Abstract
Highland barley (HB), a valuable crop thriving in challenging conditions on the Qinghai-Tibet Plateau, possesses rich nutrient contents. This study aims to investigate the nutrient profile of HB genotypes and compare the metabolic profiles of three representative genotypes exhibiting high, medium, and low quality. The screening of HB genotypes reveals significant genotype-dependent differences in starch content, protein content, amino acid content, flavonoid content, antioxidant capacity, β-glucan, and γ-aminobutyric acid. The selected genotypes, namely KKDM2021005, ZJDM012, and DCDM2020017, exemplify high, average, and low quality, respectively. Utilizing LC–MS/MS for metabolic profiling, 617 metabolites from 12 major classes, with flavonoids being the most abundant, are identified. Distinct metabolic profiles are observed among the three genotypes, with 262 DAMs for KKDM2021005 versus DCDM2020017, 261 DAMs for KKDM2021005 versus ZJDM012, and 298 DAMs for ZJDM012 versus DCDM2020017. Enrichment analysis of DAMs highlights pathways associated with anthocyanin, phenylpropanoid, flavone and flavonol, and isoflavonoid biosynthesis. Specific DAMs such as l-valine, l-isoleucine, l-leucine, trifolin, spiraeoside, ferulic acid, betanin, cyanidin-3-O-galactoside, and cyanidin-3-O-glucoside, along with others, contribute to the observed quality differences among the genotypes. These findings provide a foundation for further exploration of genotype-specific metabolic profiles to manipulate the quality aspects of HB utilizing the existing gene pool. By enhancing its nutritional value, functional properties, and potential health benefits, HB can receive significant value addition.
Article Highlights
-
1.
The study highlights the quality variation in highland barley genotypes.
-
2.
Comparative metabolomics of three distinct genotypes underpinned key differences in metabolite accumulation with flavonoids being most distinctive class.
-
3.
The results will facilitate research focused on improving highland barley quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Highland barley (HB, Hordeum vulgare var. nudum) is a prominent crop in the Qinghai-Tibet Plateau, specifically in Tibet, due to its ability to thrive in the challenging climate of high altitude, strong solar radiation, and decreasing temperatures with increasing altitude [1]. It accounts for over 50% of the arable land in Tibet and is considered a vital grain crop in the region, with the largest planting area and highest total yield [2]. HB is a type of naked barley that is known for its rich nutrient content, making it an important food source for the local population. In recent years, there has been growing interest and appreciation for highland barley worldwide due to its rich nutritional values [3]. HB possesses a unique nutritional profile characterized by high levels of protein, fiber, vitamins along with low fat, low sugar, and an array of bioactive components [4].
In recent years, HB has become the focus of considerable research interest owing to its nutritional profile being superior to conventional cereal grains [5]. It has lower starch digestibility compared to wheat, rice, and corn [6]. Eating foods containing HB on a regular basis can help provide the essential amino acids required daily by the human body [5]. HB also contains a high amount of dietary fiber (8.16–21.46%), which is higher than other grains such as wheat (10.2–15.7%), rice (2.7–7.9%), corn (13.1–19.6%), sorghum (7.55–12.30%), and millet (13.0–13.8%) [7]. Dietary fiber is a polysaccharide with significant physiological functions, and HB’s fiber content can contribute to reducing chronic diseases [8]. HB stands out for its exceptionally high β-glucan content compared to regular barley. The β-glucan levels in HB varieties range from 4 to 8%, and some genotypes contain up to 8.62% β-glucan, which is markedly higher than the β-glucan content found in common barley [4]. Additionally, HB is rich in γ-aminobutyric acid (GABA) compared to regular barley [9], and it also contains abundant essential elements and trace elements such as calcium, phosphorus, iron, zinc, and selenium [10]. These unique nutritional characteristics make HB a valuable crop with potential health benefits, and further research on its nutritional composition and health-promoting properties is warranted.
The quality traits of HB, including its nutrient composition and bioactive compounds, are important determinants of its nutritional and commercial value [11]. Therefore, to better understand the factors that contribute to the quality differences among HB genotypes, a metabolome analysis can be employed. Metabolome analysis is a powerful approach that allows for the comprehensive profiling of small molecules in biological samples, providing valuable insights into the metabolic pathways and chemical composition of crops [12]. For instance, Weng et al. provides a starting point for using metabolomics coupled with transcriptomics to assist in the breeding of pyroxslum-tolerant HB varieties [13]. However, quality characterization of barley genotypes based on metabolomics has not been fully investigated. By analyzing the specific substances that exhibit differential changes in HB with varying quality traits, a deeper understanding of the metabolic changes associated with quality differences can be obtained. This knowledge can serve as a theoretical basis for further research on HB quality and contribute to the improvement of breeding and processing strategies for this important crop.
