Abstract
Abstract
The use of sediments as soils is an area of interest for Beneficial Use of dredged sediments. In this study the impact of the transition from sediments to soils is researched by looking at the seasonal and long year (10 year) change in pore water metal chemistry of sediments which are considered clean (class A) according to the Dutch soil directive. This study is based on a combination of geohydrological, geochemical and ecotoxicological risk models and validated against measured pore water concentrations for metals over an dry/wet period. The pore water metal concentrations are compared against standards and expressed as at Risk Characterization Ratio’s (RCR) values. The RCR values are high (> 1) during the first 3 years after the application of sediments as soil, especially at the end of the summer. The multi substances Potentially Affected Fraction (ms-PAF) shows a similar trend as the RCR values, although it takes 5 years before the combined calculated potential ecotoxicity is below the legal 40% threshold level. Translated to land use, it is advised to restrict land use for farming on soils where these clean (class A) sediments are applied for a five-year transition period.
Article Highlights
-
Beneficial Use of sediments should take into account the different conditions when used as soils.
-
Use of sediments as soils lead to a predicable seasonal and multiple year trend in metal concentrations in pore water.
-
The predicted results in metal pore water concentrations are translated into an advice for temporal land use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Large scale reallocation of soils in deep under water pits has become common practise in parts of Europe as part of river restoration strategies [5]. The use of soil is seen as a beneficial application according to the EU directive 2000/60/EC [17], even when the soil contains contaminants. A recent study [56] focusses on the question on what happens to metals when soils become sediments, addressing the impact of large-scale storage of soils in sandpits and lakes and the impact of reduction kinetics on heavy metals and arsenic release to groundwater. Vink [17] takes into account the DOM characterisation, the iron chemistry and the pore water chemistry (including the main anions and cations). In this study the question is reversed, what happens to the metal availability when sediments become soils? Sediments are reduced and fully saturated with water, while soils in temperate climate regions have a water shortage in the summer and have a water excess in the winter [36]. This yearly cycle exposing the top layer of the soil to air penetration and oxidation during the summer, while in the winter the groundwater level rises causing reduction reactions to dominate.
The concept that reduced sediments with a sufficient high sulphite content pose no environmental threat with regard to metals is supported by work done on the Simultaneous Extracted Metals (SEM) to Acid Volatile Sulfides (AVS) ratio in soils and sediments [2, 14, 46]. Studies using the SEM/AVS ratio as indicator for the bioavailability of metals in sediments tend to rule out metal ecotoxicity in sediments when the SEM/AVS ratio is low [15, 48, 49].
Vink [56] shows that the underwater storage of soils as sediments can form an environmental risk for groundwater, even when the sulphide concentration in the soil is high. When large shifts in redox potential occur kinetic oxidation/reduction rates influence the speed at which (im)mobilisation of metals takes place. This can result in the temporal domination of other binding mechanisms or the emission of metals from the sediment, even when SEM/AVS of less than one would suggest that the metals are immobile [55]. DOM also plays an important role in the binding of heavy metals, especially the humic and fulvic substances of DOM. A model that incorporated such organic matter specific binding is the Non-Ideal Competitive Adsorption (NICA)—Donnan (electrostatic interaction) model [26].
A third important factor is the iron chemistry [62]. When iron oxyhydroxides minerals in the soil are reduced in the presence of sulphide the mineral iron sulphide (FeS(s)) is formed and precipitates. The formed iron sulphide binds trace metals, lowering the pore water metal concentration. The binding of metals by an excess of sulphites is in short the principle behind the SEM/AVS ratio. However, the loss of iron oxyhydroxides minerals due to Fe3+ reduction also diminish the cation sorption capacity [9, 59] and therefore increases the trace metal pore water concentration. Calcite also adsorbs trace metals, showing an initial rapid uptake of trace metals (adsorption) and slow uptake (precipitation) [8]. All these processes have kinetic restrains and often meta-stable reaction products (like the DOM-trace metals ligands).
Sediments and soils under fluctuating redox conditions therefore behave differently as predicted by equilibrium kinetics-based risk models. There is relatively little literature on soils and sediments with (seasonal) changes in redox potential. Salomons [39] looks at pore water concentrations of sulphate and iron during the early stage of diagenesis of dredged brackish sediments. Salomons [39] uses large pits (80 (width) × 30 (length) × 6 m (depth)), but the site stayed submerged and therefore did not form a soil. Weng [57] looks at a sandy soil profile, and combines a numerical multi surface model with observed pore water trace metals to look at long term (16 years) trends in the soil profile. Weng [57] shows the dominant (im)mobilisation processes of trace metals as function of soil properties and changes in soil mineralogy over time but does not give insight in the role of reaction kinetics. Pan [31, 32] looks at the mobilisation/immobilisation of trace metals in a flooding/drying cycle of paddy rice soil, using the Donnan membrane method [58] to collect trace metals from the pore water.
The groundwater conditions and the properties of the sediments and soils studies here are presented under materials and methods. This chapter also describes the background of the numerical models used to simulate the groundwater table variation, the metal behaviour in pore water, the risk of individual metals based on no-effect concentrations and the combined risk of the presence of multiple metals in pore water. The result chapter describes the outcome of the pore water measurements and the modelling results. The discussion chapter places the outcome of the measurements and models in the context of the current BU of sediment practice and soil legislation standards in the Netherlands for the application of sediments on land. In the conclusion chapter advice is given how to adapt land use during a period of transition of sediments to soils.
