Abstract
In this study, the molecular structure of single crystal containing Schiff bases has been characterized by X-ray diffraction, NMR, IR and UV‒Vis spectral techniques and compared with similar molecules in the literature. For the purpose of supporting X-ray results, geometric parameters and spectroscopic studies of the title compound were theoretically performed by Hartree‒Fock and density functional theory methods. In addition, the title compound’s molecular energies, Mulliken‒ESP‒NPA‒Hirshfeld charges, molecular electrostatic potential surface, Frontier orbitals and thermodynamic properties to elucidate intermolecular interactions were calculated. All the calculations in gas and solid phases were carried out using Gaussian 09 and Quantum Espresso programs. It was found that the studies of X-ray are more compatible with the calculations made in the solid phase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Aromatic heterocycle compounds have attracted more attention than poly ring cyclic aromatic compounds containing only carbon atoms, since they are very common in nature. Heterocyclic compounds also contain both carbon atoms and other atoms such as N, S and O in their structure. Thiazoles are also of the class of heterocycle compounds. In a ring of five, this compound is called thiazole if the sulfur and nitrogen atoms are in position 1 or 3. Many antibiotics and biomolecules containing thiazole and its derivatives are known to have biological importance. The compounds with thiazole ring have applications in pharmacology such as allergies [1], schizophrenia [2], hypertension [3], anti-HIV [4], anti-bacterial [5], anti-thrombotic activity [6], anti-protozoal [7], anti-helminthic [8], anti-microbial [9]. Furthermore, 2-aminothiazoles exhibit potential activity in many human cancer cell lines [10,11,12,13]. Thiazole rings are used as starting materials in many areas of chemistry. In addition, some thiazole compounds are used in the paint industry and in agriculture [14]. Cyclobutanes, which have a ring system with four carbon atoms, are organic compounds. There is bond tension in the cyclobutane ring, and because of this tension, the rings can easily react and be converted to straight-chain compounds. Furthermore, this tension makes the molecule more stable conformation by puckering the cyclobutane ring. Their carboxylic acid derivatives have antidepressant activity and liquid crystal properties. They are used for different purposes, especially in the synthesis of heterocyclic substances [15].
In this paper, newly synthesized chloro-acetic acid N′-(2-hydroxy-naphthalen-1-ylmethylene)-N-[4-(3-methyl-3-phenyl-cyclobutyl)-thiazol-2-yl]-hydrazide was characterized with the help of X-ray diffraction, FT-IR, NMR and UV–VIS spectroscopy. Also, these spectroscopic studies, molecular energy, net charge, Molecular Electrostatic Potential (MEP) and boundary orbitals of the title compound were carried out theoretically.
2 Materials and methods
2.1 X-ray crystallography
Diffraction data of the head crystal were collected using the STOE IPDS II (Imaging Plate Diffraction System) diffractometer. X-AREA and X-RED32 [16] programs were used for data collection and reduction processes, respectively. After the necessary corrections had been applied to the collected data, the title compound was solved by direct methods with the help of SHELXT-2015 [17] software and refined by the least squares method with the help of SHELXL-2015 [18] software included in the Olex2 [19] packet program. All non–hydrogen atom parameters were refined anisotropically and all H atom parameters were located geometrically and refined by using a riding model with Uiso (H) = xUeq, where x = 1.5 for CH3 and OH groups and 1.2 for CH2 and CH groups. The Uiso values for H atoms are in the range 0.063–0.236.
