Abstract
Purpose
To evaluate the effect of three different non-invasive ventilation (NIV) interfaces on the subjective discomfort of healthy individuals, and on a set of physiological parameters hypothesized to change in correspondence to discomfort.
Methods
Continuous pressure NIV was applied to 20 subjects using Total Face, Nasal, and Face masks for 10 min each. Tidal volume (VT) and respiratory period (RP) were estimated from respiratory inductance plethysmography. Electrodermal activity was estimated from conductance signals. Heart rate variability was measured using the time-domain indices SDNN and RMSSD, and the respiratory sinus arrhythmia amplitude (RSAp). Parameters were referenced to 5-min rest periods at beginning and end of protocol. A Likert-like scale of subjective discomfort with the masks and the ventilation was applied after 1, 5, and 9 min using each mask.
Results
RP and VT increased with the three mask models. Whereas the mean heart rate and RSAp did not change, both SDNN and RMSSD increased during NIV with Nasal and Face masks. Spontaneous electrodermal activity fluctuations were less frequent during NIV than at rest, with significant differences for Total Face and Nasal masks. Discomfort with all masks increased from minutes 1 to 9, markedly in the Total Face mask, considered most uncomfortable by 11 subjects.
Conclusion
In healthy subjects, the three masks resulted in similar respiratory responses to NIV. Correspondence between changes in physiological parameters and discomfort with NIV interface could not be detected, whereas self-report with the Likert-like scale identified progressive discomfort and the Total Face mask as the most uncomfortable interface.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute lung failure is clinically characterized by the inability of the patient to keep the necessary gas exchange autonomously (Antonelli et al. 2003; Najaf-Zadeh and Leclerc 2011). Some cases, therefore, require the institution of respiratory support to assist gas exchange, to reduce work of breathing, to increase functional residual capacity, to prevent alveolar collapse, and, consequently, to reduce respiratory discomfort (Kallet and Diaz 2009; Najaf-Zadeh and Leclerc 2011; Gaszyńska et al. 2013).
Respiratory support by positive pressure mechanical ventilation can be either invasive or non-invasive. Non-invasive ventilation (NIV), meaning ventilatory support performed without an invasive artificial airway, is a safe alternative to provide ventilatory assistance to patients with respiratory failure (Mayordomo-Colunga et al. 2009). The advantages of NIV include avoidance of complications related to orotracheal intubation; preserved defense mechanisms of the airways, as well as speech and swallowing; and more flexibility in initiation of and weaning from respiratory support (Antonelli et al. 2003).
The success of NIV intervention depends on many aspects involved in its initiation, including selection of patients according to clinical criteria, appropriate adjustment of ventilation parameters, and choice of mask interface (Navalesi et al. 2000; BaHammam et al. 2018). Low tolerance to the use of the mask, arising from leakages, skin injuries, and discomfort, is one of the causes attributed to discontinuation of NIV (Fodil et al. 2011; Papa et al. 2012). Particularly, discomfort is regarded as cause of 12 to 30% of the failures of NIV (Carron et al. 2013). Hence, selecting a comfortable interface is a clinical goal during NIV (Pisani et al. 2012; BaHammam et al. 2018).
To help in such selection, tools to assess patient discomfort are needed. This work aimed at the evaluation of the effect of 3 different NIV interfaces on the subjective discomfort of healthy individuals, and also on physiological parameters whose changes are hypothesized to be related to changes in the perception of stress, namely heart and respiratory rates, heart rate variability (HRV)—especially respiratory sinus arrhythmia (RSA)—and electrodermal activity (EDA).
Methods
The protocol was approved by the Institutional Review Board from Hospital Universitário Clementino Fraga Filho of the Federal University of Rio de Janeiro (UFRJ, Brazil, protocol number: 648.084). A group of 20 subjects (11 women), age 28.1 ± 6.6 (mean ± SD) years, and body mass index of 24.5 ± 4.21 kg/m2, reportedly non-smokers and free from respiratory or cardiac diseases, was enrolled. Subjects were requested to avoid caffeine consumption at least 6 h before the experiment, and signed informed consent prior to protocol initiation.
Experimental setup
Experiments were conducted in the Pulmonary Engineering Laboratory at UFRJ. Subjects were seated in a padded chair with back and upper limb support inside a quiet and reserved room with controlled temperature (21–24 °C) and relative humidity (40–50%). Respiratory, electrodermal, and cardiac activity were monitored during the experiment with single-use or adequately hygienized instruments. The open expiratory orifice present in the masks to be tested prevented direct measurement of airflows. Hence, tidal volume (VT) and respiratory cycle length (RP) were estimated from respiratory inductance plethysmography (RIP) signals (Inductotrace Systems, Ambulatory Monitoring, Inc., USA). Thoracic and abdominal displacements were simultaneously recorded using two elastic bands selected according to body size. For accurate measurements, RIP was calibrated against volume measurements using a variable orifice pneumotachograph before each experiment. Subjects were instructed to breathe normally for 2 min using a completely sealed mask (Vital Signs, Brazil) with the pneumotachograph (model 155,362, Hamilton Medical, Switzerland) attached to its respiratory port. VT calculated from RIP and pneumotachograph during this period were used for estimation of individual gains for the thoracic and abdominal bands (Poole et al. 2000). The pneumotachograph used as reference was calibrated with a 2-L syringe (Calibration Syringe Series 5530, Hans Rudolph, Inc., USA) using standard procedures (Gianella-Neto et al. 1998).
