Abstract
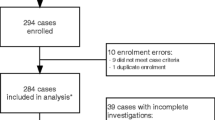
Central nervous system (CNS) infections comprise life-threatening clinical conditions in domestic species, and are commonly related to severe sequelae, disability, or high fatality rates. A set of bacterial pathogens have been identified in central nervous infections in livestock and companion animals, although the most of descriptions are restricted to case reports and a lack of comprehensive studies involving CNS-related bacterial infections have been focused on a great number of domestic species. In this scenario, we retrospectively investigated selected epidemiological data, clinical findings, bacteriological culture, and in vitro susceptibility patterns of 136 nonrepetitive neurologic cases in domestic species (2005–2021). Bacterial isolates were recovered from 25% (34/136) of the cerebrospinal fluid (CSF) sampled. The isolates were obtained from cattle (9/136 = 6.6%), dogs (7/136 = 5.1%), horses (6/136 = 4.4%), goats (3/136 = 2.2%), pigs (3/136 = 2.2%), sheep (3/136 = 2.2%), cats (2/136 = 1.5%), and asinine (1/136 = 0.7%). Among animals with bacterial isolation, Staphylococcus aureus (6/34 = 17.6%), Escherichia coli (5/34 = 14.7%), Staphylococcus beta-hemolytic (5/34 = 14.7%), and Trueperella pyogenes (3/34 = 8.8%) were predominant, in addition to a miscellaneous of other bacteria isolated in minor frequency, e.g., Corynebacterium pseudotuberculosis, Enterobacter cloacae, Mannheimia haemolytica, Pseudomonas aeruginosa, and Streptococcus equi subsp. equi. In vitro susceptibility tests of isolates revealed that amoxicillin/clavulanic acid (11/13 = 84.6%), cephalexin (9/11 = 81.8%), and florfenicol (9/12 = 75%) were the most effective antimicrobials. Conversely, isolates exhibited resistance mainly to tetracycline (6/10 = 60%), penicillin (6/11 = 54.5%), and trimethoprim/sulfamethoxazole (5/11 = 45.5%). Also, multidrug resistance to ≥ 3 classes of antimicrobials was found in 23.5% (8/34) strains. Data relative to the outcome was available in 79.4% (27/34) of animals that had bacterial isolation, and from these, the lethality rate was 92.6% (25/27). Incoordination (14/34 = 41.2%), recumbency (11/34 = 32.4%), apathy (10/34 = 29.4%), anorexia (9/34 = 26.5%), blindness (7/34 = 20.6%), seizure (6/34 = 17.6%), limb paresis (5/34 = 14.7%), head-pressing (4/34 = 11.8%), and nystagmus (3/34 = 8.8%) were the most frequent clinical signs. A variety of bacterial pathogens were identified in the CSF of domestic species showing neurologic signs, with a predominance of staphylococci, streptococci, and enterobacteria. High lethality of cases, poor in vitro efficacy of conventional antimicrobials, and a high in vitro multidrug resistance pattern of isolates were seen. Our results contribute to etiological characterization, antimicrobial resistance patterns, and clinical-epidemiological findings of bacterial infections in domestic species with neurological signs.
