Abstract
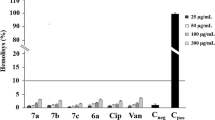
This study describes the discovery of a variety of quinoline2-one derivatives with significant antibacterial action vs a spectrum of multidrug-resistant Gram-positive bacterial strains, especially methicillin-resistant Staphylococcus aureus (MRSA). Compounds 6c, 6l, and 6o exhibited significant antibacterial activity versus the Gram-positive bacterial pathogens evaluated. In comparison to the reference daptomycin, compound 6c demonstrated the most effective activity among the assessed derivatives, with MIC concentrations of 0.75 μg/mL versus MRSA and VRE and 2.50 μg/mL against MRSE. We also reported on these compounds’ biofilm and dihydrofolate reductase inhibitory activities. Compound 6c showed the greatest antibiofilm action in a dose-dependent way and a substantial decrease of biofilm development in the MRSA ACL51 strain at concentrations of 0.5, 0.25, and 0.12 MIC, with reductions of 79%, 55%, and 38%, consecutively, whereas the corresponding values for vancomycin were 20%, 12%, and 9%. These findings imply that these quinoline compounds could be used to develop a new category of antibiotic representatives to prevent Gram-positive drug-resistant bacterial strains.


Similar content being viewed by others
References
Lomazzi M, Moore M, Johnson A, Balasegaram M, Borisch B (2019) Antimicrobial resistance–moving forward? BMC pub health 19:1–6
Chokshi A, Sifri Z, Cennimo D, Horng H (2019) Global contributors to antibiotic resistance. J Global Infect Dis 11(1):36–42
de Kraker ME, Stewardson AJ, Harbarth S (2016) Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med 13(11):e1002184
Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Aguilar GR, Gray A et al (2022) Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399(10325):629–655
Gwynn MN, Portnoy A, Rittenhouse SF, Payne DJ (2010) Challenges of antibacterial discovery revisited. Ann N Y Acad Sci 1213(1):5–19
Abushaheen MA, Fatani AJ, Alosaimi M, Mansy W, George M, Acharya S et al (2020) Antimicrobial resistance, mechanisms and its clinical significance Disease-a-Month. 66(6):100971
Rello J, Bunsow E, Perez A (2016) What if there were no new antibiotics? A look at alternatives. Expert Rev Clin Pharmacol 9(12):1547–1555
Askari S, B, Kra**ovic M. (2010) Dihydrofolate reductase gene variations in susceptibility to disease and treatment outcomes. Curr Genomics 11(8):578–583
Eshetie S, Gizachew M, Dagnew M, Kumera G, Woldie H, Ambaw F et al (2017) Multidrug resistant tuberculosis in Ethiopian settings and its association with previous history of anti-tuberculosis treatment: a systematic review and meta-analysis. BMC Infect Dis 17:1–12
Watkins RR, David MZ, Salata RA (2012) Current concepts on the virulence mechanisms of meticillin-resistant Staphylococcus aureus. J Med Microbiol 61(Pt 9):1179
Krute CN (2015) Investigation of post-translational modifications in Staphylococcus aureus. University of South Florida
Klein E, Smith DL, Laxminarayan R (2007) Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis 13(12):1840
Harris SJ, Cormican M, Cummins E (2012) Antimicrobial residues and antimicrobial-resistant bacteria: impact on the microbial environment and risk to human health—a review. Hum Ecol Risk Assess Int J 18(4):767–809
Gill SR, Fouts DE, Archer GL, Mongodin EF, DeBoy RT, Ravel J et al (2005) Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J Bacteriol 187(7):2426–2438