The overarching goal of this study was to link the nutritional composition of HB genotypes to their metabolomic profiles to gain insights into metabolic factors influencing grain quality. The specific objectives were to: (i) evaluate the starch, protein, β-glucan and GABA content in 53 different HB genotypes and identify high, medium and low quality varieties based on nutrient levels; (ii) perform metabolome analysis on selected high, medium and low quality genotypes using LC–MS/MS; (iii) compare the metabolomic landscapes of the selected varieties to identify key differential metabolites related to quality traits; (iv) map the metabolic pathways and processes enriched in each variety to reveal associations with nutritional composition; and (v) elucidate the metabolic changes underlying differences in HB grain quality. The study aims to establish connections between nutrient levels and metabolic profiles across diverse HB materials, providing a framework for future research on breeding and develo** elite HB varieties with superior nutritional quality and commercial value.
2 Materials and methods
2.1 Plant materials
We assessed a total of 53 HB genotypes sourced from various regions, including Naqqu, Lhasa, and Linzhi within Tibet prefecture. These genotypes were cultivated in glass house under control conditions at Tibet Academy of Agricultural and Animal Husbandry Sciences, Lhasa, China. The temperature was maintained at 15 °C during the night and raised to 20 °C during the day to ensure optimal growth. Relative humidity levels between 40 and 60% were maintained using humidifiers and dehumidifiers. The photoperiod was set to 16 h with daytime light intensity controlled between 300 and 400 μmol/m2s using supplemental lighting. The plants were grown in sandy loam soil with pH adjusted to 6.5 and moisture levels sustained at 60–80% field capacity by scheduled irrigation. Balanced fertilizer (NPK 15:15:15) was applied at planting and top-dressed with 50 kg/ha nitrogen at tillering to ensure adequate nutrition. Ventilation and circulation fans facilitated air exchange while preventing fungal diseases. Pest monitoring and control ensured the plants remained free of infestations. After harvesting, we collected seeds from each genotype for subsequent physiological and metabolic profiling. Here are the details of the physiological parameters analyzed:
2.2 Determination of starch contents
The starch content of HB seeds was determined through acid hydrolysis of starch into glucose followed by colorimetric measurement or commonly known as anthrone method [14]. Finely powdered seed samples (1 g) were extracted with 80% ethanol to remove soluble sugars, dried, and hydrolyzed with 2.5 N HCl at 100 °C for 3 h. After hydrolysis, the sample was neutralized using sodium carbonate and made up to 100 ml total volume with water. The glucose content in the diluted hydrolysate was then determined through a colorimetric method using anthrone reagent, which forms a colored complex with glucose that can be measured spectrophotometrically. The blue-green color developed was measured at 630 nm. Standard solutions of glucose (0–1 mg/ml) were similarly treated, and a calibration curve was constructed. The glucose concentration determined through colorimetric analysis was compared to a calibration curve prepared using glucose standards. This allowed for calculation of the starch content in the sample, by applying a dilution factor of 0.9 to account for the hydrolysis and dilution steps. The entire assay was performed in triplicates and the starch content results were averaged.
2.3 Determination of protein contents
The bicinchoninic acid (BCA) assay was utilized to determine the protein content in the HB seed samples [15]. To prepare samples for the BCA assay, 10 mg of finely ground HB seed flour was vortexed for 1 min in 1 ml of extraction buffer. The extracts were then centrifuged at 10,000 × g for 5 min and the resulting clear supernatants were used for protein estimation. The BCA working reagent was prepared by mixing reagent A, an alkaline bicinchoninic acid (BCA) solution, with reagent B, which contains copper sulfate (CuSO4), in a 50:1 ratio. For the assay, triplicates of 10 μl sample extract or bovine serum albumin standards (0.1–1.0 mg/ml) were pipetted into a 96-well microplate, followed by addition of 200 μl of the working reagent into each well. After incubating for 30 min at 37 °C, absorbance was read at 562 nm using a microplate reader. Protein concentrations were determined by comparing to the standard curve prepared using the albumin standards. Finally, the protein content was expressed in mg protein per g of flour.
