Abstract
In this present work, we described a bio-reduction method for the generation of silver nanoparticles (AgNPs) using aqueous leaf extract of Micrargeria wightii (M. wightii), which is a gifted alternative to other physicochemical routes. The prepared AgNPs were characterized by UV–visible spectroscopy (UV–vis), Fourier Transform Infrared Spectroscopy (FT-IR), X-ray diffraction (X-RD), Transmission Electron Microscopy (TEM) with EDX and Dynamic light scattering (DLS). UV–visible spectrum showed a characteristic absorption peak at 440 nm of synthesized AgNPs. FT-IR analysis confirmed the existence of plant metabolites, which are responsible for the reduction of Ag (I) ions into Ag (0) NPs. X-RD pattern studies confirm the presence of the pure face-centered cubiccrystalline nature of Ag. Energy-dispersive X-ray (E-DX) spectrum showed the elemental composition of synthesized nanoparticles. Furthermore, TEM images confirm the formation of spherical shaped nano-silver particles with sizes ranging from 30 to 70 nm and supported by particle size analyzer, Dynamic Light Scattering (DLS). Thus, the present investigation provides an easy, eco-friendly and straightforward route for the synthesis of the antibacterial agent against Bacillus subtilis subtilis and Pseudomonas aeruginosa, with 15 and 13 mm zone of inhibition (ZOI) respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the past few decades, nanotechnology is considered as one of the powerful and emerging technology with a lot of application in various fields such as agriculture, medical, textile, cosmetic and pharmaceutical research with an environmental impact [1, 2]. Nanomaterials are small particles, which are present within the nanoscale. It has unique properties such as high catalytic activity, chemical stability, thermal conductivity and optical performances [3]. The main reason behind the unique properties was the increase in large surface area to volume ratio as the size decrease. Many researchers got attracted by these unique properties, which lead to the preparation of nanomaterials [4]. Recently, nanoparticles (NPs) have been used by the researchers to improve the growth, yield, and quality, production of secondary metabolites, antioxidants, and antimicrobial agents in plants [5]. Due to its non-toxic nature, it has been used in various biological applications include diagnostic, biomolecular detections, drug delivery, waste treatment, agriculture, food production and therapeutics [6, 8]. Nanoparticles synthesised using green resources exhibit good biomedical activity due to the functional group present on the surface of the nanoparticles. The main advantage of using plant resource is easily available, low cost, safe to handle, contains a wide range of components such as alkaloids, flavonoids, terpenoids, tannins, etc. [9].
Recently, the bio-approach of fabrication of AgNPs was shifted towards less toxic and biofriendly biological methods which involve plant extracts from plants such as Artemisia quttensis Podlech [10], Agave americana, Menthaspicata and Mangiferaindica [11], Camellia sinensisL.[12], Phaseolus vulgaris[13] and Cannabis sativa [14] have also been reported. In this manuscript, we have prepared AgNPs using Micrargeria wightii (M. wightii) that belongs Orobanchaceae family. The microorganism’s P. aeruginosa is increasingly recognized as an emerging opportunistic pathogen of clinical relevance, while Bacillus subtilis subtilis is known to cause disease in severely immunocompromised patients, and it can be conversely used as probiotic in healthy individuals. Bacillus subtilis has also been implicated in several cases of food poisoning. This present work describes a bio-reduction method for generation of AgNPs using aqueous leaf extract of M. wightii. The prepared AgNPs were characterized by UV–visible spectroscopy (UV–vis), Fourier Transform Infrared Spectroscopy (FT-IR), X-ray diffraction (X-RD), Transmission Electron Microscopy (TEM) with EDX and Dynamic light scattering (DLS).
2 Experimental details
2.1 Preparation of Micrargeria wightii leaf extract
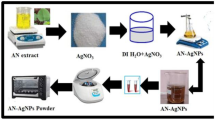
The healthy plant material was collected at tirumala hills, tirupati, Andra pradesh India. The plant M. wightii leaves were cleaned with distilled water to avoid any dust particles and then dried at room temperature. About 10 g of dry leaves powder was transferred into 100 ml conical flask with 100 ml distilled water and followed by heating at 80 °C for 30 min under magnetic hot plate stirrer for the preparation of M. wightii leaves extract. Then, the above mixture extract solution was cooled naturally and filtered with whatman No.1 filter paper without any residue. The obtained extract solution was stored at 4 °C for the preparation of AgNPs.
