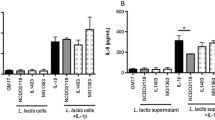
Abstract
Inflammatory bowel diseases (IBD) are gastrointestinal disorders characterized by a breakdown in intestinal homeostasis by inflammatory immune responses to luminal antigens. Novel strategies for ameliorating IBD have been proposed in many studies using animal models. Our group has demonstrated that administration of Lactococcus lactis NCDO 2118 can improve clinical parameters of colitis induced by oral administration of dextran sulphate sodium (DSS). However, it is not clear whether other strains of L. lactis can yield the same effect. The objective of present study was to analyze the effects of three different L. lactis strains (NCDO2118, IL1403 and MG1363) in the development of DSS-induced colitis in C57BL/6 mice. Acute colitis was induced in C57/BL6 mice by the administration of 2% DSS during 7 consecutive days. Body weight loss and shortening of colon length were observed in DSS-treated mice, and none of L. lactis strains had an impact in these clinical signs of colitis. On the other hand, all strains improved the global macroscopical disease index and prevented goblet cells depletion as well as the increase of intestinal permeability. TNF-α production was reduced in gut mucosa of L. lactis DSS-treated mice indicating a modulation of a critical pro-inflammatory response by all strains tested. However, only L. lactis NCDO2118 and MG1363 induced a higher frequency of CD11c+CD11b−CD103+ tolerogenic dendritic cells in lymphoid organs of mice at steady state. We conclude that all tested strains of L. lactis improved the clinical scores and parameters of colitis, which confirm their anti-inflammatory properties in this model of colitis.
Graphical abstract






Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Khor B, Gardet A, Xavier RJ (2011) Genetics and pathogenesis of inflammatory bowel disease. Nature [Internet]. 474:307–17. http://www.nature.com/articles/nature10209
De Moreno De Leblanc A, Del Carmen S, Chatel JM, Miyoshi A, Azevedo V, Langella P et al (2015) Current review of genetically modified lactic acid bacteria for the prevention and treatment of colitis using murine models. Gastroenterol Res Pract 2015. https://doi.org/10.1155/2015/146972
Melgar S, Bjursell M, Gerdin AK, Svensson L, Michaëlsson E, Bohlooly-Y M (2007) Mice with experimental colitis show an altered metabolism with decreased metabolic rate. Am J Physiol - Gastrointest Liver Physiol 292:165–172
Basso PJ, Fonseca MTC, Bonfá G, Alves VBF, Sales-Campos H, Nardini V et al (2014) Association among genetic predisposition, gut microbiota, and host immune response in the etiopathogenesis of inflammatory bowel disease. Brazilian J Med Biol Res 47:727–737
Mowat AMI (2003) Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol 3:331–341
Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M (2015) Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol 27:1–19
Melgar S, Karlsson A, Michaëlsson E (2005) Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: Correlation between symptoms and inflammation. Am J Physiol - Gastrointest Liver Physiol 288:1328–1338
Okayasu ISA (1990) Reliable experimental acute and chronic 694–702
Abraham BP, Quigley EMM (2017) Probiotics in inflammatory bowel disease. Gastroenterol Clin North Am [Internet]. Elsevier Inc;46:769–82. https://doi.org/10.1016/j.gtc.2017.08.003
FAO/WHO (2002) Guidelines for the evaluation of probiotics in food 1–11
Ishida T, Yokota A, Umezawa Y, Toda T, Yamada K (2005) Identification and characterization of lactococcal and Acetobacter strains isolated from traditional Caucasusian fermented milk. J Nutr Sci Vitaminol (Tokyo) 51:187–193
Watanabe T, Nishio H, Tanigawa T, Yamagami H, Okazaki H, Watanabe K et al (2009) Probiotic Lactobacillus casei strain Shirota prevents indomethacin-induced small intestinal injury: Involvement of lactic acid. Am J Physiol - Gastrointest Liver Physiol 297:506–513
Tang Y, Wu Y, Huang Z, Dong W, Deng Y, Wang F et al (2017) Administration of probiotic mixture DM#1 ameliorated 5-fluorouracil–induced intestinal mucositis and dysbiosis in rats. Nutrition [Internet]. Elsevier Inc. 33:96–104. https://doi.org/10.1016/j.nut.2016.05.003
Zhang F, Li Y, Wang X, Wang S, Bi D (2019) The Impact of lactobacillus plantarum on the gut microbiota of mice with DSS-induced colitis. Biomed Res Int Hindawi 2019. https://doi.org/10.1155/2019/3921315