2.4 Determination of total flavonoids
The total flavonoid content was determined using an aluminum chloride colorimetric method as described by Adom et al. [16] with minor modifications. Seed extract samples (1 ml) were mixed with 5% sodium nitrite solution and reacted for 6 min, followed by addition of 10% aluminum chloride hexahydrate and further incubation for 6 min to form complexes. The reaction was stopped by adding 2 ml of 4% sodium hydroxide. After incubating the final mixture was put at room temperature for 15 min in the dark, and then the absorbance was measured spectrophotometrically at 510 nm. Catechin standard solutions were used to construct a calibration curve. The total flavonoid concentration of samples was interpolated from the standard curve and expressed as catechin equivalents in mg per 100 g of dry weight sample. Triplicate analyses were performed, and results were reported as mean ± standard deviation (n = 3).
2.5 Determination of total amino acids
The amino acid composition of HB seed samples was analyzed by the ninhydrin colorimetric method. Finely ground seed flour samples were extracted using 70% ethanol. The obtained extracts were then spotted onto TLC plates, along with standard amino acid reference compounds. The plates were developed using an optimized solvent system and then sprayed with 0.2% ninhydrin solution. The plates were heated at 100 °C for 10 min to facilitate the reaction between ninhydrin and free amino acids to generate a purple color complex. The intensity of the colored spots produced was measured at 570 nm using a densitometer. Each amino acid produced a distinctive color spot corresponding to its Rf value which was compared to standard references for identification. The color intensity of the spots was proportional to the concentration of amino acids, as compared to a calibration curve of standards. Quantification was achieved based on the color intensity measured for each amino acid spot. Cysteine and tryptophan could not be detected by this method. The analysis was performed in three replicates for each sample.
2.6 Determination of β-glucan
Finely milled HB seed samples (100 mg each) were subjected to enzymatic hydrolysis using the β-Glucan Assay Kit as per manufacturer's protocol. The sample was incubated with lichenase enzyme solution to specifically hydrolyze β-glucan into soluble β-glucooligosaccharides. The hydrolysate was then incubated with β-glucosidase to break down the oligosaccharides into glucose units. The released glucose was specifically oxidized by glucose oxidase/peroxidase reagent to generate a red quinoneimine dye with absorbance measured at 510 nm. A calibration curve was constructed using varying concentrations of β-glucan standard provided in the kit. The β-glucan content in the sample hydrolysate was interpolated from the standard curve after making suitable dilutions. The assay was performed in three replicates for each sample and expressed in terms of percentage β-Glucan contents per 100 mg of sample.
2.7 Determination of GABA
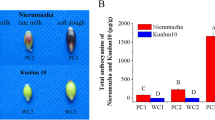
Γ-aminobutyric acid (GABA) is an important determinant of quality in HB and other cereals [3A, B, and C). These findings suggest that the differential accumulation of metabolites within these pathways may contribute to the quality variations observed among the three HB genotypes.
In the metabolic profiling of the three genotypes (KKDM2021005, ZJDM012, and DCDM2020017), flavonoids were identified as major contributors to the observed metabolic profiles. Out of the 211 identified flavonoids, 69 flavonoids exhibited a conserved differential accumulation pattern among the three genotypes. The quantification of these 69 flavonoids is depicted in Fig. 4A. Interestingly, the genotype ZJDM012 showed the highest accumulation pattern of different flavonoids compared to KKDM2021005 and DCDM2020017. This suggests that ZJDM012 genotype may possess a higher capacity for flavonoid production compared to the other two genotypes.
Among the subclasses of flavonoids, flavones, flavanols, and anthocyanins were identified as the major contributors to the flavonoid accumulation. These subclasses of flavonoids likely play a significant role in determining the metabolic differences observed among the three genotypes. These findings provide valuable insights into the variations in flavonoid accumulation among the genotypes and shed light on the potential contributions of flavonoids to the quality differences observed in the HB genotypes.
In addition to flavonoids, we also analyzed the amino acid composition and identified 48 amino acids. Among them, 10 amino acids exhibited conserved differential accumulation patterns in all three genotype comparisons (KKDM2021005 versus DCDM2020017, KKDM2021005 versus ZJDM012, and ZJDM012 versus DCDM2020017). Interestingly, all of these conserved differential accumulated metabolites (DAMs) displayed a down-accumulation pattern in the three genotype comparisons. Furthermore, specific amino acids such as l-valine, l-isoleucine, and l-leucine exhibited the highest accumulation pattern in KKDM2021005 compared to ZJDM012 and DCDM2020017. These findings indicate that there are significant differences in the accumulation of amino acids among the three genotypes, with specific amino acids showing consistent down-accumulation patterns across all genotype comparisons. L-valine, l-isoleucine, and l-leucine were particularly found to have higher accumulation in KKDM2021005 compared to the other two genotypes.