2 Material and methods
2.1 In general
This study makes use of a location where a 150 cm thick sediment layer is deposited on land. The initial sediment composition and the leachability of metals from the sediment is measured. By using the metal speciation model CHARON [11] in combination with the groundwater model SFYNXZ [11] the initial sediment conditions are used to calculate the changes in metal availability in the newly formed soil as function of soil moisture content over a period of 10 years. CHARON stands for the Chemistry Applied to the Research Of Natural systems. CHARON is an extended and modified version of the chemical equilibrium model CHEMEQ developed by the Rand Corporation [42] and is currently developed and maintained by Deltares. SFYNXZ is a 2D groundwater model based on MODFLOW [20]. During this 10-year period the transition from sediment to soil over a depth of 150 cm is simulated by a yearly fluctuation of the groundwater level over a depth of 100 cm (from the top of the soil), with a minimum pore volume soil moisture content of 50% and a maximum of 100%. During the year cycle the degradation rate of soil Organic Matter (OM) is linked to the soil temperature and soil moisture saturation, resulting in seasonal fluctuating DOM concentrations and soil oxidation/reduction potential. The CHARON/SFYNXZ model simulates the pH, Eh, DOM and metal concentrations as function of soil depth (0–150 cm) and in time (0–10 years). The CHARON/SFYNXZ model results are used to calculate the multi-substance Potentially Affected Fraction (ms-PAF) [34] with the OMEGA model [21]. The model PNEC-Pro [33] is used to calculate the pore water specific predicted no-effect concentration for Cu, Ni, Zn, and Pb, based on BLM’s.
2.2 Reference conditions; general sediment/soil parameters and groundwater balance
The reference conditions for sediment application as a soil is based on the STW project ‘Lift up of Lowlands-Beneficial use of dredged sediments to reverse land subsidence’ [30, 45]. This study is based on the ‘Lift up of Lowlands’ soil properties, with the exception of the metal concentrations. For the metals the Dutch standard for reallocation of sediments on land without restrictions [4] is used, including the corresponding standard soil organic matter (OM) and lutum content. The reason to use a risk standard concentration instead of the measured concentration is that the purpose of this study is to look into the impact of the water table variation over a longer period (10 years) on the trend in potential ecocity of the soil. Therefore, a metal concentration is chosen which is deemed safe according to the Dutch soil/sediment risk standard. Table 1 compares the measured concentrations for the Lift up of Lowlands soil with the Dutch standard for reallocation of sediments on land without restrictions.
Based on the global Dutch soil conditions for Rhine sediments, the initial soil/sediment composition was derived from literature (Table 2). The porosity and bulk density is based on expert judgement on the ripening/consolidation during the of the sediment used for Lift up of Lowlands (the initial bulk density was less than 1.100 g/dm3 due to the hydraulic pum** of the sediment slurry). Organic Matter and clay content are in accordance with Dutch standard soil [51]. The calcium carbonate content is based on the calcium carbonate for the lutum fraction of Rhine sediment [38]. The total iron content is based on Haringvliet sediment, also Rhine sediment [6]. The initial distribution of iron between iron carbonate, iron sulphite and iron hydroxide minerals is chosen by the author (1/3: 1/3:1/3). Trace metal concentrations are based on the classification of the Lift Up of Lowlands site as suitable for reallocation of sediments on land without restrictions [4]. All trace metals are assumed to be initially present as sulphide due to the anaerobic sediment conditions before placement on land. Non Ideal Competitive Adsorption (NICA) based on the Donnan potential is included in CHARON. The Nica-Donnan parameters were derived from [27].
Assuming a minimal horizontal drainage, precipitation and evaporation dictate the yearly groundwater water balance. The results are summarized in Table 3. The Dutch average precipitation and evaporation for the Western Netherlands was used [44]. Rainwater also contains trace metals. Therefore, the average background concentration was added to the precipitation [47].
The groundwater level was modelled with a 1DV transport model (SFYNXZ) [11]. The soil was divided in 15 vertical layers of 10 cm (1.5 m in total). When evaporation exceeds precipitation, air enters into the unsaturated pores. To mimic the heterogeneity of contact with air in soils when the groundwater level drops, the penetration of air is simulated as being in contact with an unsaturated (oxidised) 1DV soil layer. The model also has a saturated 1DV soil layer. Transport between the saturated and unsaturated layers is by molecular diffusion (with a diffusion distance of 1 cm). This way the soil maintains a time dependant reduced fraction, even when unsaturated.
2.3 FIAM model CHARON, including the NICA-Donnan phase, for trace metals
The FIAM model CHARON [11] is used to calculate the free metal ion concentration in the presence of a NICA-Donnan phase [24]. The metals Cd, Cu, Ni, Cr and Zn and As (a metalloid) are selected for the CHARON model. These metals are often present in soils in concentrations above soil standards and can have an anthropogenic origin. Some of these metals (cadmium and nickel) are also an EU Water Framework Directive priority substance [17]. The impact of DOM on the binding of metals in the presence of a NICA-Donnan phase, and hence the formation of metal–organic ligands, is described by Hiemstra [22]. Metal complexation constants for a large series of elements have been derived from experimental data published by Milne [27]. The FIAM model CHARON also calculates the anaerobic and aerobic formation and degradation rate of DOM [25] based on the soil OM content. Hence, the amount of metal–organic ligand formation (NICA-Donnan phase) [13] is depending on the position in the soil profile and the time of year.
2.4 BLM model PNEC-PRO
PNEC-pro [33] has been implemented in legal frameworks for EQS-compliance testing and WFD-reporting [52]. PNEC-pro calculates local, water type specific, predicted no-effect concentrations (PNEC) of Cu, Ni, Zn, and Pb based on BioLigand Models (BLMs) [53]. Local PNECs are used for compliance checks in higher-tier risk assessments [37]. PNEC-pro calculates a Risk Characterization Ratio (RCR) for each metal [54]. The RCR divides the PNEC calculated exposure levels for the measured dissolved metal concentration by the predicted no-effect concentrations based on the local water quality [28].