Chen Y, ** Y, Stanton C, Paul Ross R, Zhao J, Zhang H et al (2020) Alleviation effects of Bifidobacterium breve on DSS-induced colitis depends on intestinal tract barrier maintenance and gut microbiota modulation. Eur J Nutr [Internet]. Springer Berlin Heidelberg. https://doi.org/10.1007/s00394-020-02252-x
Berlec A, Perše M, Ravnikar M, Lunder M, Erman A, Cerar A et al (2017) Dextran sulphate sodium colitis in C57BL/6J mice is alleviated by Lactococcus lactis and worsened by the neutralization of Tumor necrosis Factor α. Int Immunopharmacol 43:219–226
Papadimitriou K, Pot B, Tsakalidou E (2015) How microbes adapt to a diversity of food niches. Curr Opin Food Sci [Internet]. Elsevier Ltd;;2:29–35. https://doi.org/10.1016/j.cofs.2015.01.001
Mancha-Agresti P, Drumond MM, Carmo FLR do, Santos MM, Santos JSC dos, Venanzi F et al (2017) A new broad range plasmid for DNA delivery in eukaryotic cells using lactic acid bacteria: in vitro and in vivo assays. Mol Ther - Methods Clin Dev [Internet]. Elsevier Ltd.;4:83–91. https://doi.org/10.1016/j.omtm.2016.12.005
Donohue DC, Gueimonde M (2011) Some considerations for the safety of novel probiotic bacteria. Lact Acid Bact Microbiol Funct Asp Fourth Ed 423–38
Song AAL, In LLA, Lim SHE, Rahim RA (2017) A review on Lactococcus lactis: from food to factory. Microb Cell Fact BioMed Central 16:1–15
Nishitani Y, Tanoue T, Yamada K, Ishida T, Yoshida M, Azuma T et al (2009) Lactococcus lactis subsp. cremoris FC alleviates symptoms of colitis induced by dextran sulfate sodium in mice. Int Immunopharmacol [Internet]. Elsevier B.V.;9:1444–51. https://doi.org/10.1016/j.intimp.2009.08.018
Luerce TD, Gomes-Santos AC, Rocha CS, Moreira TG, Cruz DN, Lemos L et al (2014) Anti-inflammatory effects of Lactococcus lactis NCDO 2118 during the remission period of chemically induced colitis. Gut Pathog 6:1–11
Gusmao-Silva G, Aguiar SLF, Miranda MCG, Guimarães MA, Alves JL, Vieira AT et al (2020) Hsp65-producing lactococcocus lactis prevents antigen-induced arthritis in mice. Front Immunol 11:1–15
Stiles ME, Holzapfel WH (1997) Lactic acid bacteria of foods and their current taxonomy. Int J Food Microbiol 36:1–29
de Moreno de LeBlanc A, del Carmen S, Zurita-Turk M, Santos Rocha C, van de Guchte M, Azevedo V et al (2011) Importance of IL-10 modulation by probiotic microorganisms in gastrointestinal inflammatory diseases. ISRN Gastroenterol 2011:1–11
Oliveira LC, Saraiva TDL, Soares SC, Ramos RTJ, Sá PHCG, Carneiro AR et al (2014) Genome Sequence of Lactococcus lactis subsp. lactis NCDO 2118, a GABA-Producing Strain. Genome Announc 2:9–10
Bolotin A, Wincker P, Mauger S, Jaillon O, Malarme K, Weissenbach J et al (2001) The complete genome sequence of the lactic acid bacterium lactococcus lactis ssp. lactis IL1403. Genome Res 11:731–53
Wegmann U, O’Connell-Motherway M, Zomer A, Buist G, Shearman C, Canchaya C et al (2007) Complete genome sequence of the prototype lactic acid bacterium Lactococcus lactis subsp. cremoris MG1363. J Bacteriol 189:3256–70
Cooper HS, Murthy SN, Shah RS, Sedergran DJ (1993) Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest [Internet]. 69:238—249. http://europepmc.org/abstract/MED/8350599
Diniz SOF, Barbosa AJA, Araújo ID, Nelson DL, da Machado LAS, Filho MB et al (2005) Assessment of bacterial translocation in obstructive jaundice using Tc-99m Escherichia coli. Brazilian Arch Biol Technol 48:45–9
Maioli TU, De Melo SB, Dias MN, Paiva NC, Cardoso VN, Fernandes SO et al (2014) Pretreatment with Saccharomyces boulardii does not prevent the experimental mucositis in Swiss mice. J Negat Results Biomed 13:1–8
Generoso SDV, Rodrigues NM, Trindade LM, Paiva NC, Cardoso VN, Carneiro CM et al (2015) Dietary supplementation with omega-3 fatty acid attenuates 5-fluorouracil induced mucositis in mice. Lipids Health Dis [Internet]. Lipids in Health and Disease.14:1–10. https://doi.org/10.1186/s12944-015-0052-z
McCafferty DM, Sihota E, Muscara M, Wallace JL, Sharkey KA, Kubes P (2000) Spontaneously develo** chronic colitis in IL-10/iNOS double-deficient mice. Am J Physiol - Gastrointest Liver Physiol 279:90–99
De Matos OG, Amaral SS, Pereira Da Silva PEM, Perez DA, Alvarenga DM, Ferreira AVM et al (2012) Dietary supplementation with omega-3-pufa-rich fish oil reduces signs of food allergy in ovalbumin-sensitized mice. Clin Dev Immunol 2012. https://doi.org/10.1155/2012/236564