Based on the differential accumulation patterns of metabolites, we identified several top-fold change differential accumulated metabolites (DAMs) in the extreme (higher and lower) accumulation patterns (Fig. 5A and Additional File 4: Table S4). In the comparison of DCDM2020017 to KKDM2021005, DAMs such as maleoyl-caffeoylquinic acid, 3-O-acetylpinobanksin, D-sedoheptuiose 7-phosphate, glucarate O-phosphoric acid, 4-methyl-5-thiazoleethanol, niacinamide, chrysoeriol-O-acetylhexoside, quercetin-7-O-(6′-O-malonyl)-β-D-glucoside, ferulic acid, and betanin showed a positive accumulation pattern in DCDM2020017. On the other hand, DAMs including tricin 7-O-glucuronide, rhamnopyranoside, thymine, cyanidin-3-O-galactoside, quercetin 4′-O-glucoside (Spiraeoside), cyanidin-3-O-glucoside (Kuromanin), petunidin-O-pentoside, 1-methyladenine, velutin O-glucuronic acid, and kaempferol 3-O-galactoside (Trifolin) exhibited an up-accumulated pattern in KKDM2021005.
These DAMs belong to various metabolite classes. In DCDM2020017, metabolites such as maleoyl-caffeoylquinic acid, ferulic acid, and betanin are phenolic acids, while 3-O-Acetylpinobanksin is a flavonoid. Additionally, D-Ssedoheptuiose 7-phosphate and glucarate O-Phosphoric acid are nucleotides and derivatives. On the other hand, DAMs in KKDM2021005, such as tricin 7-O-Glucuronide, cyanidin-3-O-galactoside, and cyanidin-3-O-glucoside, belong to the flavonoid class. Thymine and 1-methyladenine are nucleotides and derivatives, while velutin O-glucuronic acid is an organic acid. Moreover, quercetin 4'-O-glucoside and kaempferol 3-O-galactoside are flavonoids with specific sugar attachments. These findings highlight the diverse accumulation patterns of metabolites across different classes, providing insights into the specific metabolic changes associated with the quality variations observed in DCDM2020017 and KKDM2021005.
In the comparison of KKDM2021005 and ZJDM012 samples, we observed a positive accumulation pattern for several metabolites (Fig. 5B and Additional File 5: Table S5). These included maleoyl-caffeoylquinic acid, 3-O-acetylpinobanksin, D-sedoheptuiose 7-phosphate, glucarate O-phosphoric acid, 4-Methyl-5-thiazoleethanol, niacinamide, chrysoeriol-O-acetylhexoside, quercetin-7-O-(6′-O-malonyl)-β-D-glucoside, ferulic acid, and betanin in KKDM2021005. Conversely, in KKDM2021005, metabolites such as tricin 7-O-glucuronide, rhamnopyranoside, thymine, cyanidin-3-O-galactoside, quercetin 4′-O-glucoside (Spiraeoside), cyanidin-3-O-glucoside (Kuromanin), petunidin-O-pentoside, 1-methyladenine, velutin O-glucuronic acid, and kaempferol 3-O-galactoside (Trifolin) exhibited an up-accumulated pattern. These identified metabolites belong to various classes. In KKDM2021005, maleoyl-caffeoylquinic acid, ferulic acid, and betanin are phenolic acids, while 3-O-acetylpinobanksin is a flavonoid. Additionally, D-sedoheptuiose 7-phosphate and glucarate O-phosphoric acid are nucleotides and derivatives. In the case of KKDM2021005, tricin 7-O-glucuronide, cyanidin-3-O-galactoside, and cyanidin-3-O-glucoside are classified as flavonoids. Thymine and 1-Methyladenine belong to the category of nucleotides and derivatives, whereas velutin O-glucuronic acid is an organic acid. Furthermore, quercetin 4′-O-glucoside and kaempferol 3-O-galactoside are flavonoids with specific sugar attachments.
Similarly, comparison ZJDM012 versus DCDM2020017 identified metabolites such as hesperetin C-hexoside, 5-O-p-coumaroyl quinic acid O-hexoside, 3-O-acetylpinobanksin, isorhamnetin O-hexoside, 9-hydroxy-12-oxo-10-octadecenoic acid, epicatechin-epiafzelechin, epicatechin, vanillin, gramine, and epicatechin glucoside in the up-regulated category (Fig. 5C and Additional File 6: Table S6). These metabolites belong to various classes such as flavonoid carbonoside, phenolic acids, flavanols, flavonols, free fatty acids, and alkaloids. On the other hand, in the down-regulated category, we observe metabolites including cyanidin-3-O-galactoside, cyanidin O-acetylhexoside, cyanidin-3-O-glucoside (Kuromanin), petunidin-O-pentoside, pelargonidin-3-O-(6″-malonylglucoside), pelargonidin-3-O-(3″,6″-dimalonylglucoside), cyanidin-O-syringic acid, cyanidin-3-O-(3″,6″-diacetylhexoside)-O-glyceric acid, kaempferol 3-O-galactoside (Trifolin), and delphinidin-3-O-(3″,6″-dimalonylglucoside). These metabolites mainly belong to the class of anthocyanins.