2.2 Synthesis
The compound was synthesized as shown in Scheme 1 by the following procedure. To a stirred solution of 1-{[4-(3-methyl-3-mesityl-cyclobutyl)-thiazol-2-yl]-hydrazonomethyl}-naphthalen-2-ol (0.456 g, 1 mmol) in 30 mL of absolute ethanol, chloroacetyl chloride (1 mmol) in 10 mL of absolute ethanol was added dropwise in 2 h period at room temperature in the presence of 1 mmol triethylamine. After the mixture was stirred 2 h more and poured into water. Thus, precipitated solid substance was filtered off, washed with aqueous NH3 solution several times and dried in air. Suitable single crystals for crystal structure determination were obtained by slow evaporation of its ethanol solution. Yield: 67%, melting point: 428 K. Characteristic IR bands: 3390 cm−1 ν(–OH), 2957–2858 cm−1 ν(aliphatics), 1706 cm−1 ν(C = O), 1623 cm−1 ν(C = N azomethine), 1593 cm−1 ν(C = N thiazole), 736 ν(C–Cl), 639 cm−1 ν(C–S–C thiazole). Characteristic 1H NMR shifts (CDCl3, δ, ppm): 1.60 (s, 3H, –CH3 in cyclobutane), 2.18 (s, 6H, o–CH3), 2.21 (s, 3H, p–CH3), 2.66–2.70 (m, 4H, –CH2– in cyclobutane), 3.64 (q, j = 8.78 Hz, 1H, > C–H in cyclobutane), 4.62 (s, 2H, –CH2–Cl), 6.70 (s, 2H, aromatics), 6.90 (d, j = 0.73 Hz, 1H, = CH-S), 7.24 (t, j = 6.22 Hz, 2H aromatics), 7.38–7.46 (m, 2H, aromatics), 7.80–7.88 (m, 2H, aromatics), 7.90 (d, j = 9.15 Hz, 1H, aromatics), 9.82 (s, 1H, azomethine), 11.51 (s, 1H, –OH D2O exchangeable). Characteristic 13C NMR shifts (CDCl3, δ, ppm): 165.38, 160.15, 158.84, 157.05, 144.25, 135.44, 135.30, 134.88, 132.94, 130.57, 129.50, 128.57, 128.40, 124.21, 119.92, 119.23, 111.90, 107.61, 43.97, 42.79, 41.23, 31.71, 24.78, 21.65, 20.65.
2.3 Computational details
All theoretical calculations in the gas phase were performed by the Gaussian 09 program package [20] which use the density functional theory (DFT)/B3LYP [21,22,23] functional and the 6−31G(d) [24] basis set. GaussView 5 [25] program was used to visualize the obtained results. The solid phase calculations were performed using the Quantum Espresso software [26] within the density functional theory (DFT). The correlation functionals were performed by the generalized gradient approximation (GGA), as proposed by Perdew–Burke–Ernzerhof (PBE) [27] and Local Density Approach (LDA), as proposed by Perdew-Zunger (PZ) [28].
3 Results and discussion
3.1 Geometrical structure
The crystal parameters, data collection and details of refinement process are shown in Table 1 and a diagram, which the crystal drawn with experimental 20% probability ellipsoids, is shown in Fig. 1.
The molecule has non-coplanar thiazole, cyclobutane, trimethyl benzene and naphthalene rings. The angles between the cyclobutane-thiazole and the cyclobutane-trimethyl benzene rings are 55.09° and 40.60°, respectively. Experimental and calculated bond lengths, bond angles and torsion angles are also shown in Table 3. The bond lengths of O1‒C1, C11 = N1, C19‒C17, S1 = C15, C22‒C20 and C14 = N3 are experimentally 1.351 (3), 1.279 (3), 1.527 (4), 1.704 (2), 1.522 (4) and 1.303 (3) Å, respectively and these lengths are in accordance with the literature values [29, 30]. The crystal has C‒H···O intermolecular and O‒H···N and C‒H···N intramolecular hydrogen bonds. The crystal is also stabilized by normal van der Waal’s forces and by π-π stacking interactions between the naphthalene rings. The molecular structure has adopted the enol-imine form and has been stabilized by the O‒H···N type intramolecular hydrogen bonding. The bond lengths C1‒O1 showing the single bond character and C11 = N1 showing the double bond character support that the molecule adopts the enol-imine form. These bond lengths agree with the bond lengths obtained from similar enol-imine form structures [31, 32]. It is observed that C‒H···O hydrogen bond between linking adjacent molecules formed a chain sequence along the b-axis direction with graph set analysis C(10) motif (Fig. 2) and N‒H···O hydrogen bond generates an S(6) ring motif.
In addition, within the title compound, there is a π···π interactions occur between the naphthalene rings of neighboring molecules linked by symmetry. The centroid–centroid distance between Cg1 (C4–C9) and Cg2ii (C3/C4/C9/C1/C2/C10) [symmetry code: 1 − x, − 1/2 + y, 1/2 − z] is 3.959 (2) Å. As a result of the structure solution and refinement, ORTEP-3 of the obtained structure and the packaging drawing of the title compound in the unit cell are shown in Figs. 2 and 3, and the information about the hydrogen bindings are shown in Table 2.
In order that the stable molecular structure may be found, the global minimum scanning (2-dimensional PES analysis) were realized on the potential energy surfaces using AM1 semiempirical method [33]. Single point energies were calculated, which can affect the lowest energy conformation belonging to the molecule, changing the torsion angle θ (N1–N2–C14–S1) in steps of 10° ranging from − 180° to 180°. The two-dimensional single point energy profile versus the torsion angle is shown in Fig. 4. With the help of this graphic, global and local minimums were determined.