Cetinkaya Y, Falk P, Mayhall CG (2000) Vancomycin-resistant enterococci. Clin Microbiol Rev 13(4):686–707
Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK et al (2013) Health care–associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med 173(22):2039–2046
Feng T, Lu H, Ye X, Nie C, Zhang J, Yu L et al (2022) Selective inactivation of Gram-positive bacteria in vitro and in vivo through metabolic labelling. Sci China Mater 65(1):237–245
Kara Ali R, Surme S, Balkan II, Salihoglu A, Sahin Ozdemir M, Ozdemir Y et al (2020) An eleven-year cohort of bloodstream infections in 552 febrile neutropenic patients: resistance profiles of Gram-negative bacteria as a predictor of mortality. Ann Hematol 99:1925–1932
Teng P, Huo D, Nimmagadda A, Wu J, She F, Su M et al (2016) Small antimicrobial agents based on acylated reduced amide scaffold. J Med Chem 59(17):7877–7887
Stokes JM, MacNair CR, Ilyas B, French S, Côté J-P, Bouwman C et al (2017) Pentamidine sensitizes Gram-negative pathogens to antibiotics and overcomes acquired colistin resistance. Nat Microbiol 2(5):1–8
O'Connell KM, Hodgkinson JT, Sore HF, Welch M, Salmond GP, Spring DR (2013) Combating multidrug-resistant bacteria: current strategies for the discovery of novel antibacterials. Angewandte Chemie Int Ed 52(41):10706–10733
Ling LL, Schneider T, Peoples AJ, Spoering AL, Engels I, Conlon BP et al (2015) A new antibiotic kills pathogens without detectable resistance. Nature 517(7535):455–459
Lin S, Koh J-J, Aung TT, Lim F, Li J, Zou H et al (2017) Symmetrically substituted xanthone amphiphiles combat gram-positive bacterial resistance with enhanced membrane selectivity. J Med Chem 60(4):1362–1378
LaMarche MJ, Leeds JA, Brewer J, Dean K, Ding J, Dzink-Fox J et al (2016) Antibacterial and solubility optimization of thiomuracin A. J Med Chem 59(14):6920–6928
Aly AA, Ramadan M, Abuo-Rahma GE-DA, Elshaier YAMM, Elbastawesy MAI, Brown AB, et al. Chapter three-quinolones as prospective drugs: their syntheses and biological applications. Adv Heterocy Chem. 135: Academic Press; (2021).147-196.
Dib M, Ouchetto H, Ouchetto K, Hafid A, Khouili M (2021) Recent Developments of quinoline derivatives and their potential biological activities. Curr Org Synth 18(3):248–269
Srivastava V, Singh PK, Tivari S, Singh PP. Visible light photocatalysis in the synthesis of pharmaceutically relevant heterocyclic scaffolds. Organic Chemistry Front 2022;9(5):1485-1507.
Ajani OO, Iyaye KT, Ademosun OT (2022) Recent advances in chemistry and therapeutic potential of functionalized quinoline motifs - a review. RSC Adv 12(29):18594–18614
Matada BS, Pattanashettar R, Yernale NG (2021) A comprehensive review on the biological interest of quinoline and its derivatives. Bioorg Med Chem 32:115973
Al-Jumaily E, Latif A, Al-Bayati R (2016) Effect of a new quinoline-2-one derivatives (compound 3) on purified DNA gyrase from clinical isolate Pseudomonas aeruginosa PA31. IOSR J Pharmacy 12:12–18
Proisl K, Kafka S, Kosmrlj J (2017) Chemistry and applications of 4-hydroxyquinolin-2-one and quinoline-2, 4-dionebased compounds. Curr Org Chem 21(19):1949–1975
Estradé O, Vozmediano V, Carral N, Isla A, González M, Poole R et al (2022) Key factors in effective patient-tailored dosing of fluoroquinolones in urological infections: interindividual pharmacokinetic and pharmacodynamic variability. Antibiotics 11(5):641–652
Millanao AR, Mora AY, Villagra NA, Bucarey SA, Hidalgo AA (2021) Biological effects of quinolones: a family of broad-spectrum antimicrobial agents. Molecules 26(23):7153–7163
