Abstract
Study design
Retrospective study.
Objective
To describe pathogens found in SSI during pediatric-instrumented spine surgery, and to assess the relationship between pathogens and the etiology of the spinal deformity.
Summary and background data
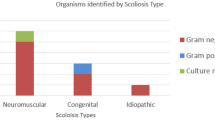
Surgical site infection (SSI) after pediatric spine fusion is a well-known complication with incidence rates between 0.5 and 42%, associated with the patient underlying disorder. Pathogens involved in SSI seem to be related to patient characteristics, such as the etiology of the spinal deformity. GNB (gram-negative bacilli) are more frequent in neuropathic, muscular, and syndromic conditions. High-risk pediatric patients with a spine deformity undergoing instrumented surgery might benefit from receiving perioperative intravenous prophylaxis for GNB.
Methods
We conducted a retrospective study at our tertiary-care pediatric hospital from January 2010 to January 2017. We reviewed records of all episodes of SSI that occurred in the first 12 months postoperatively. All patients who underwent instrumented spine surgery were included in this study.
Results
We assessed 1410 pediatric-instrumented spine surgeries; we identified 68 patients with deep SSIs, overall rate of 4.8%. Mean age at instrumented spine surgery was 12 years and 9 months. Time elapsed between instrumented surgery and debridement surgery was 28.8 days. Cultures were positive in 48 and negative in 20. Of the 48 positive culture results, 41 (72%) were GNB, 12 (21%) gram-positive cocci (GPC), three (5%) gram-positive anaerobic cocci (GPAC), and one (2%) coagulase-negative staphylococci (CoNS). Of the 68 patients with primary SSIs, 46 were considered to have a high risk of infection, which reported GNB in 81%, GPC in 15%, GPAC in 2%, and CoNS in 2%.
Conclusion
Cefazolin prophylaxis covers GPC and CoNS, but GNB with unreliable effectiveness. Gram-negative pathogens are increasingly reported in SSIs in high-risk patients. Adding prophylaxis for GNB in high-risk patients should be taken into account when considering spine surgery.
Level of evidence
IV.




Similar content being viewed by others
References
Glotzbecker MP, St Hilaire TA, Vitale MG, et al. (2017) Best practice guidelines for surgical site infection prevention with surgical treatment of early onset scoliosis and children’s spine study group; growing spine study group. J Pediatr Orthop. https://doi.org/10.1097/BPO.0000000000001079
Vitale MG, Lenke LG, Sponseller PD et al (2013) Building consensus: development of a best practice guideline (BPG) for surgical site infection (SSI) prevention in high-risk pediatric spine surgery. J Pediatr Orthop 33(5):471–478
Glotzbecker MP, Riedel MD, Saiman L et al (2013) What’s the Evidence? Systematic literature review of risk factors and preventive strategies for surgical site infection following pediatric spine surgery. J Pediatr Orthop 33(5):479–487
Glotzbecker MP, Akbarnia BA, Joshi A, et al. (2014) Surgeon practices regarding infection prevention for growth friendly spinal procedures. J Child Orthop 8:245–250
Maesani M, Doit C, Dahmani S et al (2016) Comparative microbiology of patients with idiopathic and nonidiopathic etiologies of spine deformity. Pediatr Infect Dis J 35(1):66–70
Labbé AC, Demers AM, Moore DL, et al. (2003) Surgical-site infection following spinal fusion: a case-control study in a children’s hospital. Infect Control Hosp Epidemiol 24(8):591–595
Perry JW, Montgomerie JZ, Maeder K, et al. (1997) Wound infections following spinal fusion with posterior segmental spinal instrumentation. Clin Infect Dis 24:558–561
Milstone AM, Sponseller P, Perl TM et al (2008) Timing of preoperative antibiotic prophylaxis. a modifiable risk factor for deep surgical site infections after pediatric spinal fusion. Pediatric Infectious Dis J 27(8):704–708
Vandenberg C, Niswander C, Garg S, et al. (2018) Compliance with a comprehensive antibiotic protocol improves infection incidence in pediatric spine surgery. J Pediatr Orthop 38:287–292
Li Y, Glotzbecker M, Hedequist D (2012) Surgical site infection after pediatric spinal deformity surgery. Curr Rev Musculoskelet Med 5:111–119
Cahill PJ, Warnick DE, Lee MJ, et al. (2010) Infection after spinal fusion for pediatric spinal deformity: 30 years of experience at a single institution. Spine 35:1211–1217
Vitale MG, Mackenzie WGS, Matsumoto H, et al. (2011) Surgical site infection following spinal instrumentation for scoliosis: lessons learned from a multi-center analysis of 1352 spinal instrumentation procedures for scoliosis. In: Presented at the scoliosis research society 46th annual meeting and course. Louisville, Kentucky
Sponseller PD, Shah SA, Abel MF, et al. (2010) Infection rate after spine surgery in cerebral palsy is high and impairs results: multicenter analysis of risk factors and treatment. Clin Orthop Relat Res 468:711–716
Linam WM, Margolis PA, Staat MA, et al. (2009) Risk factors associated with surgical site infection after pediatric posterior spinal fusion procedure. Infect Control Hosp Epidemiol 30:109–116
Smith JS, Shaffrey CI, Sansur CA, et al. (2011) Rates of infection after spine surgery based on 108,419 procedures: a report from the scoliosis research society morbidity and mortality committee. Spine 36:556–563