Airway opening pressure (Pao) was monitored with a pressure transducer (163PC01D48-PCB; Sensortechnics, Germany) connected between the mask and the NIV circuit. The transducer was calibrated on each day of experiment using a water column reference (Scireq, Canada). EDA was estimated from conductance signals obtained from a Electrodermal Activity Amplifier Module for the Biopac MP 100 (Biopac Systems, Inc., USA). Hollow Ag/AgCl electrodes filled with a conductive gel (Mercur, Brazil) were attached to the distal phalanges of the index and middle fingers of the non-dominant hand approximately 5 min before start of experiment. A two-point calibration with resistances of 50 kΩ and 100 kΩ was performed before each experiment. Measurements used direct current mode with a gain of 10 μS/V. A standard 3-lead electrocardiogram (ECG) was acquired from an ECG100C Electrocardiogram Amplifier Module of the Biopac MP100 system with gain of 5000, without highpass filtering.
Experimental protocol
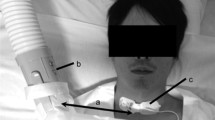
Protocol started with a 5-min rest period (pre-rest), with signal recording. Then, NIV was started using 5 cmH2O in continuous positive airway pressure (CPAP) mode applied with a single hose circuit without an expiratory valve (C-Series Tango Positive Airway Pressure Device, ResMed, USA) (Fig. 1a). This pressure level was expected to approximate the clinical scenario while imposing low levels of discomfort.
Experimental protocol. a The protocol consisted in 5-min rest periods before (pre-rest) and after (post-rest) a sequence on non-invasive ventilation (NIV) using three different interfaces: Total Face mask (b), Nasal mask (c), and Face mask (d). Each mask was used for 10 min with a 5-min washout period between two masks. After 1 min (T1), 5 min (T5) and 9 min (T9) using each mask subjects were asked to grade their discomfort with the mask and the ventilation. These time points were signaled by a beep sound and the questions were presented in a computer screen
Three NIV interfaces were used for 10-min periods by each subject (Fig. 1b–d): FitLife Total Face Mask (Respironics Inc., USA); ComfortClassic Nasal Mask (Respironics Inc.); and FullLife Face Mask (Respironics Inc.). These masks are commonly commercialized and have been approved by the bodies responsible for surveillance of health products. The body of the masks (faceplate) is made of polycarbonate and the cushion is made of silicone. Equal materials and manufacturer were chosen to emphasize differences in mask type. Subjects were monitored during a 5-min washout period after removal of one interface and before placement of the next, in an attempt to reduce carryover effects. Effects of time on comparative discomfort among masks were controlled by use of three randomized sequences: Total Face-Nasal-Face; Nasal-Face-Total Face; and Face-Total Face-Nasal (Fig. 1a). The masks’ expiratory outlet was kept open (intentional leak), and mask was fitted following the manufacturer recommendations. Non-intentional leaks (i.e., leaks around the interface) were assessed from patient report.
Discomfort due to the mask and the CPAP were evaluated independently using a 5-level Likert-like scale to answer the questions “How is your level of discomfort related to the mask?” and “How is your level of discomfort related to the ventilation?” (in Portuguese). The extremes of the scale were “1 - no discomfort” and “5 - unbearable discomfort,” with three intermediate levels (“light discomfort,” “moderate discomfort,” and “intense discomfort”). Progress of discomfort was evaluated by repeating both questions at three time points after start of ventilation with each mask: after 1 min (T1), after 5 min (T5), and after 9 min (T9). Experiment timing was kept by a sequence of slides that changed color (from black to white) and showed the above questions. Simultaneously to the change in color, subjects were stimulated with a 100-Hz short beep.
The experiment ended with a 5-min rest period (post-rest), after which the subjects were asked to choose the masks that caused most discomfort.
Data acquisition and processing
The PTC, RIP, Pao, ECG, and EAD signals were recorded with an 11-bits analog-to-digital converter (USB-6009; National Instruments, USA) at 1000 samples per second using a program written in LabView (National Instruments). Recorded signals were processed using programs written in MATLAB R2007b (Mathworks, USA) as follows:
PTC
Flow signals were filtered in forward and backward direction with a 10-Hz Butterworth low-pass digital filter. The beginning of each respiratory phase was identified using a zero-crossing detection algorithm and imposing a minimum of 50 ml to accept a true transition. The detected cycles were then manually verified and RP was calculated from the time difference between consecutive inspirations.
RIP
A flow signal was estimated by numerical differentiation of the estimated volume signal filtered by a digital first-order Butterworth low-pass (3 Hz) filter. Cycle detection and RP calculation used the same methods described for PTC signal. The tidal volume (VTRIP) was considered the maximum volume within each cycle.
Pao
Pressure signals were summarized by the cycle peak pressure (peakP) and difference between mean inspiratory and expiratory pressures (dPao).
ECG
To reduce the baseline fluctuations and high frequency noise, ECG signals were pre-processed with a 4th-order Butterworth band-pass filter between 5 and 200 Hz. The peaks of the R waves were detected by the zero-crossing of the filtered ECG first derivative considering a refractory period of 250 ms and threshold of 0.3 V to validate a peak (Beda et al. 2007). Detected R waves were manually verified.
