):e415. https://doi.org/10.1002/ctm2.415
Zhang N, Zhang P, Chen Q, Zhou K, Liu Y, Wang H, **e Y, Ren L, Hou W, Yang J, Yu Y, Zheng Y, Shi L (2022) Quartet metabolite reference materials for assessing inter-laboratory reliability and data integration of metabolomic profiling. bioRxiv:2022.2011.2001.514762. https://doi.org/10.1101/2022.11.01.514762
Zheng Y, Liu Y, Yang J, Dong L, Zhang R, Tian S, Yu Y, Ren L, Hou W, Han J, Zhang L, Jiang H, Lin L, Lou J, Li R, Lin J, Liu H, Wang D, Dai F, Bao D, Cao Z, Chen Q, Chen Q, Chen X, Gao Y, Jiang H, Li B, Li B, Li J, Liu R, Qing T, Shang E, Shang J, Sun S, Wang H, Wang X, Zhang N, Zhang P, Zhang R, Zhu S, Scherer A, Gloerich J, Wang J, Wang J, Xu J, Hong H, **ao W, ** L, The Quartet Project Team, Ding C, Li J, Fang X, Tong W, Shi L (2023) Multi-omics data integration using ratio-based quantitative profiling with Quartet reference materials. Nat Biotechnol. https://doi.org/10.1038/s41587-023-01934-1
Zhou JY, Chen L, Zhang B, Tian Y, Liu T, Thomas SN, Chen L, Schnaubelt M, Boja E, Hiltke T, Kinsinger CR, Rodriguez H, Davies SR, Li S, Snider JE, Erdmann-Gilmore P, Tabb DL, Townsend RR, Ellis MJ, Rodland KD, Smith RD, Carr SA, Zhang Z, Chan DW, Zhang H (2017) Quality assessments of long-term quantitative proteomic analysis of breast cancer xenograft tissues. J Proteome Res 16(12):4523–4530. https://doi.org/10.1021/acs.jproteome.7b00362
Zook JM, Chapman B, Wang J, Mittelman D, Hofmann O, Hide W, Salit M (2014) Integrating human sequence data sets provides a resource of benchmark SNP and indel genotype calls. Nat Biotechnol 32(3):246–251. https://doi.org/10.1038/nbt.2835
Zook JM, Catoe D, McDaniel J, Vang L, Spies N, Sidow A, Weng Z, Liu Y, Mason CE, Alexander N, Henaff E, McIntyre AB, Chandramohan D, Chen F, Jaeger E, Moshrefi A, Pham K, Stedman W, Liang T, Saghbini M, Dzakula Z, Hastie A, Cao H, Deikus G, Schadt E, Sebra R, Bashir A, Truty RM, Chang CC, Gulbahce N, Zhao K, Ghosh S, Hyland F, Fu Y, Chaisson M, **ao C, Trow J, Sherry ST, Zaranek AW, Ball M, Bobe J, Estep P, Church GM, Marks P, Kyriazopoulou-Panagiotopoulou S, Zheng GX, Schnall-Levin M, Ordonez HS, Mudivarti PA, Giorda K, Sheng Y, Rypdal KB, Salit M (2016) Extensive sequencing of seven human genomes to characterize benchmark reference materials. Sci Data 3:160025. https://doi.org/10.1038/sdata.2016.25
Zook JM, McDaniel J, Olson ND, Wagner J, Parikh H, Heaton H, Irvine SA, Trigg L, Truty R, McLean CY, De La Vega FM, **ao C, Sherry S, Salit M (2019) An open resource for accurately benchmarking small variant and reference calls. Nat Biotechnol 37(5):561–566. https://doi.org/10.1038/s41587-019-0074-6
Zook JM, Hansen NF, Olson ND, Chapman L, Mullikin JC, **ao C, Sherry S, Koren S, Phillippy AM, Boutros PC, Sahraeian SME, Huang V, Rouette A, Alexander N, Mason CE, Hajirasouliha I, Ricketts C, Lee J, Tearle R, Fiddes IT, Barrio AM, Wala J, Carroll A, Ghaffari N, Rodriguez OL, Bashir A, Jackman S, Farrell JJ, Wenger AM, Alkan C, Soylev A, Schatz MC, Garg S, Church G, Marschall T, Chen K, Fan X, English AC, Rosenfeld JA, Zhou W, Mills RE, Sage JM, Davis JR, Kaiser MD, Oliver JS, Catalano AP, Chaisson MJP, Spies N, Sedlazeck FJ, Salit M (2020) A robust benchmark for detection of germline large deletions and insertions. Nat Biotechnol 38(11):1347–1355. https://doi.org/10.1038/s41587-020-0538-8
Acknowledgements
This study was supported in part by Shanghai Sailing Program (22YF1403500), the National Natural Science Foundation of China (32300536, 31720103909 and 32170657), the National Key R&D Project of China (2018YFE0201603 and 2018YFE0201600), State Key Laboratory of Genetic Engineering (SKLGE-2117), and the 111 Project (B13016). Some of the illustrations in this paper were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
Manuscript writing: LR, LS, and YZ. All authors approved this manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing financial interests.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Ethical Approval
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ren, L., Shi, L. & Zheng, Y. Reference Materials for Improving Reliability of Multiomics Profiling. Phenomics (2024). https://doi.org/10.1007/s43657-023-00153-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43657-023-00153-7