Fair RJ, Tor Y (2014) Antibiotics and bacterial resistance in the 21st century. Perspect med chem 6; PMC. S14459
Hooper DC, Jacoby GA (2015) Mechanisms of drug resistance: quinolone resistance. Ann N Y Acad Sci 1354(1):12–31
Elbastawesy MA, Mohamed FA, Zaki I, Alahmdi MI, Alzahrani SS, Alzahrani HA et al (2023) Design, synthesis and antimicrobial activity of novel quinoline-2-one hybrids as promising DNA gyrase and topoisomerase IV inhibitors. J Mol Struct 1278:134902
Youssif BGM (2013) Synthesis and biological evaluation of some new coumarin derivatives as antimicrobial agents. Bullet Pharmaceut Sci Assiut 36(2):105–116
Youssif B, Abdel-Moty SG, Sayed IM (2014) Synthesis and biological evaluation of some novel 1, 2, 3-triazol-N-arylidene Acetohydrazide incorporating benzimidazole ring moiety as potential antimicrobial agents. J Curr Chem Pharm Sci 4:54–64
Hofny HA, Mohamed MF, Gomaa HA, Abdel-Aziz SA, Youssif BG, El-Koussi NA et al (2021) Design, synthesis, and antibacterial evaluation of new quinoline-1, 3, 4-oxadiazole and quinoline-1, 2, 4-triazole hybrids as potential inhibitors of DNA gyrase and topoisomerase IV. Bioorg Chem 112:104920
Frejat FOA, Cao Y, Zhai H, Abdel-Aziz SA, Gomaa HA, Youssif BG et al (2022) Novel 1, 2, 4-oxadiazole/pyrrolidine hybrids as DNA gyrase and topoisomerase IV inhibitors with potential antibacterial activity. Arab J Chem 15(1):103538
Frejat FOA, Zhai H, Cao Y, Wang L, Mostafa YA, Gomaa HA et al (2022) Novel indazole derivatives as potent apoptotic antiproliferative agents by multi-targeted mechanism: synthesis and biological evaluation. Bioorg Chem 126:105922
Al-Wahaibi LH, Amer AA, Marzouk AA, Gomaa HA, Youssif BG, Abdelhamid AA (2021) Design, synthesis, and antibacterial screening of some novel heteroaryl-based ciprofloxacin derivatives as DNA gyrase and topoisomerase IV inhibitors. Pharmaceut 14(5):399
Abdelhameed RM, Abu-Elghait M, El-Shahat M (2020) Hybrid three MOFs composites (ZIF-67@ ZIF-8@ MIL-125-NH2): enhancement the biological and visible-light photocatalytic activity. J Environ Chem Eng 8(5):104107
Rashid N, Thapliyal C, Chattopadhyay PC (2016) Dihydrofolate reductase as a versatile drug target in healthcare. J Proteins Proteom 7(4)
Gomaa HA, Shaker ME, Alzarea SI, Hendawy O, Mohamed FA, Gouda AM et al (2022) Optimization and SAR investigation of novel 2, 3-dihydropyrazino [1, 2-a] indole-1, 4-dione derivatives as EGFR and BRAFV600E dual inhibitors with potent antiproliferative and antioxidant activities. Bioorg Chem 120:105616
Gomaa HA, El-Sherief HA, Hussein S, Gouda AM, Salem OI, Alharbi KS et al (2020) Novel 1, 2, 4-triazole derivatives as apoptotic inducers targeting p53: synthesis and antiproliferative activity. Bioorg Chem 105:104369
Acknowledgements
Professor Dr. Bahaa G. M. Youssif, and Dr. Elbastawesy M.A., Pharmaceutical Organic Chemistry Department, Faculty of Pharmacy, Assiut University, Assiut, Egypt, are gratefully acknowledged for providing compounds 6c, 6i, 6l, and 6o for biological assays.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Responsible Editor: Ilana Camargo
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alzahrani, H.A. Quinoline-2-one derivatives as promising antibacterial agents against multidrug-resistant Gram-positive bacterial strains. Braz J Microbiol 54, 2799–2805 (2023). https://doi.org/10.1007/s42770-023-01132-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-023-01132-w