2.2 Synthesis of silver nanoparticles
For the preparation of AgNPs, 90 ml of 0.001 M AgNO3 precursor solution was prepared in 150 ml of round bottle flask, followed by stirring on magnetic stirrer to obtain for a clear solution. Then, 10 ml of M. wightii extract was added drop wise to the above 90 ml of AgNO3 precursor solution and stored at the dark place for overnight for the complete reduction of AgNPs. The colour of the solution was turned into the dark brown colour. This colour indicates the formation of AgNPs.
2.3 Characterization
The prepared AgNPs structural analysis was confirmed by powder X-Ray diffraction (model PAN analytic BV). The maximum absorbance wavelength of the AgNPs was confirmed by using the UV–Visible spectroscopy (model Shimadzu UV). The functional groups of prepared AgNPs were carried out by using the Fourier transform infrared spectra (FTIR). To make KBr pellet, a small quantity of the AgNPs powder was mixed in dry potassium bromide (KBr), the mixture was thoroughly mixed in a mortar and pressed at a pressure of 6 bars within 2 min to form a KBr thin disc. Then the disc was placed in a sample cup of a diffuse reflectance accessory. The IR spectrum was obtained using Bruker, Alpha T, and Germany 70 infrared spectrometer. The sample was scanned from 4000 to 400 cm−1. The size of AgNPs was analysed by Zeta Sizer model Nano-S90 (Malvern U.K). The morphology and elemental analysis of AgNPs confirmed by Transmission electron microscopy images, taken using a JEOL 3010 at 200 kV and Energy dispersive X-ray spectroscopy (EDAX) spectra were done by SUPRATM 55.
2.4 Antibacterial actvity of Ag NPs
The synthesized AgNPs by the extract of M. wightii have shown significant antibacterial activity against Bacillus subtilis (1134) and Pseudomonas aeruginosa (4996), were tested by disc diffusion method [15]. The bacterial strains were inoculated into nutrient agar medium. A set of three different amounts (10, 20, and 30 µl) of AgNPs colloidal solution in disc were prepared and used in study. At four corners of the petriplates, three different concentrations of the AgNPs in respected discs were placed over the lawn of bacterial culture along with drug 1% streptomycin (s) 30 µl. The study was carried out with 24 h active cultures of the selected bacterial strains. The zone of inhibition was found after 24 h and the results are placed in Table 1.
The study was carried out with 24 h active cultures of the selected bacterial strains. The zone of inhibition was found after 24 h and the results are placed in Table1. This experiment was conducted in triplicates to attain consistent outcomes.
3 Results and discussion
3.1 UV–Visible spectral results
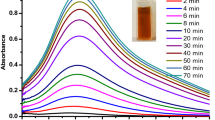
UV–Visible spectrum of AgNPs M. wightii leaf extract is shown in Fig. 1a. The prepared AgNPs were confirmed by using the UV–visible spectrum as shown in Fig. 1b. The colloidal solution was examined with UV–Vis in the wavelength ranging from 300-600 nm for proximate analysis with is diluted to 1:10 (v:v) with the same solvent. UV–Vis spectrum is an essential technique to observe the formation of metal nanoparticles in aqueous solution. The maximum absorbance of AgNPs at wavelength about 440 nm was appeared due to surface plasmon resonance of AgNPs [16].
3.2 X-ray diffraction
The crystal structure and average crystalline size were confirmed by X-ray diffraction pattern of as synthesized AgNPs by using M. wightii leaf extract as shown in Fig. 2. The AgNPs showed good crystalline nature through the green synthesis approach. The AgNPs clearly showed face-centered cubic (FCC) structure and the diffraction peaks appeared at 2θ values 38.32°, 44.47°, 64.66° and 77.55° were well matched with their corresponding miller indices (111), (200), (220) and (311) respectively [19]. The average crystalline size of AgNPs at all diffraction peaks was calculated by using the Debye–Scherrer formula with the average crystalline size of AgNPs about 29.13 nm through the green synthesis method [20]. In XRD pattern, extra peaks appeared due to organic molecules present in the plant extract [16].
3.3 Dynamic light scattering
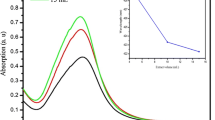
The particle size distribution of the AgNPs synthesized by using M. wightii leaf extract is examined by the DLS is shown in Fig. 3. From this figure, the particle size ranges from 20 to 50 nm. The majority percentage of the AgNPs size distribution was around the 25 nm range. The particle size distribution of AgNPs with DLS results is well matched by XRD and TEM results.