2.5 Ms-PAF model OMEGA
The concept of calculating the Potentially Affected Fraction (PAF) is based on the observed median lethal concentration [LC50] or no-observed-effect concentration [NOEC] for individual components and individual species (single contaminant Specie Sensitivity Distribution (SSD)). The potential toxic risk for multiple substances is then calculated using the distribution of the LC50 or NOEC values [34] following the methodologies as proposed by De Zwart and Posthuma [12]:
-
(i)
Response Addition (RA), or
-
(ii)
Concentration Addition (CA).
The resulting multi substances Potentially Affected Fraction (ms-PAF) gives the fraction of the population that is potentially at risk. The characterisation of risk can be by an increase in mortality, degraded reproduction rates or growth deformation [34]. The definition of risk can be either acute or chronic, depending in the duration of the exposure. Ecotoxicity tests are therefore divided in acute or chronic exposure [43].
The numerical model ‘Optimal Modeling for Ecotoxicological Assessment’ (OMEGA) incorporates these methodologies to calculate the ecotoxicological risk for multiple contaminants [21]. The model used the ecotoxicological data for approximately 200 substances [4]. The data is derived by establishing dose effect relations for a toxicant under standard water conditions defined by DSW [1].
In addition to the metals used for the BLM model PNEC-Pro, cadmium and arsenic were also included. The ms-PAF was calculated for acute toxicity (less than 24 h exposure).Response Addition (RA) was used for the metals, concentration addition for the metalloid arsenic.
3 Results
3.1 General sediment/soil parameters, model results
3.1.1 Groundwater level variation during multiple year cycles
The groundwater level variation is 100 cm. The minimum saturated groundwater level is at 100 cm below surface level. The maximum groundwater level is at 10 cm below the surface level. In Fig. 1 the soil profile saturation level (as % of the pore volume) and the soil profile redox potential (mV) is given over a period of 10 years after the sediment has been put on land, illustrating the impact of the groundwater level variation.
A dry period (summer) is characterized by a drop in the groundwater level and a rise in the redox potential (above 0 mV, indicating oxidizing conditions). During a wet period the groundwater level rises and the pe declines. Due to the degradation of DOM produces during the summer period (see paragraph 3.1.2.) the pe drops to slightly lower values during multiple year cycles.
3.1.2 pH and DOM concentrations in the groundwater during multiple year cycles
The pH and the production and degradation of DOM of depends on the saturation level and the correlated aerobic/anaerobic conditions within the soil profile (see the pe fluctuation in Fig. 1). Figure 2 shows the calculated pH and DOM concentration over a period of 10 years.
Soil pH and DOM concentration during a period of 3650 days (10 year cycles). Time (in days) on the x-axis, the soil depth (in cm) on the y-axis. The DOM concentration varied between 110 mg/l during summer in the upper 10 cm of the profile, to < 5 mg/l for the intermediate (> 50 cm) and deeper (> 100 cm) part of the soil profile during the whole year
In the top 100 cm of the soil profile, the pH drops to 6.0 during dry period (summer) and rises to 7.6 during the wet period (winter). In the deeper part of the soil profile (> 100 cm) the pH varies between 5.3 and 6.1.
The DOM production is highest during the dry period (summer), especially in the top 10 cm of the soil profile. The DOM concentration varies between less than 5 mg/l in the deeper parts of the soil profile (> 100 cm), up to 110 mg/l in the top 10 cm of the soil profile during a dry period (summer).
3.2 FIAM model CHARON, dissolved trace metals in the groundwater during multiple year cycles
The soil pore water profile for Cu, Ni, Zn, and Pb (the four PNEC-pro metals) over a period of 10 years is given in Fig. 3.
All four metals (Cu, Ni, Zn and Pb) follow the same pattern. The first 3–5 years after putting the sediment on land, the concentrations are high over in the whole soil profile, declining after 3–5 years in the top 100 cm of the soil. During dry periods (summer), the concentrations spike, but to a far lesser extent than during the first 3–5 years.
To illustrate that not all metal like elements follow this pattern, the concentration of arsenic (a metalloid) over 10 years is given in Fig. 4.
During dry periods (summer) arsenic is mobilized in the unsaturated top part of the soil profile. When the groundwater level rises (during autumn), arsenic is transported to the lower part of the soil profile, where it is bound (mainly to the iron oxides and soil OM). The high arsenic mobility in the summer is mainly due to the DOM production in the top soil layer (upper 10 cm). As-DOM ligands make up for 50% of the dissolved arsenic concentration.
3.3 BLM model PNEC-PRO in the groundwater during multiple year cycles, model results
The BLM model PNEC-pro uses the local water quality parameters DOM, Ca2 +, Mg2 + and Na + to calculate the predicted no-effect concentrations (PNEC) for each of the four metals. In the chosen soil schematisation Mg2 + was not taken into account since calcite and not dolomite is the dominant buffering mineral for the reference location (Lift up of Lowlands). Na + was modelled, but not reacting. The Na + concentration is constant and defined by the boundary input (rainwater). Mg2 + and Na + are therefore chosen as a constant based on the closest rainwater station (RIVM station 444) [3].
Figure 5 shows the calculated PNEC RCR’s up to 1.0 as function of soil profile depth over a period of 10 years on a monthly base.
The initial (first year) RCR for all four metals is high in the lower part of the soil profile. Due to an increase in pH and the DOM concentration in the top 50 cm of the soil profile, the RCR rapidly (within a year) drops to values below one (below the NOEC concentration).