These findings highlight the diverse accumulation patterns of metabolites across different classes in HB genotypes DCDM2020017, KKDM2021005, and ZJDM012. The variations in metabolite accumulation suggest underlying metabolic differences and potential implications for the quality attributes of these genotypes. Understanding the specific metabolic changes associated with different genotypes can contribute to the development of targeted breeding strategies to enhance desirable quality traits in HB. It is important to note that further investigations, such as functional validation and pathway analysis, are necessary to elucidate the precise roles of the identified metabolites and their corresponding pathways in determining the observed quality variations. Additionally, considering a larger sample size and conducting sensory evaluations could provide a more comprehensive understanding of the impact of these metabolites on the overall quality characteristics of highland barley genotypes.
4 Discussion
A screening of 53 highland barley (HB) genotypes was conducted to investigate variations in quality parameters such as starch content, protein content, amino acid content, flavonoid content, antioxidant capacity, β-Glucan, and GABA. Significant genotype-dependent differences were found for all the examined traits as previously observed in several studies [11, 24,25,26]. We identified β-Glucan with an average of 5.83%, in line with previous reports suggesting 5–8% in HB genotypes [4, 27]. Obadi et al. [7] found that HB flour has lower protein and β-glucan levels, but higher lipid, crude fiber, and ash levels compared to waxy or high-amylose HB varieties. In contrast, a previous study by Kinner et al. demonstrated that HB varieties have better nutritional value due to their higher content of proteins, lipids, crude fiber, and β-glucan [28]. Both HB and oats contain higher β-glucan compared to other cereals, although the actual content can vary based on genotype and environmental factors [29]. HB primarily consists of starch, which accounts for approximately 65% of its weight. Approximately 80% of the total dry weight of HB grains consists of various carbohydrates, encompassing sugars and non-starch polysaccharides like β-glucan and arabinoxylan [30]. The quantity and composition of these carbohydrates vary subject to the grain type, genotype, and environmental conditions during crop development [30].
Starch, being the major constituent of HB, plays a crucial role in determining the functional properties of HB products. We identified starch contents ranging from 529 to 614 mg/g. these results confirm previous findings of identification of starch contents in HB genotypes [31]. The pasting characteristics of starch, which include parameters such as pasting temperature and peak viscosity, directly influence the thickening and gelling behavior of food products. The digestibility of starch components is also important for understanding the nutritional characteristics of food, such as glycemic index [32]. Compared to starches from other cereals, HB starch exhibits distinctive properties. It displays higher pasting temperatures, peak viscosities, and improved retrogradation and gelatinization characteristics [33]. These unique properties make HB starch suitable for specific applications in the food industry where higher thermal stability, viscosity, and texture are desired.
Three genotypes, KKDM2021005, ZJDM012, and DCDM2020017, were selected to represent high, average, and low quality, respectively and used for metabolic profiling. Metabolic profiling using LC–MS/MS revealed variations in metabolite accumulation among the genotypes, with 617 metabolites from 12 major classes quantified. Differential accumulated metabolites (DAMs) were identified among the three genotypes. Previously, several studies have utilized metabolomics to observe metabolic profile of HB genotypes under stress conditions [13, 44,45]. Furthermore, HB stands out by presenting increased levels of essential amino acids, particularly lysine and threonine, in comparison to wheat and hulled barley. Concerning the protein amino acid composition, no substantial differences have been observed among different hull-less barley type [46]. Barley is recognized for its richness in prolamin-type proteins, which are soluble in alcohol and contain sulfur-containing amino acids, although they are limited in lysine. In the specific case of HB, its protein content has been documented at approximately 11.31%, accompanied by elevated levels of eight essential amino acids, with a particular emphasis on lysine and tryptophan. These characteristics set HB apart from other commonly consumed grains such as wheat, rice, and maize [47].
HB also possesses noteworthy levels of γ-aminobutyric acid (GABA), a naturally occurring compound present in plant sources. A study conducted by Waleed et al. [20] explored several treatment approaches for HB, including soaking, germination, fermentation, germination before fermentation, and fermentation before germination. This investigation revealed that all of these methods led to a substantial increase in the GABA content within HB. In a broader context, HB varieties are characterized by elevated protein content and contain essential amino acids such as lysine and tryptophan, distinguishing them from hulled barley and other cereal grains. Additionally, HB stands out as a promising reservoir of GABA, with its potential for augmentation through diverse processing methods [48].