As seen in Fig. 4, the two most stable conformer (conf1 and conf2) were identified corresponding to global and local values at nearly − 120° and 100° angles. As a result of the re-optimization of the obtained two conformer, the corresponding total energy values were determined as − 2334.315800 a.u. (conf1) (Fig. 5a) and − 2334.310532 a.u. (conf2) (Fig. 5b). The conf1 conformation, which has a lower total energy, is more stable and has been used in all theoretical calculations.
Experimental and theoretical bond lengths, bond angles and torsional angles in the gas and solid phases of the title compound are listed comparatively in Table 3. When the calculated values in the Table 3 are examined, it is seen that HF method for bond lengths and B3LYP method for bond angles are more successful in representing experimental geometry in gas phase. Likewise, the GGA method has yielded better results than the LDA method for all parameters in the solid phase.
Figure 6 shows the overlap of X-ray and calculated molecular geometries. The RMS values resulting from the superposition of the experimental and theoretically structures were found to be 0.631 Å for HF level and 0.470 Å for B3LYP level. According to these results, DFT method has less error and characterizes the three dimensional structure of molecular geometry better than HF method.
3.2 Periodic boundary calculations (PBC)
The coordinates of 268 atoms in the unit cell were obtained from the cif file of the title compound and the kinetic energy cut-off (Ecut) value to model the system in the Plane Wave Self-Compliance field program was selected as 80 Ry. Furthermore, lattice constant was taken as 31.0155 a.u. and the numbers of k-point mesh for the Brillouin region were determined by MP (Monkhorst–Pack) method [34] and taken as 2 × 2 × 2. In order to increase the accuracy of the results of the total energy calculation, a convergence value of 10−6 Rydberg (Ry) was used. The optimized parameters of the unit cell were obtained using QE–VC-Relax method and BFGS (Broyden–Fletcher–Goldfarb–Shanno) quasi-Newton optimization algorithm. Table 4 shows the unit cell parameters obtained from X-ray and calculated using GGA and LDA methods while Fig. 7a–c is shown the distribution of atoms within the unit cell. In addition, the total lattice energy values per unit cell were obtained using the scf (self-consistent field) set included in Quantum-Espresso packet program for GGA and LDA methods. The energy values according to GGA and LDA methods were found to be − 2208.76400880 Ry and − 2201.94258365 Ry, respectively, which indicate that the GGA method has reached a more stable structure than the LDA method.
3.3 Spectroscopic studies
The experimental and calculated IR spectra of the title compound is shown in Fig. 8 and vibration frequency values are given in Table 5. The molecule has 195 normal modes of vibration. The essential characteristic vibrations of the title compound are C–H (aromatic), C = O, C = N (thiazole) and N–C = S vibrational frequencies connected to the ring groups were experimentally observed to be 3098, 1706, 1593 and 1207 cm−1, respectively, as compatible with the literature [35, 36]. These modes have been calculated at 3083, 1781, 1476 and 1192 cm−1 for HF level and at 3148, 1726, 1472 and 1217 cm−1 for B3LYP level. While the O–H group, which does not contain an intramolecular or intermolecular hydrogen bonding, has a free vibration frequency between 3700 and 3550 cm−1, this vibration frequency containing hydrogen bonding is observed between 3550 and 3200 cm−1 [37]. In our study, this frequency value was observed at 3293 cm−1, while it was calculated at 3459 cm−1 for HF level and at 3224 cm−1 for B3LYP level and compares well with the value reported previously as 3436 cm−1 for experimentally and 3406 cm−1 for B3LYP level [38].
Experimental and calculated 1H and 13C chemical shift values for the title molecule are given comparatively in Table 6. The peaks of the C14, C15 and C 16 atoms in the 13C-NMR spectrum (Fig. 9) may indicate that the molecule has a thiazole ring. The chemical shift values of these atoms are higher than other carbon atoms. Due to deshielding by the electronegative property of the N and S atoms, the chemical shift values of C14, C15 and C16 atoms are greater than the other carbon atoms and were experimentally found to be 165.38, 107.61 and 153.3 ppm, respectively which is consistent with the values reported previously (168.54, 99.58 and 151.07 ppm) [38]. Similarly, the C1 and C12 carbon peaks linked to O1 and O2 atoms were experimentally found to be 157.05 and 160.15 ppm. These values agree with similar molecules [39]. The C1, C12, C14, C15 and C16 peaks were calculated at 162.95, 174.60, 173.11, 109.32 and 151.23 ppm for HF theory, respectively and at 153.3, 159.31, 155.56, 109.34 and 146.55 ppm for DFT theory.