3.4 Ms-PAF model OMEGA in the groundwater during multiple year cycles
The ms-PAF results indicate how many of the organisms are at risk of experiencing an observable toxicological impact due to the presence of a mixture of metals. The calculations have been carried out for the following contaminants in the soil pore water; arsenic, cadmium, copper, nickel, zinc and lead. The resulting calculated chronic ms-PAF is given in Fig. 6 during a period of 10 years.
4 Discussion
While the FIAM model geochemical soil and pore water constants are calibrated for steady state sediments with a similar composition [60] it is difficult to validate a FIAM model under dynamic conditions [41]. This is especially the case in the presence of an unsaturated zone with a high DOM production rate, and hence a potential large influence of the NICA phase. This study has been done in collaboration with Wageningen University & Research (WUR), The Netherlands, as part of the NWO program “Lift up of Lowlands” providing the generic NICA-Donnan model parameters [27]. More detailed studies on the relation between measured DOM concentration levels and the speciation of dissolved metals in soils with alternating flooding and drainage were carried out by the WUR [31, 32]. The main drawback of these studies in relation to this study is that the soil composition was fundamentally different, and the period of flooding and drainage is shorter (two cycles in 128 days) as compared to the year cycle simulated here. The soil with the best matching properties is the FY soil (from the vicinity of Fuyang City, China) since this soil has clay (25%), organic matter (9.2%) and calcite (21%), resulting in a pH of 7.5 and a DOC content of 189 mg C per litre (equivalent of 473 mg/l DOM). The modelled Lift up of Lowlands soil has more or less similar properties (see Table 2), although a slightly lower organic matter content. The DOC/DOM content is not measured for the Lift up of Lowlands soil, but similar peatlands in North-eastern Germany show DOC release ranged from 4 to 123 mg/l (10 to 308 mg/l DOM). The degree of decomposition and pH were found to be the major driving factors for DOC release, with the highest DOC concentrations in the most degraded part of the peat [40].
We are not aware of more recent similar work on alternating flooded/wetted recently applied sediment as a soil, measuring changes in redox conditions, pH, DOM and dissolved metals in correlation with FIAM modelling which includes the formation of DOM-metal ligands considering non ideal competitive adsorption (NICA) conditions. Earlier work [23] looks at seasonal variation of metals in soils during flooding and drainage but lacks a predictive modelling element. Schroder [41] takes pore water measurements in a floodplain over a period of 1½ year and derives a stochastic model for metal availability based on the measurements and FIAM model results, but the study is focusses on historical flood plain soils. Du Laing [16] presents a review on processes influencing the change in trace metal behaviour in floodplains, including the influence of kinetic restrains on redox-sensitive processes, but is based on more marine sediments. We take these processes and kinetic restrains into account in the FIAM model during the ripening phase of the sediment to become a soil (over a 10 year period). For the soil composition and direct comparison with measurements we compare our results with the FY soil measurements by Pan [32].
4.1 General soil parameters
The observed seasonal trend that the top soil Eh increases and pH decreases with a lower water table in the summer is observed in flood plains [16], seasonal flooded peatlands [40] and rice paddies [23]. An increase in DOM concentrations in the top 10 cm of peat soil during summer was also observed by Clark [7]. In years with a significant drop in the water table, the pH in the top soil also dropped due to the oxidation of organic/organic sulphur stored in the peat (measured by an increase in the sulphate release). These processes are also present in the FIAM model (sulphate concentrations are not presented in this paper), and the modelled trends for Eh, pH and DOM follow the trends described in literature. Table 4 gives an overview of the measured general soil parameters for the FY soil [32] versus the outcome of the FIAM calculations for the currently studied Lift op of Lowlands soil.
The trends in Eh, pH and DOM as function of the soil oxidation state (flooded/drained) follow a similar pattern.
4.2 Dissolved trace metals
A direct comparison with the FY soil [32] (Table 5) shows that the Lift up of Lowland soil is in general less contaminated with trace metals, with the exception of zinc and lead.
More important than the absolute pore water trace metal concentrations are the seasonal trends as function of soil saturation. The FY soil spikes when flooded for the first time (which is the reverse from the Lift up Lowlands soil, which is saturated at the start and drains when applied as a land soil). What is similar on both the FY soil and the Lift up of Lowlands soil is that at low water level (drained state) the pore trace metal concentration normally rises. The exception is that in the last test period for the FY soil (85 to 100 days, drained state) some trace metals seem to be depleted. The Lift up of Lowlands soil shows a similar trend over the years but needs more time to reach a lower pore water trace metal concentration. The lower DOM concentrations in the Lift up Lowlands soil, and hence a lower leaching of DOM bound trace metals during flooding/saturation might be part of the reason for the difference in timescale. Due to the difference in test time scale (the FY soil experiment lasted 120 days in total, with two flooded and two drained periods), it is difficult to compare the multi cycle trends. The kinetic processes as described by Du Laing [16] better fit with the timescale of this paper (a decade) as compared to the 128 days for the FY soil.
Looking at multiple year cycles, the initial high dissolved trace metal concentrations in the top soil (upper 50 cm) drop after one to three year (depending on the trace metal). There is some seasonal variation, also due to the continuous yearly DOM production/degradation cycle with strong DOM-metal ligand formation in the dry summer period (high DOM concentrations in the top soil), declining during the wet winter period. The drop in the trace metal concentrations is due to leaching of the pore water (flushing out the freely dissolved metals and DOM bound metals), and fixation of trace metals over time of newly formed iron oxides with a high sorption capacity in the top soil. Arsenic is the exception due to continuous changes in the iron sulphide/iron oxide minerals, influencing the release of arsenic.