In summary, our study revealed significant genotype-dependent variations in quality parameters such as starch content, protein content, amino acid content, flavonoid content, antioxidant capacity, β-Glucan, and GABA in HB genotypes. Flavonoids, specifically flavones, flavanols, and anthocyanins, emerged as prominent contributors to the metabolic profile of HB genotypes, offering diverse health benefits including antioxidant, anticancer, anti-inflammatory, and antiallergic effects. Future research should focus on exploring the genetic and environmental factors influencing the observed variations in quality parameters, particularly investigating the specific genes and pathways involved in starch, protein, and flavonoid biosynthesis in HB genotypes. Furthermore, the impact of post-harvest processing methods on the nutritional composition and bioactive compounds of HB warrants further investigation. With appropriate variety selection and processing, HB holds promise as a nutritionally balanced food crop and a component of healthy diets. Additionally, studying the functional properties and potential applications of HB starch in the food industry would be valuable. Continued research on HB will contribute to a comprehensive understanding of its nutritional value, functional properties, and potential health benefits.
Data availability
All data used in this work can be found inside the text and in the supplementary Tables.
References
Feng X, Wang G, Wang J. Space distribution of highland barley GNS and its relationship with environmental factors in the Qinghai-Tibet Plateau. Am J Biochem Biotechnol. 2018;14:137–44.
d’AlpoimGuedes JA, Lu H, Hein AM, Schmidt AH. Early evidence for the use of wheat and barley as staple crops on the margins of the Tibetan Plateau. Proc Natl Acad Sci. 2015;112:5625–30.
Lyu Y, Ma S, Liu J, Wang X. A systematic review of highland barley: ingredients, health functions and applications. Grain Oil Sci Technol. 2022;5:35–43.
Guo T, Horvath C, Chen L, Chen J, Zheng B. Understanding the nutrient composition and nutritional functions of highland barley (Qingke): a review. Trends Food Sci Technol. 2020;103:109–17.
Wang CP, Pan ZF, Nima ZX, Tang YW, Cai P, Liang JJ, Deng GB, Long H, Yu MQ. Starch granule-associated proteins of hull-less barley (Hordeum vulgare L.) from the Qinghai-Tibet Plateau in China. J Sci Food Agric. 2011;91:616–24.
Moza J, Gujral HS. Starch digestibility and bioactivity of high altitude hulless barley. Food Chem. 2016;194:561–8.
Obadi M, Sun J, Xu B. Highland barley: Chemical composition, bioactive compounds, health effects, and applications. Food Res Int. 2021;140:110065.
Deng J, **ang Z, Lin C, Zhu Y, Yang K, Liu T, **a C, Chen J, Zhang W, Zhang Y. Identification and quantification of free, esterified, and insoluble-bound phenolics in grains of hulless barley varieties and their antioxidant activities. LWT. 2021;151:112001.
Farag MA, **ao J, Abdallah HM. Nutritional value of barley cereal and better opportunities for its processing as a value-added food: a comprehensive review. Crit Rev Food Sci Nutr. 2022;62:1092–104.
Petrova P, Petrov K. Lactic acid fermentation of cereals and pseudocereals: ancient nutritional biotechnologies with modern applications. Nutrients. 2020;12:1118.
Dang B, Zhang WG, Zhang J, Yang XJ, Xu HD. Evaluation of nutritional components, phenolic composition, and antioxidant capacity of highland barley with different grain colors on the Qinghai Tibet Plateau. Foods. 2025;2022:11.
Pavagadhi S, Swarup S. Metabolomics for evaluating flavor-associated metabolites in plant-based products. Metabolites. 2020;10:197.
Weng H, Yan J, Guo L, Chen H. Integrated transcriptomic and metabolomic analyses of the molecular mechanisms of two highland barley genotypes with pyroxsulam responses. Front Plant Sci. 2022;13:1030578.
Buckan DS. Estimation of glycemic carbohydrates from commonly consumed foods using modified anthrone method. Indian J Appl Res. 2015;3:45–7.
Chan K, Wasserman BP. Rapid solid-phase determination of cereal protein using bicinchoninic acid. Cereal Chem. 1993;70:27–27.
Adom KK, Sorrells ME, Liu RH. Phytochemical profiles and antioxidant activity of wheat varieties. J Agric Food Chem. 2003;51:7825–34.
Zheng Q, Wang Z, **ong F, Song Y, Zhang G. Effect of pearling on nutritional value of highland barley flour and processing characteristics of noodles. Food Chem X. 2023;17:100596.