In the 1H-NMR spectrum (Fig. 10), the hydroxy hydrogen peak in the naphthalene ring was experimentally observed as a single proton peak at 11.51 ppm due to the formation of O–H···N intramolecular hydrogen bonding. In theoretical calculations, it was found that this peak was close to the experimental results at 10.85 ppm for HF and at 10.04 ppm for DFT.
3.4 Total charge distribution, electronic properties and MEPs of the title compound
Mulliken, ESP, NPA and Hirshfeld charge distributions of the title compound were calculated using the DFT/B3LYP/6−31G(d) method and are shown in Table 7. When the charge distributions are examined, it is seen that the most negative charges are collected on N, O and Cl atoms while the most positive charges are on C11, C12, C14, C16 and H1 (OH) atoms with neighboring electronegative atoms. Since these atoms are used in a strong intramolecular hydrogen bonding, the charge densities may be increased. As a result of theoretical calculations, the fact that both the N1 and N3 atoms are more negatively charged than the N2 atom and the O1 atom than the O2 atom support the intermolecular interactions within the crystal obtained by X-ray diffraction. According to these results, it is possible to say that these atoms play an important role in the formation of intermolecular hydrogen bonds.
Schiff bases obtained from aldehydes containing o-hydroxy group have two types of tautomeric structures: enol-imine and keto-amine. The presence of these two types structures was also determined by UV–VIS spectral analysis. It is known that electronic transitions occurring below 400 nm are enol-imine transitions and electronic transitions above 400 nm are keto-amine forms [40].
In our study, UV–VIS spectra (Fig. 11) of C30H30N3O2SCl molecule were recorded in ethanol and it was determined that C = C, N–N and C = O groups may belong to π → π* and n → π* transitions. The two major maximum peaks observed in the experimental UV–VIS spectra are at 306.57 nm (molar absorption coefficient; ε = 2800 L/mol cm) and 277.24 nm (molar absorption coefficient; ε = 3050 L/mol cm). These values correspond to the transitions n → π* and π → π*, respectively. This result supports that the molecule crystallizes in enol-imine form and agree with similar molecule [41]. Using the DFT method, these peaks were theoretically obtained at 366.93 nm (ε = 20,089 L/mol cm) and 249.13 nm (ε = 18,833 L/mol cm) (Fig. 12) and the two peaks correspond to the electron transitions HOMO → LUMO (98%) for 366.93 nm and HOMO-6 → LUMO (9%), HOMO-5 → LUMO (60%), HOMO-4 → LUMO (2%), HOMO-4 → LUMO + 2 (3%), HOMO-3 → LUMO + 1 (2%), HOMO-3 → LUMO + 2 (4%), HOMO → LUMO + 3 (16%) for 249.13 nm. The experimental and calculated two major maximum peaks of UV–Vis spectral data (excitation energies, wavelength, oscillator strength) were showed comparatively in Table 8.
The HOMO and LUMO orbitals and energy values of the title compound are given in Fig. 13. As it is seen, the orbitals, which the molecule react by giving electron, are located on thiazole and naphthalene rings and the orbitals, which the molecule react by taking electron, are located on naphthalene, thiazole rings and N atoms. These orbitals on the compound indicate the regions where nucleophilic and electrophilic attacks occur.
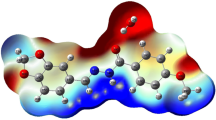
The MEP map of the molecule was obtained using the DFT/B3LYP/6–31G(d) method and is shown in Fig. 14a, b. In the MEP map, negative regions (red, yellow) are on O and S atoms, while positive regions (blue) are on hydrogen atoms. The most negative region is on O atoms and the maximum MEP value is − 0.050 a.b., while the MEP value for S atom is − 0.035 a.b. and these results indicate that O and S atoms are the most suitable regions for electrophilic reaction. The maximum MEP value in the positive region on the H atoms bound to the benzene and naphthalene rings was calculated as + 0.023 a.b.. According to these results, the nucleophilic and electrophilic regions determined by net charge analysis, Frontier orbitals (FMO) and MEP analysis are in good agreement with each other.