3.4 FT-IR spectra
Fourier transform infrared spectrum was used to identify the characteristic functional groups involved in the formation of AgNPs. It provides the about the active groups of a molecule, frequently obtained from its absorption spectrum. The synthesized AgNPs by using M. wightii leaf extract contain functional groups were analyzed through FT-IR spectrum as shown in Fig. 4a and AgNPs is presented in Fig. 4b. The FT-IR spectral peaks appeared at 3380.81 cm−1 assigned to the hydroxyl (–OH) stretching due to the presence of polyphenols and flavonoids [17]. A peak at 1077 cm−1 is due to vibrations of C-O stretching of alcoholic groups in phenols and polyphenols.The stretching vibrations at 1513 cm−1 and 1609 cm−1 were attributed to N–H and C = O stretching of amine and amide bonds associated with the protein molecules [18]. Therefore, the bioreduction of silver (I) into AgNPs may be attributed to the presence of phenols, proteins, aminogroups, alkanes, alkenes and flavonoids present in the M. wightii aqueous leaf extract.
3.5 Morphology and elemental studies
TEM analysis of synthesized AgNPs was prepared by drop-coating biosynthesized nanoparticles solution on carbon-coated copper TEM grid. Samples were dried and kept under vacuum in desiccators before loading on to a specimen holder. The AgNPs prepared by green synthesis method TEM images is shown in Fig. 5a–c.The formations of Ag NPs are spherical in shape without any accumulation and the majority of the particles were uniform distribution. The particle size of Ag was ranging from 20 to 50 nm. The elemental composition of AgNPs was confirmed by EDAX as shown in Fig. 5d. The Ag element is shown in the EDAX image so it confirmed the AgNPs but some other extra elemental peaks are shown in Fig. 5d because these elements are indicated as extracted biomolecules.
3.6 Antibacterial activity
Antibacterial activity results of AgNPs were presented in Fig. 6. The highest activity was 15 mm diameter of zone inhibition observed against Bacillus subtilis, followed by 13 mm of zone inhibition against P. aeruinosa was observed to compare with the standard streptomycin. Metallic silver is additionally a potent antimicrobial agent with high potential of activity. The high reactivity and high binding efficiency with proteins by altering the structure of bacterial cell membrane and nuclear membrane, which results in cell disruption and damage. AgNPs with sizes ranging of 10–100 nm showed high bactericidal activity against gram-positive and gram-negative bacteria [21]. The high reactivity and high binding efficiency with proteins followed by with changes within the structure of bacterial cell membrane and nuclear membrane. Finally, result in cell disruption and death. Silver ions have the power to restrain the replication of bacteria, by binding and denaturing bacterial DNA, which incorporates the reaction of silver ions with the thiol group of proteins followed by DNA condensation resulting in apoptosis [22]. Nanoform of silver is more reactive and they cross through cell membranes leading to the deposition of intracellular nanoparticles, which results in the disfunction of cells [23]. It has been already found that AgNPs produce reactive oxygen species and free radicals, which will lead to apoptosis resulting in necrobiosis. AgNPs can easily penetrate into the microbial cell wall due to their small size than the microorganisms. AgNPs are also utilized in food industry for the packaging to stop damage of food products by pathogens [24]. There have been Electron spin resonance spectroscopy (ESR) studies which proposed that there is a generation of free radicals by the AgNPs on interaction with the bacteria and these free radicals have the capability to damage the bacterial cell membrane and make it porous which can finally lead to cell death [25]. Silver is classified as a soft acid, and there is a usual tendency of acid to react with a base, in this case, a soft acid to react with a soft base. Another fact is that the DNA has phosphorus and sulphur as its major constituents; the AgNPs can react with soft bases and damage the DNA, which would ultimately lead to cell death as shown in Fig. 7 [26,27,28,29,30,31,32,33].
4 Conclusion
In this current work, non-hazardous, simple, biosynthesis method was employed for the successful synthesis of Ag nanoparticles. The formed Ag nanoparticles were characterized for their UV–visible absorption, structural, morphological and FTIR analysis. The antibacterial potential of the synthesized samples was studied against Bacillus subtilis and Pseudomonas aeruginosa. The Ag nanoparticles shows very good antibacterial activity may be used for controlling of bacterial associated diseases.