While there are differences in absolute values for the FY soil (measurements) as compared to the c soil (calculations), the similarities in trends when flooding/draining (FY soil) or wetting/drying (Lift up of Lowlands soil) for both the general soil parameters as also for the trace metal concentrations is striking. The FIAM model can reproduce the impact of the dominant soil processes on the main soil parameters and trace metals when a soil is seasonally varying in groundwater level. However, without pore water samples there are no guaranties that the calculated values are 100% representative for real field conditions.
4.3 Comparison FIAM model results with leaching experiments
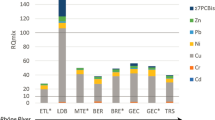
The ‘Lift up of Lowlands’ program is part of the EU INTERREG project CEAMaS (2012–2015). The CEAMaS projects focussed on the use of sediments, including the use of sediments for the formation of land soils. Five EU sediment quality guidelines were used for this evaluation (French, Irish, Flemish, Dutch and German). Two of these five sediment standards (the Dutch and Flemish) include a leaching test for metals according to NEN standards [29]. While the methodology of a leaching test is different from a natural dry/wetting cycle, the leaching test gives an indication if metals become mobile when the soil is saturated and flushed with oxygen containing water. The leaching test [29] for the ‘Lift up of Lowlands’ sample was performed by Deltares. Figure 7 illustrates the results of the leaching tests during seven leaching steps. Each steps represents an increased Liquid/Solid ratio (L/S) ratio between sediment/soil and the added water, up to a 10/1 ratio. The leaching results are compared with the FIAM results for year with a monthly interval. Figure 7 only plots the results for nickel and zinc since the other metals like copper and lead were below the limit of detection (LOD).
The soil sample for the Lift up of Lowlands’ leaching test was taken roughly one year after the deposition of the sediment, therefore FIAM calculation for year two is chosen as reference.
When comparing the concentration range, the nickel concentration of the leaching test eluate is in the same range as the FIAM model results in the top 10 cm of the soil during the simulated year cycle (0–9 µg/l). However, the measured zinc concentration in the eluate is much higher (range 0–70 µg/l) compared to the FIAM model results (0–2.5 µg/l). The high zinc concentrations in the eluate samples dilutes over timer, dwindling to almost zero for fraction k7. This indicates that the zinc contamination in the leaching experiment might be the result of a flushing of a soil adsorbed zinc fraction instead of the result of an ongoing oxidation and therefore continuous mobilisation.
While there are similarities between the leaching test and the FIAM model simulated natural year cycle when it comes to introducing oxygen into the soil, the FIAM results in Fig. 7 illustrate that there are more processes in natural soils that influence the mobility and leachability of metals. Therefore the leaching test rests are not suitable to validate the FIAM model. The reverse can also be said; the leaching test are not suitable to evaluate the use of sediments for the formation of soils.
4.4 Sensitivity analyses FIAM model
The FIAM model has a great number of parameters that are soil and situation specific, and therefore uncertain. When grouped, the main uncertainties are:
-
The equilibrium thermodynamic constants (including the sorption to OM and soil minerals like clays) for redox sensitive elements like metals
-
The kinetic exchange rates when changes occur
-
The complexation of metals, including the complexation with DOM
-
The formation and degradation of DOM
-
The water content in different parts of the unsaturated soil profile
This study is therefore does not represent ‘the’ truth for the impact of wetting/drying on a freshly formed soil like the former sediments used for the “Lift up of lowlands” project. The FIAM model calculations illustrate the dynamics and timescale of change in pore water concentrations caused by multiple wetting/drying cycles over multiple years.
For the sensitivity analyses, the FIAM model parameters (thermodynamic constants, kinetic rates, DOM production and degradation rates) were kept constant. The focus is on the impact of a period of dry (100 mm per year less rainfall, 100 mm per year more evaporation) and wet (100 mm per year more rainfall, 100 mm per year less evaporation) years on the dynamics of the metals pore water concentration (see Table 6).
The difference between a wet and dry year is most relevant during the spring period (with a relative high water level, see Fig. 1), where the water level is lower in the upper 20 cm of the soil (see Fig. 8).
The impact of a dry year is most severe in the summer, when the upper 20 cm of the soil is almost completely dried out, as is shown in Fig. 9 for cadmium.
The high concentration factor increases for cadmium (factor 10.000) is due to the drop in the groundwater level in late winter/early spring (see Fig. 8), causing an earlier oxygen penetration in the top soil (± upper 20 cm), and hence longer period of oxidation of the metal sulphides. The oxidation of sulphides releases cadmium (and other trace metals) to the pore water. The early oxygen penetration also enhances the OM degradation, causing a higher DOM production and higher DOM concentrations in the pore water, as is shown by Pan [32] for the drainage stage of rice paddy soils. The DOM forms a metal–ligand complex, enhancing the solubility of the trace metals. Evaporation of the pore water in the top soil during the summer and lasting until late autumn (from 180 to 330 days) causes the concentration levels of dissolved cadmium to further increase. In the autumn/winter there is a net water surplus (more rain then evaporation), transporting the dissolved cadmium deeper into the soil profile (± 50 cm). At this depth the redox potential is low, the cadmium immobilized as cadmium sulphide (solid).
While the FIAM model might overpredict the summer concentrations for metals like cadmium in the top soil under long term dry conditions, the effect of an enhanced oxidation on an increase of dissolved trace metals in sediments is something which is also observed in natural systems [18]. Studies on the impact of the duration of the drainage stage (with soil oxidation) of paddy soils on cadmium solubility also shown an increased dissolved cadmium concentration with longer drainage periods [10].
4.5 BLM model, PNEC levels
The trend in dissolved metal concentrations with depth over time is mimicked by the PNEC. After the first year(s) (depending on the trace metal) the PNEC does not rise above 1.0 in the top 50 cm of the soil profile. Based on the individual trace metal concentrations and the water quality conditions in the top 50 cm of the soil, the trace metals are therefore no longer considered a biological risk based according to the BLM model.