Gomes-Santos AC, Moreira TG, Castro-Junior AB, Horta BC, Lemos L, Cruz DN et al (2012) New insights into the immunological changes in IL-10-deficient mice during the course of spontaneous inflammation in the gut mucosa. Clin Dev Immunol 2012. https://doi.org/10.1155/2012/560817
Herzenberg LA, Tung J, Moore WA, Herzenberg LA, Parks DR (2006) Interpreting flow cytometry data: a guide for the perplexed - Supplementary Information. Nat Immunol 7:681–685
Poritz LS, Garver KI, Green C, Fitzpatrick L, Ruggiero F, Koltun WA (2007) Loss of the tight junction protein ZO-1 in Dextran sulfate sodium induced colitis. J Surg Res 140:12–19
O’Sullivan DJ (2001) Screening of intestinal microflora for effective probiotic bacteria. J Agric Food Chem 49:1751–1760
Brandtzaeg P (1998) Development and basic mechanisms of human gut immunity. Nutr Rev 56
Muzaki ARBM, Tetlak P, Sheng J, Loh SC, Setiagani YA, Poidinger M et al (2016) Intestinal CD103+ CD11b- dendritic cells restrain colitis via IFN-γ-induced anti-inflammatory response in epithelial cells. Mucosal Immunol 9:336–351
Annacker O, Coombes JL, Malmstrom V, Uhlig HH, Bourne T, Johansson-Lindbom B et al (2005) Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med 202:1051–1061
Coombes JL, Siddiqui KRR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y et al (2007) A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β -and retinoic acid-dependent mechanism. J Exp Med 204:1757–1764
Mercadante ACT, Perobelli SM, Alves APG, Gonçalves-Silva T, Mello W, Gomes-Santos AC et al (2014) Oral combined therapy with probiotics and alloantigen induces B Cell–dependent long-lasting specific tolerance. J Immunol 192:1928–1937
Forkel M, Mjösberg J (2016) Dysregulation of group 3 Innate lymphoid cells in the pathogenesis of inflammatory bowel disease. Curr Allergy Asthma Rep [Internet]. Curr Allergy Asthma Rep 16. https://doi.org/10.1007/s11882-016-0652-3
Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W (1993) Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75:263–274
Mosser DM, Edwards JP (2009) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8:958–969
Aziz M, Holodick NE, Rothstein TL, Wang P (2015) The Role of B-1 Cells in Inflammation. Immunol Res 63:153–66
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B et al (2014) Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514
Koelink PJ, Bloemendaal FM, Li B, Westera L, Vogels EWM, van Roest M, Gloudemans AK, van’tWout AB, Korf H, Vermeire S, Te Velde AA, Ponsioen CY, D’Haens GR, Verbeek JS, Geiger TL, Wildenberg ME, van den Brink GR (2020) Anti-TNF therapy in IBD exerts its therapeutic effect through macrophage IL-10 signalling. Gut 69(6):1053–1063. https://doi.org/10.1136/gutjnl-2019-318264
Acknowledgements
The authors would like to thank Ilda Marçal de Souza and Hermes Oliveira for the excellent care of the animals.
Funding
This study was financially supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnologico), FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais), and PRPq-UFMG (Pró Reitoria de Pesquisa da UFMG), Brazil. Some of the authors are recipients of scholarships (J.L.A., L.L, M.C.C., A.C.G-S.) from CNPq and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), Brazil, and research fellowships (A.M.C.F., D.C.C., V.A., T.U.M.) from CNPq, Brazil.
Author information
Authors and Affiliations
Contributions
Experimental procedures were performed by JLA, LL, NMR, MCC, PAVB, VBP and MG. Data analysis was done by JLA, LL, NMR, PAVB, DCC, TUM and ACGS. Bacteria Strains were provided by AM, VAA and VBP. Manuscript writing was prepared by JLA and AMCF. Manuscript revision was performed by JLA and AMCF.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
All authors consent to participate in this publication.
Consent for publication
All authors consent to publish the manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Mariana X Byndloss
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alves, J.L., Lemos, L., Rodrigues, N.M. et al. Immunomodulatory effects of different strains of Lactococcus lactis in DSS-induced colitis. Braz J Microbiol 54, 1203–1215 (2023). https://doi.org/10.1007/s42770-023-00928-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-023-00928-0