The time intervals between two R waves (RRi) were used to quantify heart rate variability using three time domain indices (Task Force Of The European Society Of Cardiology And The North American Society Of Pacing And Electrophysiology 1996): standard deviation of the intervals (SDNN); root mean square of differences between consecutive intervals (RMSSD); and the proportion of intervals with at least 50 ms (pNN50).
The identified R waves and respiratory cycle onsets from the RIP were analyzed together to quantify the RSA. An average pattern of the change in RRi within a breath cycle for each period using masks or while resting was calculated with the filtering method proposed elsewhere (Gilad et al. 2005). Briefly, the RRi within each respiratory cycle were interpolated into 50 equispaced time intervals, and a 50-point transversal average of the resulting series was calculated. For each series, its deviation from the transversal average was quantified as the sum of the squared deviations of each interpolated point from the respective point in the transversal average. The cycles with 10% larger deviations were excluded. For the remaining cycles, each of the 50 points was assigned an angle value from 0 to 2π radians, and the phase of the RSA of each cycle was defined as the angle assigned to the maximum value of the respective interpolated series. The average RSA pattern was the mean of the 80% of cycles with the lowest absolute phase deviation from the mean phase. From this average pattern, the RSA was characterized by its amplitude (peak-to-peak difference, RSAp). For the sake of comparison, we tested the Spearman correlation (0.996, p < 0.001) and the difference (0.006 s CI [0.008 s, 0.004 s], Wilcoxon signed test, p < 0.001) between the mean RRi and the mean of the average RSA pattern.
EDA
The EDA signal was filtered with a first-order Butterworth low-pass filter (1 Hz) and the local maxima and minima were manually identified. An electrodermal response was defined as a local raise of the EDA and was characterized by its amplitude (maximum-minimum) and rise time (time interval between minimum and maximum). Only responses with amplitude of at least 0.01 μS were considered for analysis. Each response was classified as spontaneous or evoked. An evoked response was the first response within 10 s of the beep stimuli (at T1, T5, and T9 in Fig. 1a) and was additionally characterized by its latency (time interval from the stimulus to the EDA minimum). The spontaneous responses were additionally summarized by their frequency (number of responses per minute) during the use of each mask.
Statistical analysis
Statistical analysis used GraphPad Prism 6.0 (GhaphPad Software, USA) and significance threshold was p = 0.05. Data was presented as mean, SD, and median of subjects, and each protocol stage was represented by the average value of each variable within subject. Respiratory and cardiac variables (RPRIP, VTRIP, RSAp, SDNN, RMSSD, and pNN50) were compared among the masks and rest periods. Parameters derived from the PTC (RPPTC, dPao and peakP) were compared only among masks. EDA variables (amplitude, rise time, latency, and frequency) were compared among T1, T5, and T9 within each mask, and among masks within T1, T5, and T9. All those comparisons used Friedman test followed by Dunn’s post hoc test. RPRIP was validated against RPPTC using a paired Wilcoxon test.
Descriptive statistics of subjective evaluations of discomfort were reported as the proportion of choices of each discomfort level, considering changes in time within mask and aggregated answers compared among masks.
Results
All subjects completed the protocol without complications or adverse effects of ventilation, or the use of mask interfaces. No subject reported the presence of non-intentional leaks.
Respiratory variables
Presence of NIV changed the ventilatory pattern independently of the mask interface, resulting in increased RPRIP and VTRIP compared to rest periods (Fig. 2). While VTRIP decreased at post-rest to the same level as in pre-rest, the RPRIP was still elevated at the end of the experiment. However, such differences in RPRIP were only consistent for the Nasal and Face masks (Fig. 2). Measured RPRIP was close to the corresponding values calculated with the pneumotacograph during mask use, supporting the proposed method for respiratory cycle detection (see Table S1 in Online Resource). Airway opening pressure had lowest peakP values with the Face mask (p < 0.001), although tidal changes in pressure (dPao) were not different for Face mask compared to the others (Online Resource Tab. S1).
Respiratory period (RPRIP) and tidal volume (VTRIP) during the protocol. RPRIP and VTRIP were measured using respiratory inductance plesthymography calibrated against a variable orifice pneumotachograph. The use of non-invasive ventilation with any of the three masks tested (Total Face, Nasal, and Face) resulted in increased RPRIP and VTRIP relative to pre-rest. VTRIP was also increased when using the masks compared to post-rest. Subjects used the masks in three different orders and the figure uses a single one for simplification. Data is shown as mean (circles) ± SD (vertical lies), and median (squares). *vs pre-rest, #vs post-rest
Cardiac variables
Heart rate variability (HRV) increased after the first rest period. While the mean RRi did not change during the experiment (p = 0.765, Online Resource Tab. S1), both SDNN and RMSSD indexes of HRV increased during NIV periods and did not always returned to the same values (Fig. 3). Differences were significant only for the Nasal (SDNN—p < 0.001 and RMSSD—p = 0.015) and Face masks (SDNN—p < 0.001). No difference was observed among the 5 experimental stages for the pNN50 and the RSA amplitude RSAp (Online Resource Tab. S1). These variations could indicate a higher contribution of the parasympathetic system to the HRV.