Al-Ansi W, Zhang Y, Alkawry TAA, Al-Adeeb A, Mahdi AA, Al-Maqtari QA, Ahmed A, Mushtaq BS, Fan M, Li Y. Influence of germination on bread-making behaviors, functional and shelf-life properties, and overall quality of highland barley bread. LWT. 2022;159:113200.
Wang S, Zhou S, Wang L, Liu X, Ma Y, Tong L, Zhang Y, Wang F. Effect of an environment friendly heat and relative humidity approach on γ-aminobutyric acid accumulation in different highland barley cultivars. Foods. 2022;11:691.
Waleed A-A, Mahdi AA, Al-Maqtari QA, Mushtaq BS, Ahmed A, Karrar E, Mohammed JK, Fan M, Li Y, Qian H. The potential improvements of naked barley pretreatments on GABA, β-glucan, and antioxidant properties. LWT. 2020;130:109698.
Ikram S, Zhang H, Ming H, Wang J. Recovery of major phenolic acids and antioxidant activity of highland barley brewer’s spent grains extracts. J Food Process Preserv. 2020;44:e14308.
Wang Y, Zeng X, Xu Q, Mei X, Yuan H, Jiabu D, Sang Z, Nyima T. Metabolite profiling in two contrasting Tibetan hulless barley cultivars revealed the core salt-responsive metabolome and key salt-tolerance biomarkers. AoB Plants. 2019;11:plz021.
Cao H, Ji Y, Li S, Lu L, Tian M, Yang W, Li H. Extensive metabolic profiles of leaves and stems from the medicinal plant Dendrobium officinale Kimura et Migo. Metabolites. 2019;9:215.
Roohi E, Mohammadi R, Niane AA, Niazian M, Niedbała G. Agronomic performance of rainfed barley genotypes under different tillage systems in highland areas of dryland conditions. Agronomy. 2022;12:1070.
Terefe D, Desalegn T, Ashagre H. Effect of nitrogen fertilizer levels on grain yield and quality of malt barley (Hordeum vulgare L.) varieties at Wolmera District, Central Highland of Ethiopia. Int J Res Stud Agric Sci. 2018;4:29–43.
Tsige T, Shiferaw T, Gezahegn S, Taye K. Assessment of malt barley genotypes for grain yield and malting quality traits in the central highlands of Ethiopia. Assessment. 2020;10.
Tian M, Song J, Liu P, Su L, Sun C, Li Y. Effects of beta glucan in highland barley on blood glucose and serum lipid in high fat-induced C57 mouse. Zhonghua yu Fang yi xue za zhi [Chinese Journal of Preventive Medicine]. 2013;47:55–8.
Kinner M, Nitschko S, Sommeregger J, Petrasch A, Linsberger-Martin G, Grausgruber H, Berghofer E, Siebenhandl-Ehn S. Naked barley—optimized recipe for pure barley bread with sufficient beta-glucan according to the EFSA health claims. J Cereal Sci. 2011;53:225–30.
Ehrenbergerová J, BřezinováBelcredi N, Psota V, Hrstková P, Cerkal R, Newman C. Changes caused by genotype and environmental conditions in beta-glucan content of spring barley for dietetically beneficial human nutrition. Plant Foods Hum Nutr. 2008;63:111–7.
Gous PW, Fox GP. Amylopectin synthesis and hydrolysis–Understanding isoamylase and limit dextrinase and their impact on starch structure on barley (Hordeum vulgare) quality. Trends Food Sci Technol. 2017;62:23–32.
Waleed AA, Mahdi AA, Al-Maqtari QA, Sajid BM, Al-Adeeb A, Ahmed A, Fan M, Li Y, Qian H, **xin L. Characterization of molecular, physicochemical, and morphological properties of starch isolated from germinated highland barley. Food Biosci. 2021;42:101052.
Asare EK, Jaiswal S, Maley J, Baga M, Sammynaiken R, Rossnagel BG, Chibbar RN. Barley grain constituents, starch composition, and structure affect starch in vitro enzymatic hydrolysis. J Agric Food Chem. 2011;59:4743–54.
Yangcheng H, Gong L, Zhang Y, Jane J-L. Pysicochemical properties of Tibetan hull-less barley starch. Carbohyds Polym. 2016;137:525–31.
Tang Y, **ao L, Wu X, Li W, Wu T, Zhang P. Impact of germination pretreatment on the polyphenol profile, antioxidant activities, and physicochemical properties of three color cultivars of highland barley. J Cereal Sci. 2021;97:103152.