References
Kazemzadeh Y, Shojaei S, Riazi M, Sharifi M (2019) Chin J Chem Eng 27:237
Nazarzadeh ZE, Pooyan M, Assunta B, Franklin TR, A. B. de, and V. T. Vinod, (2019) Chem Commun 55:14871
Ahmadi Z (2019) Prog Org Coa 132:445
Raza A, Sime FB, Cabot PJ, Maqbool F, Roberts JA, Falconer JR (2019) Drug Discov Today 24:858
Zaka M, Abbasi BH, Rahman L, Shah A, Zia M (2016) IET Nanobiotech 10:1
Cristina BA, Oana G, Mihai GA, Laurentiu M, Anton F, Ecaterina A (2018) Nanomaterials 8:681
**-Feng Z, Zhi-Guo L, Wei S, Sangiliyandi G (2016) Int J Mol Sci 17:1534
Zhi G, Kang** C, Guangming Z, Jiajia W, **ngpan G (2018) Sci Total Environ 643:1325
Zeqing B, Christopher LQ (2019) Colloids Surf. B. 184:110519
Farinaz G, Amir M, Fatemeh A, Hassan N, Mojgan DJ, Soheil S, Shandiz SAS (2017) IET Nanobiotechnol 11:485
Bashir A, Farah S, Bashir S, Ibrar K, Sadiq A (2016) IET Nanobiotechnol 10:1
Masooleh AK, Ahmadikhah A, Saidi A (2019) IET Nanobiotechnol 13:183
Pooja R, Kumar V, Singh PP, Matharu AS, Zhange W, Kim KH, Singh J, Rawat M (2020) Environ. Int. 143:105924
Sonam C, Sanjay G (2020) Mater. Sci. Energy Technol. 3:536
Ahluwalia V, Kumar J, Sisodia R, Shakil NA, Walia S (2014) Ind Crops Prod 55:202
Siva SS, Lakshman KD (2018) Mater Lett 226:47
Chouhan S, Guleria S (2020) Mater. Sci. Energy Technol. 3:536
Pooja R, Vanish K, Pal SP, Singh MA, Wei Z, Hyun KK, Jagpreet S, Mohit R (2020) Environ. Int. 143:105924
Siwar J, Jenana RKB, Dridi C (2020) Mater. Chem. Phys. 248:122898
Tanmoy D, Nath GN, Mahuya D, Rajsekhar A, Vivekananda M, Asoke PC (2020) J. Environ. Chem. Eng. 8:104019
Jamee B, Bond CM, Worthington RJ, Smith CA, Gifford JC, Simpson CA, Carter CJ, Wang G, Hartman J, Osbaugh NA, Shoemaker RK, Melander C, Feldheim DL (2014) J Am Chem Soc 136:5295
Sunitha S, Rao AN, Abraham LS, Dhayalan E, Thirugnanasambandam R, Kumar VG (2015) J. Chem. Pharm. 7:191
Dhas TS, Kumar VG, Karthick V, Angel KJ, Govindaraju K (2014) SpectrochimicaActa 120:416
Pal S, Tak YK, Song JM (2015) J Biol Chem 290:1712
Singh A, Jain D, Upadhyay MK, Khandelwal N (2010) Dig J Nanomater Biostructures 5:483
Raza M, Kanwal Z, Rauf A, Sabri A, Riaz S, Naseem S (2016) Nanomaterials 6:74
Sadeghi B, Rostami A, Momeni SS (2015) Spectrochim. Acta Part A Mol Biomol Spectrosc 134:326
Shivananda CS, Lakshmeesha-Rao B, Pasha A, Sangappa Y (2016) IOP Conf. Ser. Mater. Sci. Eng. 149:012175
Shivananda CS, Asha S, Madhukumar R, Satish S, Narayana B, Byrappa K, Wang Y, Sangappa Y (2016) Macromol Res 24:684–690
Shivananda CS, Madhu-Kumar R, Narayana B, Byrappa K, Renu P, Wang Y, Sangappa Y (2017) Mater. Res. Innovat. 21:210–214
Shivananda CS, Asha S, Madhukumar R, Satish S, Narayana B, Byrappa K, Wang Y, Sangappa Y (2016) Biomed. Phys. Eng. Express 2:035004
Shivananda CS, Lakshmeesha-Rao B, Rajesh-Shetty BG, Sangappa Y (2018) AIP Confer. Proc. 1953:030254
Shivananda CS, Rao BL, Nagi-Reddy BK, Sangappa Y (2020) AIP Confer. Proc. 2220:020024
Author information
Authors and Affiliations
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dogiparthi, L.K., Sana, S.S., Shaik, S.Z. et al. Phytochemical mediated synthesis of silver nanoparticles and their antibacterial activity. SN Appl. Sci. 3, 631 (2021). https://doi.org/10.1007/s42452-021-04641-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04641-1