Heart rate variability during the protocol. The variability in length of the intervals between each consecutive heart beat (RRi) identified in the electrocardiogram signal was analyzed in the time domain. Both the standard deviation (SDNN) and the root mean square of successive differences (RMSSD) of the RRi increased after pre-rest. However, the differences were significant only for the Nasal mask, and for the SDNN with Face mask. Data is shown as mean (circles) ± SD (vertical lies), and median (squares). *Difference to pre-rest; #difference to post-rest
Electrodermal activity
The amplitude, latency, and rise time of the electrodermal response to the beep stimuli did not show marked differences between and within masks (Fig. 4). Only the Nasal mask showed differences between time points, with largest rise time at T9 (p = 0.002) and lowest latency at T5 (p = 0.023). There was no difference between masks when they were compared within time points. The temporal changes seen with the Nasal mask may be related to a measurement artifact.
Electrodermal activity evoked by the beep sound at three time points during the use of each mask. At 1 min (T1), 5 min (T5), and 9 min (T9) of mask use, a beep sound was used to warn subjects to evaluate their discomfort. Variations in electrodermal activity within 10 s from this stimuli were considered to be a response to it and were evaluated by their amplitude, time to onset after stimuli (latency), and time to reach the peak after onset (rise time). The use of Nasal mask (gray) leads to an increase in rising time and latency at T9. The use of Total Face (dark gray) and Face (light gray) masks had no significant effect on electrodermal evoked activity. Data is shown as mean (circles) ± SD (vertical lies), and median (squares). *Difference to T9
The spontaneous electrodermal fluctuations were less frequent and with larger rise times during NIV than at rest, with significant differences for Total Face (frequency—p < 0.001 and rise time—p = 0.002) and Nasal masks (frequency—p < 0.001) (Fig. 5). These differences in electrodermal activity may be related to subject’s focus level at the different protocol stages. There was no difference in the amplitude of the spontaneous fluctuations (Fig. 5).
Spontaneous electrodermal fluctuations during the protocol. The frequency of fluctuations decreased during the use of non-invasive ventilation (NIV) independent of the interface, although not significant for the Face mask. The amplitude of these fluctuations did not change between rest and NIV, while the mean time to reach a peak after a valley (rise time) was significantly different from rest only for Total Face mask. Data is shown as mean (circles) ± SD (vertical lies), and median (squares). *Difference to pre-rest; #difference to post-rest
Subjective discomfort
The discomfort with the masks was between none and moderate with exception of one unbearable classification for the Nasal and one intense for the Face mask (Fig. 6). Discomfort changed with time, and with all masks, the number of classifications as none decreases from T1 to T9. The Total Face mask changed from a majority of evaluations as none to moderate. The Nasal mask was always mostly classified with light discomfort, with an increase in this answer at T9. The Face mask showed a decrease in the number of evaluations as none, although this level was always the most often chosen (Fig. 6).
Evaluation of discomfort with mask and with ventilation. When using each mask, the subjects were asked about the discomfort generated by the use of the mask and the continuous positive airway pressure ventilation, at 1 min (T1), 5 min (T5), and 9 min (T9). Discomfort was graded in a 5-level Likert-like scale: none (lightest gray), light (light gray), moderate (gray), intense (dark gray), or unbearable (black). Each level of discomfort is represented by the fraction it was assigned at each time point, and the rightmost panel sums up the answers of all 3 time points (T1 + T5 + T9)
While discomfort with ventilation was more classified as intense than discomfort with masks, most of the subjects classified discomfort with ventilation from none to moderate (Fig. 6). The changes in time of discomfort with ventilation were less clear than for discomfort with mask. Putting all 60 answers (20 subjects, 3 time points) together revealed that Face and Total Face masks led to similar levels of discomfort with ventilation, while the Nasal mask had a small increase in the number of moderate evaluations (Fig. 6).
At the end of the protocol, subjects chose the most uncomfortable mask as Total Face (11/20), followed by Nasal (6/20) and Face (3/20). The aggregate evaluations of discomfort agreed with this choice showing the Total Face with largest number of moderate ratings and Nasal with largest number of light ratings (Fig. 6).
Discussion
Positive-pressure NIV is an effective therapeutic strategy to avoid or delay the use of invasive mechanical ventilation (Kallet and Diaz 2009). However, long time use of NIV may lead to complications such as dry skin and pressure wounds that may be reduced by a periodic rotation of interfaces (Brill 2014). For an effective rotation, all interfaces should have similar physiological effect and should not cause high discomfort to avoid early discontinuation (Pisani et al. 2012). The choice of the most appropriate interface should take various aspects into account, like patient’s features (shape of the face, mouth, and nose), breathing pattern, ventilatory mode, respiratory failure type, experience of the medical staff, and patient’s preference (BaHammam et al. 2018). Herein, the evaluation of short-term effects of different NIV interfaces on cardiorespiratory and electrodermal variables, as well as subjective discomfort, on health subjects showed similar degrees of discomfort for the 3 interfaces studied. Moreover, in general, when compared to rest periods without ventilation, the use of NIV increased tidal volume and respiratory period; affected HRV; and decreased the frequency of electrodermal activity spontaneous fluctuations. Subjects evaluated the Total Face mask as the most uncomfortable, and repeated Likert-like survey hints at the effect of time on discomfort associated with the interface, but not with the ventilation itself. Overall, these results suggest adaptation of respiratory pattern to NIV, and progressive discomfort, which should be evaluated through extended periods of use.