Similar content being viewed by others
References
Callanan J (2021) Meningitis, encephalitis, and encephalomyelitis in animals. Aiello, S. (Ed.) In: MSD Veterinary Manual. Merck & Co., Ney Jersey, USA. Available: https://www.msdvetmanual.com/nervous-system/meningitis,-encephalitis,-and-encephalomyelitis/meningitis,-encephalitis,-and-encephalomyelitis-in-animals
Hertzsch R, Richter A (2022) Systematic review of the pharmacological evidence for the selection of antimicrobials in bacterial infections of the central nervous system in the dogs and cats. Front Vet Sci 8:1–17
Giovane R, Lavender PD (2018) Central nervous system infections. Prim Care: Clin Off Pract 45:505–518
Bystritsky RJ, Chow FC (2022) Infectious meningitis and encephalitis. Neurol Clin 40:77–91
Viu J, Monreal L, Jose-Cunilleras E, Cesarini C, Añor S, Armengou L (2012) Clinical findings in 10 foals with bacterial meningoencephalitis. Equine Vet J 44, suppl(41):100–104
Carvalho PC, Eckstein C, de Moura LL, Heleno NVR, da Silva LA, Santos DO, Souza LR, Oliveira AR, Xavier RGC, Thompson M, Silva ROS, Santos RL (2022) Staphylococcus aureus-induced pyogranulomatous dermatitis, osteomyelitis, and meningitis with Splendore-Hoeppli reaction in a cat coinfected with the feline leukemia virus and Leishmania sp. Rev Bras Patol Anim 15:31–37
Salat J, Ruzek D (2020) Tick-borne encephalitis in domestic animals. Acta Virol 64:226–232
Elbert JA, Rissi DR (2022) Neuropathologic changes associated with systematic bacterial infection in 28 dogs. J Vet Diagn Invest 34:752–756
Messer JS, Wagner SO, Baumwart RD, Colitz CM (2008) A case of canine streptococcal meningoencephalitis diagnosed using universal bacterial polymerase chain reaction. J Anim Hosp Assoc 44:205–209
Komatsu T, Yoshida E, Shigenaga A, Yasuie N, Uchiyama S, Takamura Y, Sugie K, Kimura K, Haritani M, Shibahara T (2021) Fatal suppurative meningoencephalitis caused by Klebsiella pneumoniae in two calves. J Vet Med Sci 83:1113–1119
Ribeiro MG, Risseti RM, Bolaños CAD, Caffaro KA, Morais ACB, Lara GHB, Zamprogna TO, Paes AC, Listoni FJP, Franco MMF (2015) Trueperella pyogenes multispecies infections in domestic animals: a retrospective study of 144 cases (2002 to 2012). Vet Q 35:82–87
Messer JS, Kegge SJ, Cooper ES, Colitz CMH, Abramson CJ (2006) Meningoencephalitis caused by Pasteurella multocida in a cat. J Vet Intern Med 20:1033–1036
Cecco BS, Carossino M, Piero FD, Wakamatsu N, Mitchell MS, Fowlkes NW, Langohr IM (2021) Meningoencephalomyelitis in domestic cats: 3 cases of Pasteurella multocida infection and literature review. J Vet Diagn Invest 33:1156–1162
Wang W, Cai M, Hu J, Zhang Z, Wang X, Chang X, Zhang F, Guo C, Wang X (2020) Mechanisms of blood-brain barrier disruption by an Escherichia coli from lambs with severe diarrhea and meningoencephalitis. Microb Pathog 147:e104288
Wu CC, Chang YP (2015) Cerebral ventriculitis associated with otogenic meningoencephalitis in a dog. J Am Anim Hosp Associatinon 51:272–278
Rupprecht CE, Mani RS, Mshelbwala PP, Recuenco SE, Ward MP (2022) Rabies in the Tropics. Emerg Trop Dis 9:28–39
Iwanaga M, Imai N, Kamikawa A, Shimada K, Okura M, Takamatsu D, Ueda D, Nakayama M, Shibahara T (2022) Suppurative meningoencephalitis and perineuritis caused by Streptococcus gallolyticus in a Japanese black calf. J Vet Med Sci 84:53–58
Ribeiro MG, Morais ABC, Alves AC, Bolaños CAD, de Paula CL, Portilho FVRP, Nardi Júnior G, Lara GHB, Martins LSA, Moraes LS, Risseti RM, Guerra ST, Bello TS, Siqueira AK, Bertolini AB, Rodrigues CA, Paschoal NR, Almeida BO, Listoni FJP, Sánchez LFG, Paes AC (2022) Klebsiella-induced infections in domestic species: a case-series study in 697 animals (1997–2019). Braz J Microbiol 53:455–464
Johnson PJ, Constantinescu GM (2000) Collection of cerebrospinal fluid of horses. Equine Vet Educ 12:7–12
Rusbridge C (1997) Collection and interpretation of cerebrospinal fluid in cats and dogs. In Pract 19:322–331