For zinc the conditions to calculate the PNEC were outside the PNEC-Pro calibrated boundary conditions for the deeper part (> 50 cm) of the soil after the first year. This is mainly due to the pH, which drops below 6.0.
Since the Lift up of Lowlands soil is considered an uncontaminated (class A) soil, the PNEC levels should not exceed 1.0. The fact that the PNEC exceeds 1.0 in the top 50 cm of the soil during the first year(s), and longer form the deeper parts of the soil, illustrates that the transition from sediment to soil introduces a temporal exposure risk. This temporal risk is further explored with the ms-PAF model.
4.6 Ms-PAF
For reuse of sediments on land, the guiding principle in the Netherlands is that the chronic ms-PAF for metals should not exceed 50% [35, 50]. The chronic ms-PAF for metals in this study is based on the calculated pore water concentrations for cadmium, copper, nickel, zinc, lead and arsenic. Figure 6 illustrates that the 50% value is exceeded in the complete soil profile during the first year. The chronic ms-PAF level for metals drops after the first year in the top (upper 50 cm) of the soil during the dry period (summer), shortly spiking in the top soil to values of 42% during the start of the wet period (autumn to winter).
An earlier study on the impact of putting sediments on land [19] shows a chronic ms-PAF value close to 50%. Harmsen [19] used the total sediment metal concentrations instead of pore water concentrations. The ms-PAF calculations were based on equilibrium partitioning, not taking into account the seasonal trends in pore water chemistry. In this study we show that the ms-PAF in the transition phase of sediment to soil can be improved when taking into account the variation in local pore water quality parameters like DOM and pH. The correction of dissolved metal concentrations based on water quality parameters is the base for the BLM concept (see paragraph 4.3).
The variation in groundwater level not only influences the pore water quality parameters and therefore the BLM PNEC, but also influences the total dissolved pore water metal concentration. The total dissolved concentration is used for the ms-PAF calculation. While the spatial pattern over soil depth and time for the individual metals according to BLM PNEC calculation (Fig. 5) and the combined metals chronic ms-PAF calculation (Fig. 6) is somewhat different, the trend that the potential ecotoxicological impact decreases over multiple dry–wet year cycles is the same. This is especially the case in the top 50 cm of the soil, where the ms-PAF does not exceed the 50% threshold after the second year. The reason why the ms-PAF shows a strong repeating short spike in the top 50 cm of the soil over the years is due to the incorporation of the arsenic pore water concentrations (see Fig. 4) in the ms-PAF calculation.
5 Conclusion
Putting sediments on land to form a new soil is a geochemical dynamic process. Taking a location where a layer of 200 cm of sediment (before consolidation) was put on land (Lift up of Lowlands) and simulating the development of this newly formed soil over a 10 year period shows that, especially during the first few years, the soil is changing in ecotoxicity. This change is most profound in the top 50 cm of the soil, where there is an unsaturated zone for more than 50% of the year. During the sediment to soil transition time the metals pore water concentrations spike, the BLM RCR values for the metals exceed 1.0 and the chronic ms-PAF for metals is over 50%. This enhanced ecotoxicological effect is somewhat tempered during wet years but enhanced during dry years.
The duration of the transition time varies somewhat based on the chosen evaluation criterium (being the dissolved metal concentration, the BLM RCR or the ms-PAF), but all criteria congregate that uptake risk in the upper 50 cm of the soil has dropped to acceptable levels after 3 years. Legislation on sediment use on land should take this transition time into account, adapting the land use during this period of 3 years on the potential higher exposure for crops and cattle in the top 50 cm of the soil.
References
Aldenberg T, Jaworska JS, Traas TP (2001) Normal species sensitivity distributions and probabilistic ecological risk assessment. https://doi.org/10.1201/9781420032314.ch5
Berry WJ, Hansen DJ, Mahony JD, Robson DL, Di Toro DM, Shirley BP, Rogers B, Corbin JM, Boothman WS (1996) Predicting the toxicity of metals-spiked laboratory sediments using acid-volatile sulfide and interstitial water normalization. Environ Toxicol Chem 15:2067–2079
Boschloo DJ, Stolk AP (1999) Landelijk Meetnet Regenwatersamenstelling Meetresultaten (1995). RIVM Rapp 723101:046
Bruijn de J, Crommentuijn T, van Leeuwen K, van der Plassche E, Sijm D, van der Weiden M (1998) Environmental Risk Limits in The Netherlands, RIVM Report 601640001
Buijse AD, Coops H, Staras M, Jans LH, van Geest GJ, Grift RE, Ibelings BW, Oosterberg W, Roozen FCJM (2002) Restoration strategies for river floodplains along large lowland rivers in Europe. Freshw Biol 47:889–907
Canavan RW, Van Cappellen P, Zwolsman JJG, van den Berg GA, Slomp CP (2007) Geochemistry of trace metals in a fresh water sediment: field results and diagenetic modelling. Sci Total Environ 381:263–279
Clark JM, Chapman PJ, Adamson JK, Lane SN (2005) Influence of drought-induced acidification on the mobility of dissolved organic carbon in peat soils. Glob Change Biol 11(5):791–809