Peak Pao was lower for the Face mask, without significant changes in delta Pao. These results could be explained by larger intentional leak in the Face mask. Measurement at 14 cmH2O with the same models of mask used in this study showed 40% more intentional leak in Face mask than in Nasal mask (Borel et al. 2009). Thus, the Face mask may require slightly higher pressure settings on the ventilator to achieve the same effects of Nasal and Total Face masks. Respiratory period and tidal volume were increased during NIV as compared to rest periods. These increases agree with measurements in patients with acute respiratory insufficiency or chronic obstructive pulmonary disease at different pressure levels (Criner et al. 1994; Antón et al. 2003; Conti et al. 2007). Nonetheless, no difference in respiratory period or tidal volume was found in healthy individuals spontaneously breathing for 5 min with or without PEEP of 5 cmH2O (Vaschetto et al. 2014). Such disagreement could be explained by differences in instrumentation. Whereas herein RIP measured tidal volume and respiratory period, Vaschetto et al. (2014) used a mouth-piece and nose-clip known to affect the breathing pattern during spontaneous breathing (Weissman et al. 1984; Perez and Tobin 1985). Of note, the differences in respiratory variables during NIV observed in this study may be in part related to the presence of the interfaces.
Increased parasympathetic activity on heart rate seemed to be one of the consequences of NIV in this study. Mean heart rate was constant during the protocol, which would be expected in a cohort of young, healthy, seated subjects at rest. However, RMSSD and SDNN changed during NIV compared to the initial rest period. This change in HRV could be related to inhibition of sympathetic stimuli due to increased lung stretch (Reis et al. 2010), or increased parasympathetic stimuli due to deactivation of heart filling sensors with decreased transmural pressure gradient (Fietze et al. 2004; Garet et al. 2006). The observed changes in HRV agree with previous measurements in healthy adults breathing through an external resistance (Calabrese et al. 2000), but not with spectral analysis of RRi during CPAP using nasal mask interface, when high frequency component of HRV decreased (Valipour et al. 2005). These differences may be explained by the use of controlled breath rate by the latter. Calabrese et al. (2000) showed that increase in HRV was correlated with increased RP resulting from adaptation to the external respiratory load, similar to the increase in RP we observed during NIV. Moreover, HRV measures were similar when subjects breathed with a given RP independent of the presence of the external resistance (Calabrese et al. 2000). Thus, the measured changes in RP may be linked or act as confounding factor for conclusions about sympathovagal balance from HRV.
This study did not find statistically significant differences in RSAp among the studied conditions, despite increased VT and RP during NIV, factors that are both expected to contribute to a larger amplitude of RSA (Ritz et al. 2001). Although not monitored in these experiments, arterial partial pressure of carbon dioxide (PaCO2) likely reduced during NIV as an effect of increased VT in the healthy subjects; that may have had a reducing effect on RSA amplitude via diminished vagal efference (Sasano et al. 2002). Direct or indirect measurements of PaCO2 may help clarifying this issue. Of note, Calabrese et al. (2000) observed a difference in RSA related to increased respiratory load and RP, without a noticeable change in end-tidal carbon dioxide concentration.
The low to moderate levels of subjective discomfort during NIV appear to have not affected the evoked EDA response. We observed differences in rise time and latency at the last minute of NIV with the Nasal mask. However, there was no difference among masks within each of the three points of assessments of discomfort. Measurement of EDA is sensitive to ambient temperature and humidity, and the position of the electrodes (Boucsein et al. 2012; Silva et al. 2012). Our experiments were in a closed room with an air conditioner and followed a standardized protocol for placing the electrodes, minimizing those effects. Other experimental factors affecting EDA are pressure of electrode fixation and amount of conductor gel used, which have progressive effects and may explain the results with Nasal mask (Boucsein et al. 2012). The evoked EDA response is related to the sympathetic reactivity and together with the significant change in RMSSD with the Nasal mask may indicate a physiological change. Further studies are needed to confirm if the change in rise time and latency with Nasal mask were an artifact or a real effect.
The frequency of spontaneous fluctuations on the EDA decreased during NIV compared to both rest periods. Amplitude and frequency of these spontaneous fluctuations are also linked to the excitation of the sympathetic system and respond to both positive and negative emotional stimuli (Bauer 1998; Tronstad et al. 2013). Thus, the observed changes in spontaneous EDA response may be connected to the changes in HRV and support a change in sympathovagal balance. Additionally, our results would suggest that frequency of fluctuations may be more sensitive than their amplitudes at this level of sympathetic activity variation. Nevertheless, the increased number of spontaneous fluctuations during rest could be due to stimuli unrelated to the experiment. During the NIV subjects may have an increased level of attention due to the use of interface and ventilation, and the focus required to answer the questions about discomfort. Hence, during rest periods subjects could have responded more to uncontrolled external stimuli.
Patient discomfort with the mask may result in early discontinuation (Pisani et al. 2012; Silva et al. 2013), and patients should try different types of interfaces to choose the most comfortable (BaHammam et al. 2018). There is an agreement that comfort, in general, has a subjective nature, is influenced by different factors (physical, physiological, psychological), and is a reaction to the environment (De Looze et al. 2003). Thus, comfort may be defined as a well-being state of physiological, psychological, and physical balance between a person and the environment (Slater 1985 APUD De Looze et al. 2003). Because of its subjective nature, this sensation is related to experience and self-perception, and varies from one person to other. In this study, we tried to semi-quantify self-perception of discomfort using a Likert-like scale and associate this with physiological measurements. The assessment of subjective discomfort with a Likert-like scale suggested that discomfort with the mask is progressive with rate of change varying among mask types. The Total Face mask seems to have the highest rate of increase in discomfort, changing from mostly none to mostly moderate discomfort within the 10 min tested. Accordingly, the Total Face mask was chosen as the most uncomfortable by 11 subjects. This subjective assessment suggests that, regarding discomfort, mask rotation could consider longer periods with the Face mask interspersed with Nasal or Total Face masks.