Tang Y, Zhang B, Li X, Chen PX, Zhang H, Liu R, Tsao R. Bound phenolics of quinoa seeds released by acid, alkaline, and enzymatic treatments and their antioxidant and α-glucosidase and pancreatic lipase inhibitory effects. J Agric Food Chem. 2016;64:1712–9.
Irakli M, Lazaridou A, Mylonas I, Biliaderis CG. Bioactive components and antioxidant activity distribution in pearling fractions of different Greek barley cultivars. Foods. 2020;9:783.
Abdel-Aal E-SM, Young JC, Rabalski I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J Agric Food Chem. 2006;54:4696–704.
Abdel-Aal EM, Choo T-M, Dhillon S, Rabalski I. Free and bound phenolic acids and total phenolics in black, blue and yellow barley and their contribution to free radical scavenging capacity. Cereal Chem. 2012;89:198–204.
Kim MJ, Hyun JN, Kim JA, Park JC, Kim MY, Kim JG, Lee SJ, Chun SC, Chung IM. Relationship between phenolic compounds, anthocyanins content and antioxidant activity in colored barley germplasm. J Agric Food Chem. 2007;55:4802–9.
Yang XJ, Dang B, Fan MT. Free and bound phenolic compound content and antioxidant activity of different cultivated blue highland barley varieties from the Qinghai-Tibet Plateau. Molecules. 2018;23:879.
Idehen E, Tang Y, Sang S. Bioactive phytochemicals in barley. J Food Drug Anal. 2017;25:148–61.
Quinde-Axtell Z, Baik B-K. Phenolic compounds of barley grain and their implication in food product discoloration. J Agric Food Chem. 2006;54:9978–84.
Sterna V, Zute S, Jansone I, Kantane I. Chemical composition of covered and naked spring barley varieties and their potential for food production. Pol J Food Nutr Sci. 2017;67:151–8.
Sturite I, Kronberga A, Strazdina V, Kokare A, Aassveen M, Bergjord Olsen AK, Sterna V, Straumite E. Adaptability of hull-less barley varieties to different crop** systems and climatic conditions. Acta Agric Scand Sect B Soil Plant Sci. 2019;69:1–11.
Wirkijowska A, Rzedzicki Z, Zarzycki P, Sobota A, Sykut-Domanska E. Chemical composition of naked barley for production of functional food. Acta Agrophys. 2016;23:287–301.
Helm CV, de Francisco A, Gaziola SA, Francisco Fornazier R, BertoniPompeu G, AntunesAzevedo R. Hull-less barley varieties: storage proteins and amino acid distribution in relation to nutritional quality. Food Biotechnol. 2004;18:327–41.
Yan WW, Yao HYY, Nie SP, Li YJ. Mineral analysis of hulless barley grown in different areas and its β-glucan concentrates. Cogent Food Agric. 2016;2:1186139.
Ma Y, Wang P, Wang M, Sun M, Gu Z, Yang R. GABA mediates phenolic compounds accumulation and the antioxidant system enhancement in germinated hulless barley under NaCl stress. Food Chem. 2019;270:593–601.
Funding
This work was funded by the National Crop Germplasm Resource Bank Operation Service—Tibet Branch Operation (NCGRC-2023-28), the Operation of the Tibet Scientific Observation Experimental Station for Crop Genetic Resources and Germplasm Creation by the Ministry of Agriculture (XZNKYNYS-2023-C-009) and the Crop Breeding project (XZ202201ZY0013N-003).
Author information
Authors and Affiliations
Contributions
HH: Writing. WL: Project administration, Funding acquisition. HH, YL, XG, PT, NY: Investigation, Data curation. HH, LZ, ZC, ZY: Sampling, Investigation. W.L.: Project Supervision. ZY, WL: Writing-review & editing. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Additional file 1: Table S1.
Quality characterization of 53 highland barley genotypes.
Additional file 2: Table S2.
Characteristics of three genotypes selected for metabolomic analysis.
Additional file 3: Table S3.
Identification and characterization of metabolites identified in three distinct highland barley genotypes.
Additional file 4: Table S4.
Differential metabolic landscape of KKDM2021005 vs. DCDM2020017.
Additional file 5: Table S5.
Differential metabolic landscape of KKDM2021005 vs. ZJDM012.
Additional file 6: Table S6.
Differential metabolic landscape of ZJDM012 vs. DCDM2020017.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, H., Li, Y., Gao, X. et al. Metabolic profiling of highland barley (Hordeum vulgare var. nudum) genotypes. Discov Appl Sci 6, 83 (2024). https://doi.org/10.1007/s42452-024-05710-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-05710-x