Comans RNJ, Middelburg JJ (1987) Sorption of trace metals on calcite: applicability of the surface precipitation model. Geochim Cosmochim Acta 51(9):2587–2591
Davranche M, Bollinger J-C (2000) Release of metals from iron oxyhydroxides under reductive conditions: effect of metal/solid interactions. J Colloid Interface Sci 232:165–173
De Livera J, McLaughlin MJ, Hettiarachchi GM, Kirby JK, Beak DG (2011) Cadmium solubility in paddy soils: effects of soil oxidation, metal sulfides and competitive ions. Sci Total Environ 409:1489–1497
De Rooij NM (1991) Chemistry Applied to the Research Of Natural systems, WL Delft Hydraulics, R1310–10
De Zwart D, Posthuma L (2005) Complex mixture toxicity for single and multiple species proposed methodologies. Environ Toxicol Chem 24:2665–2676
Dijkstra JJ, Meeussen JC, Comans RN (2009) Evaluation of a generic multisurface sorption model for inorganic soil contaminants. Environ Sci Technol 43:6196–6201
Di Toro DM, Mahony JD, Hansen DJ, Scott KJ, Carlson AR, Ankley GT (1992) Acid volatile sulfide predicts the acute toxicity of cadmium and nickel in sediments. Environ Sci Technol 26:96–101
Di Toro D, McGrath J, Hansen DJ, Berry WJ (2005) Predicting the toxicity of metals in sediments using organic carbon normalised SEM and AVS. Environ Toxicol Chem 24:2410–2427
Du Laing G, Rinklebe J, Vandeccasteele E, Meers MG, Tack FMG (2009) Trace metal behaviour in estuarine and riverine floodplain soils and sediments: a review. Sci Total Environ 407(13):3972–3985
European Communities (2000) Directive 2000/60/EC of the European Parliament and of the Council of 3.23 October 2000 establishing a framework for Community action in the field of water policy, EU Official Journal 22/12/2000(327):1–73
Förstner U (2006) Contaminated sediments: lectures on environmental aspects of particle-associated chemicals in aquatic systems. Springer-Verlag, Berlin Heidelberg GmnH
Harmsen J, Rietra RPJJ, Groenenberg JE, Lahr J, van den Toorn A, Zweers HJ (2012) Verspreiden van bagger op het land in klei- en veengebieden, Alterra report 2282, ISSN 1566–7197
Harbaugh AW (2005) MODFLOW-2005, the U.S. Geological Survey modular ground-water model -- the Ground-Water Flow Process: U.S. Geological Survey Techniques and Methods 6-A16
Hendriks J, van de Guchte K (1997) Optimal modeling and monitoring in ecotoxicological assessments: choosing instruments for applied research and management with examples from the Rhine-Meuse delta. Environ Toxicol Water Qual 12(4):321–333
Hiemstra T, van Riemsdijk WH (2006) Biogeochemical speciation of Fe in ocean water. Mar Chem 102:181–197
Jung MC, Thornton I (1997) Environmental contamination and seasonal variation of metals in soils, plants and waters in the paddy fields around a Pb Zn mine in Korea. Sci Total Environ 198(2):105–121
Kinniburgh DG, Milne CJ, Benedetti MF, Pinheiro JP, Filius J, Koopal LK, Riemsdijk V, Willem H (1996) Metal ion binding by humic acid: application of the NICA-Donnan model. Environ Sci Technol 30:1687–1698
Kinniburgh DG et al (1999) Ion binding to natural organic matter: competition, heterogeneity, stoichiometry and thermodynamic consistency. Colloids Surf A 151(1–2):147–166
Koopal LK, Saito T, Pinheiro JP, van Riemsdijk WH (2005) Ion binding to natural organic matter: general considerations and the NICA-Donnan model. Colloids Surf A 265:40–54
Milne CJ, Kinniburgh DG, van Riemsdijk WH, Tip** E (2003) Generic NICA−Donnan model parameters for metal-ion binding by Humic substances. Environ Sci Technol 37:958–971
Muñoz I, Gómez-Ramos MJ, Agüera A, Fernández-Alba AR, García-Reyes JF, Molina-Díaz A (2009) Chemical evaluation of contaminants in wastewater effluents and the environmental risk of reusing effluents in agriculture. TrAC Trends Anal Chem 28(6):676–694
NEN 7373 (2004), Leaching characteristics - Determination of the leaching of inorganic components from granular materials with a column test - Solid earthy and stony materials, ICS-CODE 13.030.10 91.100.01
Oliveira BRF (2017) Lift up of Lowlands: beneficial use of dredged sediments to reverse land subsidence. PhD. Thesis Wageningen University, ISBN-978-94-6257-883-8
Pan Y, Koopmans GF, Bonten LT, Song J, Luo Y, Temminghoff EJ, Comans RN (2015) In-situ measurement of free trace metal concentrations in a flooded paddy soil using the Donnan Membrane Technique. Geoderma 241:59–67
Pan Y (2015) Speciation of trace metals and their uptake by rice in paddy soils. PhD. thesis Wageningen University, ISBN-978-94-6257-274-4
PNEC-Pro V6 (2016) BLM tool for calculation of bioavailability of metals. http://www.pnec-pro.com, Deltares
Posthuma L, Sutter GW, Traas TP (2002) Species sensitivity distributions in ecotoxicology. CRC Press, Lewis Publishers, Florida
Posthuma L, Lijzen JPA, Otte PF, de Zwart D, Wintersen A, Oste L, Beek M, Harmsen J, Groenenberg BJ (2006) Decisioning on sediment deposition on land. Part 3. Modeling risks after sediment deposition, RIVM rapport 711701046
Rodriguez-Iturbe I, Porporato A, Ridolfi L, Isham V, Coxi DR (1999) Probabilistic modelling of water balance at a point: the role of climate, soil and vegetation. Proc R Soc Lond A Math Phys Eng Sci 455:3789–3805
Rüdel H, Muñiz CD, Garelick H, Kandile NG, Miller BW, Pantoja ML, Peijnenburg WJGM, Purchase D, Shevah Y, van Sprang P, Vijver MG, Vink JPM (2015) Consideration of the bioavailability of metal/metalloid species in freshwaters: experiences regarding the implementation of biotic ligand model-based approaches in risk assessment frameworks. Environ Sci Pollut Res Int. https://doi.org/10.1007/s11356-015-4257-5