The apparent dissociation between subjective discomfort and physiological effects of a NIV interface was observed before in healthy subjects. While two different types of helmet interfaces showed significant differences in ventilator synchrony compared to a Face mask, all interfaces were evaluated with similar low levels of discomfort, and were not different in inspiratory muscle unloading and work of breathing (Vaschetto et al. 2014). However, in that study, subjects used the masks for 5 min and results may have been different due to progressive discomfort. Holanda et al. (2009) evaluated subjective discomfort, and the incidence and intensity of different types of acute adverse effects during the use of three NIV masks in low and moderate/high pressure levels of Bilevel Positive Airway Pressure (BIPAP) ventilation. There was no difference among masks in physiological response and reported comfort, while the moderate/high pressure level was considered more uncomfortable. In acute respiratory failure patients, Ozsancak et al. (2011) reported that during the first 3 h of NIV, Nasal and Total Face masks were evaluated with similar levels of discomfort and resulted in the same levels of increase in respiratory period and oxygen saturation. Following patients during treatment showed a trend of higher discontinuation rate for the Total Face mask (57% vs 40%), which agrees with our observation of faster progress of discomfort with Total Face mask in some subjects. On the other hand, Criner et al. (1994) observed that patients with chronic respiratory failure that were considered to not tolerate NIV with Nasal or Facial masks, would adapt for the use of a Total Face mask. In agreement, in our study, both Nasal and Face masks were also selected as the most uncomfortable by some subjects. Thus, individual assessment of discomfort is needed and our results reinforce that this assessment should be repeated periodically.
Limitations
Results from healthy subjects may not be translated directly to patients with respiratory failure in an Intensive Care Unit whose discomfort with the NIV interface is superimposed with limitations from the disease and possible benefits of NIV. However, healthy subjects were previously enrolled in this type of study (Chiumello et al. 2003; Costa et al. 2005; Holanda et al. 2009; Vaschetto et al. 2014). Future studies must confirm our findings in patients with respiratory failure. Our results may be limited to the CPAP mode, the single pressure used, the models and manufacturer of the masks, and the time frame of measurements. Thus, the results should be viewed as guidance for methods and hypothesis for larger studies, and a later stabilization of subjective discomfort cannot be ruled out.
We used RIP for respiratory measurements because the required leak on the masks hindered the correct estimation of tidal volumes using a pneumotachograph. RIP signal is known to change with variations in respiratory pattern (abdominal/thoracic) and to be sensitive to movement artifacts (Lanatà et al. 2010). The measurements of healthy, seated subjects during a relatively short time should minimize these limitations. Additionally, we calibrated the RIP against pneumotachograph measurements for each subject, and comparison of RIP and pneumotachograph after calibration showed satisfactory agreement.
The aim of the study was to evaluate subjective discomfort with the NIV interface. However, the instructions to the subjects to answer about this when prompted on the screen may have worked like an attention task, affecting part of the physiological measurements. Moreover, to better understand those measurements other variables such as intentional air leak, end-tidal carbon dioxide concentration and systemic arterial pressure would need to be measured. Lastly, we did not ask subjects to report what was the cause of their discomfort. While some causes of discomfort may be solved in a clinical setting (Silva et al. 2013), presence of some adverse effects and subjective assessment of discomfort may not agree (Holanda et al. 2009).
Translation of this research into evaluation of patient’s discomfort with NIV interface in clinical settings requires special considerations regarding sample selection and confounding factors. Environmental factors and emotional state of the patient may influence discomfort in the ICU. De Leur et al. (2004) reported that the prevalence of self-reported discomfort during the ICU stay was 54%, and the most cited sources were endotracheal tube (for patients in invasive mechanical ventilation), hallucination experiences, medical procedures, and the acoustic noise in the ICU. Thus, multiple groups or additional questions to each patient may be needed in order to disentangle the discomfort due specifically to NIV interface. Moreover, larger leaks from the mask have been linked to worse synchrony between patient and NIV ventilator (Schettino et al. 2001), which may in turn worsen overall discomfort. Directly asking patients about leaks, as we did in this study, may not be feasible, and monitoring leakage may be necessary to understand sources of discomfort. Another point to consider is that mood and affective states, for instance anxiety, a transient emotional state characterized by physiological excitement (Endler and Kocovski 2001), may influence subjective assessments of discomfort. The use of standardized tools (for instance, Spielberger State Anxiety Inventory and Face Anxiety Scale; McKinley and Madronio 2008) during patient screening may help to standardize the study sample.
Conclusion
In healthy subjects in controlled environment, constant-pressure NIV resulted in similar levels of increased respiratory period and tidal volume for Total Face, Nasal, and Face mask interfaces. The effect of NIV over the sympathetic activity, suggested by time-domain HRV analysis and measurements of EDA, needs further validation. Correspondence of physiological measurements and reporting of discomfort with NIV interface could not be confirmed, and should be evaluated in longer experiments that may result in larger changes in comfort. Nevertheless, a 5-level Likert-like scale could capture progressive discomfort that matched overall perception of most uncomfortable interface. In view of these results, we suggest further studying instruments—such as the Likert-like scale employed herein—to assess adaptation throughout prolonged use of NIV, and the potential progressive discomfort elicited thereof. Provided that other sources of discomfort are monitored and the emotional state prior to the NIV adaptation and during its use can be assessed, techniques and measurements such as those herein described may help evaluating discomfort in patients, particularly those under critical care, with the NIV masks in health care environments.