Quinn PJ, Markey BK, Leonard FC, Fitzpatrick ES, Fanning S, Hartigan PJ (2011) Veterinary microbiology and microbial disease, 2nd edn. Wiley-Blackwell, Chichester, West Sussex, UK
Clinical and Laboratory Standards Institute-CLSI (2020a) Performance standards for antimicrobial disk and dilution susceptibility test for bacteria isolated from animals (CLSI VET 015), 5th edn. Wayne, p 250
Clinical and Laboratory Standards Institute-CLSI (2020b) Performance standards of antimicrobial susceptibility testing, 30th edn. Wayne, p 332
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Kahlmeter HJFG, Liljequist BO, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–81
Gelman A, Jakulin A, Pittau MG, Su Y (2008) A weakly informative default prior distribution for logistic and other regression models. Ann Appl Stat 2:1360–1383
Olivo G, Lucas TM, Borges AS, Silva ROS, Lobato FCF, Siqueira AK, Leite DS, Brandão PE, Gregori F, Oliveira-Filho JP, Takai S, Ribeiro MG (2016) Enteric pathogens and coinfections in foals with and without diarrhea. Biomed Res Int e2016:1–12
Siqueira AK, Ribeiro MG, Leite DS, Tiba MR, Moura C, Lopes MD, Prestes NC, Salerno T, da Silva AV (2009) Virulence factors in Escherichia coli strains isolated from urinary tract infection and pyometra cases and from feces of healthy dogs. Res Vet Sci 86:206–210
Guerra ST, Orsi H, Joaquim SF, Guimarães FF, Lopes BC, Dalanezi FM, Leite DS, Langoni H, Pantoja JC, Rall VM, Hernandes RT, Lucheis SB, Ribeiro MG (2020) Investigation of extra-intestinal pathogenic Escherichia coli virulence genes, bacterial motility, and multidrug resistance pattern of strains isolated from dairy cows with different severity scores of clinical mastitis. J Dairy Sci 103:3606–3614
Li X, Zhang Z, Chang X, Wang X, Hu J, Lin Q, Jia Y, Yang X, Wang X (2019) Disruption of blood-brain barrier by an Escherichia coli isolated from canine septicemia and meningoencephalitis. Comp Immunol Microbiol Infect Dis 63:44–50
Zamprogna TO, Ribeiro D, Azevedo VAC, Lara GHB, Motta RG, da Silva RC, Siqueira AK, de NardiJúnior G, Listoni FJP, Martins LSA, da Silva AV, Portilho FVR, Mota AR, Rodrigues CA, Almeida BO, Ribeiro MG (2021) Bacteriological, cytological, and molecular investigation of Corynebacterium pseudotuberculosis, mycobacteria, and other bacteria in caseous lymphadenitis and healthy lymph nodes of slaughtered sheep. Braz J Microbiol 52:431–438
Giguère S, Prescott JF, Dowling PM (2013) Antimicrobial therapy in veterinary medicine, 5th edn. Wiley & Sons Inc, Blackwell, Iowa, p 704
Clarke LL, Hawkins IK, Rissi DR (2019) Central nervous system diseases of cattle in Georgia, 2001–2017. J Vet Diagn Invest 31:588–593
Wright WF, Casey NP, Palisoc K, Baghli S (2019) Viral (aseptic) meningitis: a review. J Neurologic Sci 398:176–183
Soto RA, Hughes ML, Staples JE, Lindsay NP (2022) West Nile virus and other domestic nationally notificable arboviral diseases – United States 2020. Morb Mortal Wkly Rep 71:628–632
Ungureanu A, Van der Meer J, Bicvic A, Abbuehl L, Chiffi G, Jaques L, Suter-Riniker F, Leib SL, Bassetti CLA, Dietmann A (2021) Meningitis, meningoencephalitis, and encephalitis in Bern: an observational study of 258 patients. BMC Neurol 21:e474
Acknowledgements
The authors thank the National Council for Scientific and Technological Development (CNPq), Brazil, for research productivity fellowships given to Márcio Garcia Ribeiro, Jane Megid, Helio Langoni, Alexandre Secorun Borges, and José Paes de Oliveira Filho.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Luis Nero
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ribeiro, M.G., Pereira, T.T., de Lima Paz, P.J. et al. Bacterial identification in cerebrospinal fluid of domestic species with neurologic signs: a retrospective case-series study in 136 animals (2005–2021). Braz J Microbiol 54, 449–457 (2023). https://doi.org/10.1007/s42770-022-00891-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-022-00891-2