Sponseller PD, LaPorte DM, Hungerford MW, et al. (2000) Deep wound infections after neuromuscular scoliosis surgery: a multicenter study of risk factors and treatment outcomes. Spine 25:2461–2466
Aleissa S, Parsons D, Grant J, et al. (2011) Deep wound infection following pediatric scoliosis surgery: incidence and analysis of risk factors. Can J Surg 54:263–269
Ho C, Skaggs DL, Weiss JM, et al. (2007) Management of infection after instrumented posterior spine fusion in pediatric scoliosis. Spine 32:2739–2744
McLeod LM, Keren R, Dormans JP, et al. (2013) Perioperative antibiotic use for spinal surgery procedures in US children’s hospitals. Spine 38(7):609–616
Horan MP, Williams BA, Vitale, MG. (2011) Can infection following scoliosis instrumentation surgery be a ‘never event’?: Dramatic reduction in infection rates at one institution after implementation of a multimodal prevention protocol. Read at the Annual Meeting of the American Academy of Pediatrics, Boston
Hatlen T, Song K, Shurtleff D, et al. (2010) Contributory factors to postoperative spinal fusion complications for children with myelomeningocele. Spine (Phila Pa 1976) 35:1294–1299
Wright ML, Skaggs DL, Vitale MG, et al. (2016) Does the type of metal instrumentation affect the risk of surgical site infection in pediatric scoliosis surgery? Spine Deformity 4:206–210
Warner SJ, Uppstrom TJ, Perlman SL, et al. (2017) Epidemiology of deep surgical site infections after pediatric spinal fusion surgery. Spine 42(3):E163–E168
Abdul-Jabbar A, Berven SH, Hu SS, et al. (2013) Surgical site infections in spine surgery. Spine (Phila Pa 1976) 38:E1425–E1431
Farley FA, Li Y, Gilsdorf JR, et al. (2014) Postoperative spine and VEPTR infections in children: a case-control study. J Pediatr Orthop 34:14–21
Mackenzie WGS, Matsumoto H, Williams BA, et al. (2013) Surgical site infection following spinal instrumentation for scoliosis: a multi- center analysis of rates, risk factors, and pathogens. J Bone Joint Surg Am 95:800–806
Goede WJ, Lovely JK, Thompson RL, et al. (2013) Assessment of prophylactic antibiotic use in patients with surgical site infections. Hosp Pharm 48:560–567
Brook I, Frazier EH (2000) Aerobic and anaerobic microbiology of wound infection following spinal fusion in children. Pediatr Neurosurg 32:20–23
Weinstein MA, McCabe JP, Cammisa FP (2000) Postoperative spinal wound infection: a review of 2,391 consecutive index procedures. J Spinal Disord 13:422–426
Hahn F, Zbinden R, Min K (2005) Late implant infections caused by Propionibacterium acnes in scoliosis surgery. Eur Spine J 14:783–788
Ballard MR, Miller NH, Nyquist A, et al. (2012) A multidisciplinary approach improves infection rates in pediatric spine surgery. J Pediatr Orthop 32:266–270
Sponseller PD, Shah SA, Marks MC, et al. (2013) Deep wound infections after spinal fusion in children with cerebral palsy. A prospective cohort study. Spine 38(23):2023–2027
Croft LD, Pottinger JM, Herwaldt LA, et al. (2015) Risk factors for surgical site infections after pediatric spine operations. Spine 40(2):E112–E119
Gans I, Dormans JP, Baldwin KD, et al. (2013) Adjunctive vancomycin powder in pediatric spine surgery is safe. Spine 38(19):1703–1707
Sweet FA, Roh M, Sliva C (2011) Intrawound application of vancomycin for prophylaxis in instrumented thoracolumbar fusions: efficacy, drug levels, and patient outcomes. Spine (Phila Pa 1976) 36:2084–2088
Glotzbecker MP, Vitale MG, Flynn JM, et al. (2013) Surgeon practices regarding infection prevention for pediatric spinal surgery. On behalf of the POSNA committee on the quality, safety, value initiative (QSVI). J Pediatr Orthop. 33:694–699
Taketomo CK, Hodding JH, Kraus DM et al. (2016) Pediatric dosage handbook, 16th, Ed. pag 302–315
Vigilancia de la Resistencia a los Antimicrobianos. Red WHONET- Red SIREVA II Argentina: 2010–2018 parcial. Servicio Antimicrobianos. Laboratorio Nacional de Referencia en Resistencia a los Antimicrobianos Instituto Nacional de Enfermedades Infecciosas—ANLIS « Dr. Carlos G. Malbrán » . Buenos Aires. Argentina. 2018
Acknowledgment
We thank the Infections Control Specialists for the retrospective evaluation of SSIs. None of the authors has any conflict of interest to report related to this study.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Design of study: LP, CAT, RR, MN. Consulting references: LP, IAFW, EG, ESB. Participation in draft manuscript: LP, IAFW, MN, EG, ESB. Revise manuscript: IAFW, CAT, RR, ESB, MN. Approved the final version of the paper: LP, CAT, RR, IAFW, EG, ESB, MN
Corresponding author
Ethics declarations
Conflicts of interest
No conflicts of interest received during the conduct of this study
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
IRB: The study was approved by the hospital Institutional Review Board (IRB), because of the retrospective observational nature of the study IRB waived the informed consent.
Rights and permissions
About this article
Cite this article
Piantoni, L., Tello, C.A., Remondino, R.G. et al. Antibiotic prophylaxis in high-risk pediatric spine surgery: Is cefazolin enough?. Spine Deform 8, 669–676 (2020). https://doi.org/10.1007/s43390-020-00092-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43390-020-00092-7