References
Antón A, Tárrega J, Giner J, Güell R, Sanchis J. Acute physiologic effects of nasal and full-face masks during noninvasive positive-pressure ventilation in patients with acute exacerbations of chronic obstructive pulmonary disease. Respir Care. 2003;48(10):922–5.
Antonelli M, Pennisi MA, Conti PG. New advances in the use of noninvasive ventilation for acute hypoxaemic respiratory failure. Eur Respir J. 2003;22(supl.42):65s–71s.
BaHammam AS, Singh TD, Gupta R, Pandi-Perumal SR. Choosing the proper interface for positive airway pressure therapy in subjects with acute respiratory failure. Respir Care. 2018;63(2):227–37.
Bauer RM. Physiologic measures of emotion. J Clin Neurophysiol. 1998;15(5):388–96.
Beda A, Jandre FC, Phillips DIW, Gianella-Neto A, Simpson DM. Heart-rate and blood-pressure variability during psychophysiological tasks involving speech: influence of respiration. Psychophysiology. 2007;44:767–78.
Borel JC, Sabil A, Janssens J-P, Couteau M, Boulon L, Lévy P, et al. Intentional leaks in industrial masks have a significant impact on efficacy of bilevel noninvasive ventilation: a bench test study. Chest. 2009;135:669–77.
Boucsein W, Fowles DC, Grimnes S, Ben-Shakhar G, Roth WT, Dawson ME, et al. Committee report: publication recommendations for electrodermal measurements. Psychophysiology. 2012;49:1017–34.
Brill A-K. How to avoid interface problems in acute noninvasive ventilation. Breathe. 2014;10:230–42.
Calabrese P, Perrault H, Dinh TP, Eberhard A, Benchetrit G. Cardiorespiratory interactions during resistive load breathing. Am J Phys Regul Integr Comp Phys. 2000;279:R2208–13.
Carron M, Freo U, Bahammam AS, Dellweg D, Guarracino F, Cosentini R, et al. Complications of non-invasive ventilation techniques: a comprehensive qualitative review of randomized trials. Br J Anaesth. 2013;110(6):896–914.
Chiumello D, Pelosi P, Carlesso E, Severgnini P, Aspesi M, Gamberoni C, et al. Noninvasive positive pressure ventilation delivered by helmet vs. standard face mask. Intensive Care Med. 2003;29:1671–9.
Conti G, Cavaliere F, Costa R, Craba A, Catarci S, Festa V, et al. Noninvasive positive-pressure ventilation with different interfaces in patients with respiratory failure after abdominal surgery: a matched-control study. Respir Care. 2007;52(11):1463–71.
Costa R, Navalesi P, Antonelli M, Cavaliere F, Craba A, Proietti R, et al. Physiologic evaluation of different levels of assistance during noninvasive ventilation delivered through a helmet. Chest. 2005;128:2984–90.
Criner GJ, Travaline JM, Brennan KJ, Kreimer DT. Efficacy of a new full face mask for noninvasive positive pressure ventilation. Chest. 1994;106:1109–15.
De Leur JPV, der Schans CPV, Loef BG, Deelman BG, Geertzen JHB, Zwaveling JH. Discomfort anda factual recollection in intensive care unit patients. Crit Care. 2004;8:R467–73.
De Looze MP, Kuijt-Evers LFM, Dieën JV. Sitting comfort and discomfort and the relationships with objective measures. Ergonomics. 2003;46(10):985–97.
Endler NS, Kocovski NL. Stait and trace anxiety revisited. Anxiety Disord. 2001;15:231–45.
Fietze I, Romberg D, Glos M, Endres S, Theres H, Witt C, et al. Effects of positive-pressure ventilation on the spontaneous baroreflex in healthy subjects. J Appl Physiol. 2004;96:1155–60.
Fodil R, Lellouche F, Mancebo J, Sbirlea-Apiou G, Isabey D, Brochard L, et al. Comparison of patient-ventilator interfaces based on their computerized effective dead space. Intensive Care Med. 2011;37(2):257–62.
Garet M, Barthélémy J-C, Degache F, Pichot V, Duverney D, Roche F. Modulations of human autonomic function induced by positive pressure-assisted breathing. Clin Physiol Funct Imaging. 2006;26:15–20.
Gaszyńska E, Roguszka K, Stankiewicz-Rudnicki M, Wieczorek A, Gaszyński T. Comparison of haemodynamic effects of noninvasive ventilation delivered via ResQGARD and CPAP boussignac masks. Polski Przegląd Chirurgiczn. 2013;85(3):129–32.
Gianella-Neto A, Bellido C, Barbosa RB, Melo MFV. Design and calibration of unicapillary pneumotachographs. J Appl Physiol. 1998;84:335–43.
Gilad O, Swenne CA, Davrath LR, Akselrod S. Phase-averaged characterization of respiratory sinus arrhythmia pattern. Am J Physiol Heart Circ Physiol. 2005;288:504–10.
Holanda MA, Reis RC, Winkeler GFP, Fortaleza SCB, Lima JWO, Pereira EDB. Influence of total face, facial and nasal masks on short-term adverse effects during noninvasive ventilantion. J Bras Pneumol. 2009;35(2):164–73.