Salomons W (1975) Chemical and isotopic composition of carbonates in recent sediments and soils from Western European. J Sediment Petrol 45:440–449
Salomons W (1985) Sediments and water quality. Environ Technol Lett 6:315–326
Schwalm M, Zeitz J (2015) Concentrations of dissolved organic carbon in peat soils as influenced by land use and site characteristics—A lysimeter study. CATENA 127:72–79
Schröder TJ (2005) Solid-solution partitioning of heavy metals in floodplain soils of the river Rhine and Meuse, thesis Wageningen University, ISBN 90-8504-310-7
Shapley M, Cutler L (1970) Rand's chemical composition program: a manual Rand, R-495-PR
Stephen CE, Mount DI, Hansen DJ, Gentile JR, Chapman GA, Brungs WA (1985) Guidelines for deriving numerical national water quality criteria for the protection of aquatic organisms and their uses. EPA PB85-227049
Sluijter R (2011) Klimaatatlas. Langjarige gemiddelden 1981–2010/2011, Koninklijk Nederlands Meteorologisch Instituut. Groningen : Noordhoff Uitgevers
Tollenaar GRN (2017) experimental investigation on the desiccation and fracturing of clay. PhD. thesis Technical University of Delft, ISBN 978-94-92516-59-6
Van Den Hoop M, Den Hollander H, Kerdijk HN (1997) Spatial and seasonal variations of acid volatile sulphide (AVS) and simultaneously extracted metals (SEM) in Dutch marine and freshwater sediments. Chemosphere 35:2307–2316
Van Den Nguyen AGA, Merks PV (1990) Atmospheric deposition of acid, heavy metals, dissolved organic carbon and nutrients in the dutch Delta area in 1980–1986. Sci Total Environ 99:77–91
Vangheluwe ML, Heijerick D, Van Sprang P (2003) Probabilistic assessment of zinc bioavailability in sediments. Final report February 2003. Commissioned by the International Lead and Zinc Research Organization
Van Griethuysen C, van Baren J, Peeters ET, Koelmans AA (2004) Trace metal availability and effects on benthic community structure in floodplain lakes. Environ Toxicol Chem 23:668–681
Van Noort P, Cuypers C, Wintersen A, de Zwart D, Peijnenburg WJGM, Posthuma L, Groenenberg BJ, Harmsen J (2006) Decisioning on sediment deposition on land. Part 2. Modeling compound behaviour and predicted soil concentrations resulting from sediment deposition on land, RIVM rapport 711701045
van Straalen NM, Denneman CA (1989) Ecotoxicological evaluation of soil quality criteria. Ecotoxicol Environ Saf 18(3):241–251
Verschoor A, Vink JPM (2010) Biotic Ligand Models: availability, performance and applicability for water quality assessment, Deltares report 1203842-000
Verschoor A, Vink JPM, de Snoo GR, Vijver MG (2011) Spatial and temporal variation of watertype-specific no-effect concentrations and risks of Cu Ni, and Zn. Environ Sci Technol 45(14):6049–6056
Verschoor A, Vink JPM, Vijver MG (2012) simplification of biotic ligand models of Cu, Ni, and Zn by 1-, 2-, and 3-parameter transfer functions. Integr Environ Assess Manag 8(4):738–748
Vink JPM, Harmsen J, Rijnaarts H (2010) Delayed immobilization of heavy metals in soils and sediment under reducing and anaerobic conditions; Consequences for flooding and storage. J Soils Sediments 10:1633–1645
Vink JPM, van Zomeren A, Dijkstra JJ, Comans RNJ (2017) When soils become sediments: Large-scale storage of soils in sandpits and lakes and the impact of reduction kinetics on heavy metals and arsenic release to groundwater. Environ Pollut 227:146–156
Weng L, Temminghoff EJM, Van Riemsdijk WH (2001) Contribution of individual sorbents to the control of heavy metal activity in sandy soil. Environ Sci Technol 35:4436–4443
Weng L, Temminghoff EJM, Van Riemsdijk WH (2001) Determination of the free ion concentration of trace metals in soil solution using a soil column Donnan membrane technique. Eur J Soil Sci 52:629–637
Weng L, Van Riemsdijk WH, Hiemstra T (2008) Cu2+ and Ca2+ adsorption to goethite in the presence of fulvic acids. Geochim Cosmochim Acta 72(24):5857–5870
Wijdeveld A, Schipper C, Heimovaara T (2018) Variation in the availability of metals in surface water, an evaluation based on the dissolved, the freely dissolved and Biotic Ligand Model bioavailable concentration. CATENA 166:260–270
Wijdeveld, Scientific progress in sediment and water quality assessment, implementation of practical case studies, PhD. thesis Delft University of Technology, ISBM 9789081-13604
Zhang YGE, Yao H, Chen X, Minkun Hu (2012) Iron oxidation-reduction and its impacts on cadmium bioavailability in paddy soils: a review. Front Environ Sci Eng 6:509–517
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This research is supported by the Dutch Technology Foundation STW under award number 11344 (“Lift up of Lowlands”). The authors have no competing interests to declare that are relevant to the content of this article. The author (Dr. A. Wijdeveld) is holder of the copy rights of the PhD. thesis [61] of which this paper is a chapter and explicitly allows the reproduction for scientific purposes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wijdeveld, A.J., Schipper, C.A. & Heimovaara, I.T.J. From sediments to soils: changes in pore water metal bioavailability. SN Appl. Sci. 4, 145 (2022). https://doi.org/10.1007/s42452-022-05030-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-022-05030-y