Kallet RH, Diaz JV. The physiologic effects of noninvasive ventilation. Respir Care. 2009;54(1):102–14.
Lanatà A, Scilingo EP, Nardini E, Loriga G, Paradiso R, De-Rossi D. Comparative evaluation of susceptibility to motion artifact in different wearable systems for monitoring respiratory rate. IEEE Transact Inform Technol Biomed. 2010;14(2):378–86.
Mayordomo-Colunga J, Medina A, Rey C, Díaz JJ, Concha A, Los Arcos M, et al. Predictive factors of non invasive ventilation failure in critically ill children: a prospective epidemiological study. Intensive Care Med. 2009;35:527–36.
McKinley S, Madronio C. Validity of the Faces Anxiety Scale for the assessment of state anxiety in intensive care patients not receiving mechanical ventilation. J Psychosom Res. 2008;64:503–7.
Najaf-Zadeh A, Leclerc F. Noninvasive positive pressure ventilation for acute respiratory failure in children: a concise review. Ann Intensive Care. 2011;1(15):2–10.
Navalesi P, Fanfulla F, Frigerio P, Gregoretti C, Nava S. Physiologic evaluation of noninvasive mechanical ventilation delivered with three types of masks in patients with chronic hypercapnic respiratory failure. Crit Care Med. 2000;28(6):1785–90.
Ozsancak A, Sidhom SS, Liesching TN, Howard W, Hill NS. Evaluation of the total face mask for noninvasive ventilation to treat acute respiratory failure. Chest. 2011;139(5):1034–41.
Papa GFS, Di Marco F, Akoumianaki E, Brochard L. Recent advances in interfaces for non-invasive ventilation: from bench studies to practical issues. Minerva Anestesiol. 2012;78:1146–53.
Perez W, Tobin M. Separation of factors responsible for change in breathing pattern induced by instrumentation. J Appl Physiol. 1985;59:1515–20.
Pisani L, Carlucci A, Nava S. Interfaces for noninvasive mechanical ventilation: technical aspects and efficiency. Minerva Anestesiol. 2012;78:1154–61.
Poole KA, Thompson JR, Hallinan HM, Beardsmore CS. Respiratory inductance plethysmography in healthy infants: a comparison of three calibration methods. Eur Respir J. 2000;16:1084–90.
Reis MS, Sampaio LMM, Lacerda D, De Oliveira LVF, Pereira GB, Pantoni CBF, et al. Acute effects of different levels of continuous positive airway pressure on cardiac autonomic modulation in chronic heart failure and chronic obstructive pulmonary disease. Arch Med Sci. 2010;6(5):719–27.
Ritz T, Thöns M, Dahme B. Modulation of respiratory sinus arrhythmia by respiration rate and volume: stability across postures and volume variations. Psychophysiology. 2001;38:858–62.
Sasano N, Vesely AE, Hayano J, Sasano H, Somogyi R, Preiss D, et al. Direct effect of PaCO2 on respiratory sinus arrhythmia in conscious humans. Am J Physiol Heart Circ Physiol. 2002;282:973–6.
Schettino GPP, Tucci MR, Sousa R, Barbas CSV, Amato MBP, Carvalho CRR. Mask mechanics and leak dynamics during noninvasive pressure support ventilation: a bench study. Intensive Care Med. 2001;27:1887–91.
Silva H, Fred A, Lourenço A. Electrodermal response propagation time as a potential psychophysiological marker. 34th Annual International Conference of the IEEE EMBS, 2012, San Diego, California, USA, 28 August - 1 September.
Silva RM, Timenetsky KT, Neves RCM, Shigemichi LH, Kanda SS, Maekawa C, et al. Adaptation to different noninvasive ventilation masks in critically ill patients. J Bras Pneumol. 2013;39(3):469–75.
Task Force Of The European Society Of Cardiology And The North American Society Of Pacing And Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–81.
Tronstad C, KalvøY H, Grimnes S, Martinsen øG. Waveform difference between skin conductance and skin potential responses in relation to electrical and evaporative properties of skin. Psychophysiology. 2013;50:1070–8.
Valipour A, Schneider F, Kössler W, Saliba S, Burghuber OC. Heart rate variability and spontaneous baroreflex sequences in supine healthy volunteers subjected to nasal positive airway pressure. J Appl Physiol. 2005;99:2137–43.
Vaschetto R, De Jong A, Conseli M, Galia F, Mahul M, Coisel Y, et al. Comparative evaluation of three interfaces for non-invasive ventilation: a randomized cross-over design physiologic study on healthy volunteers. Crit Care. 2014;18:R2.
Weissman C, Askanazi J, Milic-Emili J, Kinney JM. Effect of respiratory apparatus on respiration. J Appl Physiol. 1984;57:475–80.
Funding
This work was partially funded by the Brazilian state agencies Coordination for the Improvement of Higher Education Personnel (CAPES), National Council for Scientific and Technological Development (CNPq), and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 54 kb)
Rights and permissions
About this article
Cite this article
da Cunha-Martins, B.S.M., Motta-Ribeiro, G.C. & Jandre, F.C. Short-term usage of three non-invasive ventilation interfaces causes progressive discomfort in healthy adults. Res. Biomed. Eng. 37, 289–298 (2021). https://doi.org/10.1007/s42600-020-00114-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42600-020-00114-3