3.5 Thermodynamic parameters of the title compound
Scheme 1 shows the complete synthesis scheme of the title compound. Heat capacity (\(C_{m}^{0}\)), entropy (\(S_{m}^{0}\)) and enthalpy (\(H_{m}^{0}\)) values, which are the standard thermodynamic functions for each reagent and products in the formation reaction, were calculated and are listed in Table 9, while enthalpy change (ΔH), Gibbs free energy change (ΔG) and entropy change (ΔS) values were calculated and are listed in Table 10. As can be seen in Table 9, the standard heat capacity, entropy and enthalpy of reactance and products were calculated between 100 and 500 K temperature and it is seen that the (\(C_{m}^{0}\)), (\(S_{m}^{0}\)) and (\(H_{m}^{0}\)) values increase as the temperature increases. In Table 10, the ΔS values of the title compound are negative in all temperature and the ΔH values are positive. This result indicates that the formation of the title compound occurs between 100 and 500 K by an endothermic process. Using the ΔG = ΔH − TΔS equation, Gibbs free energy change (ΔG) was also obtained between 100 and 500 K temperatures. In Table 10, the ΔG values above 100 K temperature are positive (ΔG > 0), indicating that the formation process of the title compound is not spontaneous.
According to the data in Table 9, The correlations equations of the \(C_{m}^{0}\), \(S_{m}^{0}\) and \(H_{m}^{0}\) versus T temperatures were obtained as follows;
The values \(C_{m}^{0}\), \(S_{m}^{0}\) ve \(H_{m}^{0}\) can be obtained at all other temperatures with these equations. Also, their correlation graphs are shown in Fig. 15.
The sign and size of these thermodynamic parameters are useful for determining the intermolecular binding mode (particularly receptor-ligand interactions). It has been reported that a hydrophobic interaction is characterized by ΔH > 0 and ΔS > 0 and van der Waals force or hydrogen bond formation is characterized by ΔH < 0 and ΔS < 0. However, ΔH ≃ 0 and ΔS > 0 are caused by an electrostatic force [42]. In our study, negative values of calculated ΔH and ΔS at temperatures corresponding to 100–500 K range indicate that van der Waals force or hydrogen bond interactions may play an important role in binding the title compound to a receptor. In addition, negative values of ΔG indicate the nature of the spontaneous formation of the binding process [43].
4 Conclusions
In this study, molecular structure of chloro-acetic acid N′-(2-hydroxy-naphthalen-1-ylmethylene)-N-[4-(3-methyl-3-phenyl-cyclobutyl)-thiazol-2-yl]-hydrazide crystal has been determined by X-ray diffraction and IR, NMR and UV–Vis spectroscopic methods. In addition, spectroscopic spectra were obtained theoretically. Finally, the Mulliken, ESP, NPA and Hirshfeld charge distributions, molecular electrostatic potential and FMO were investigated. The geometric parameters of the title compound in the gas and solid phases were calculated, and generally good agreement were observed when compared with the experimental structure. It was observed that the experimental and calculated IR values were consistent with the literature values. The presence of O–H and C = N stretching vibrations in the experimental IR spectrum supports that these molecules are in enol-imine form. When the charge distributions and molecular electrostatic potential surface were examined, it was observed that negative charges were generally located on electronegative atoms. The red and blue regions on the molecular electrostatic potential map represent regions that are prone to chemical reaction and are unstable to react.
References
Hargrave KD, Hess FK, Oliver JT (1983) N-(4-Substituted-thiazolyl) oxamic acid derivatives, new series of potent, orally active antiallergy agents. J Med Chem 26(8):1158–1163
Jaen JC, Wise LD, Caprathe BW, Tecle H, Bergmeier S, Humblet CC, Heffner TG, Meltzer LT, Pugsley TA (1990) 4-(1, 2, 5, 6-Tetrahydro-1-alkyl-3-pyridinyl)-2-thiazolamines: a novel class of compounds with central dopamine agonist properties. J Med Chem 33(1):311–317
Patt WC, Hamilton HW, Taylor MD, Ryan MJ, Taylor DG Jr, Connolly CJ, Doherty AM, Klutchko SR, Sircar I (1992) Structure-activity relationships of a series of 2-amino-4-thiazole-containing renin inhibitors. J Med Chem 35(14):2562–2572
Bell FW, Cantrell AS, Hoegberg M, Jaskunas SR, Johansson NG, Jordan CL, Kinnick MD, Lind P, Morin JM Jr (1995) Phenethylthiazolethiourea (PETT) compounds, a new class of HIV-1 reverse transcriptase inhibitors. 1. Synthesis and basic structure-activity relationship studies of PETT analogs. J Med Chem 38(25):4929–4936
Tsuji K, Ishikawa H (1994) Synthesis and anti-pseudomonal activity of new 2-isocephems with a dihydroxypyridone moiety at C-7. Bioorg Med Chem Lett 4(13):1601–1606
Badorc A, Bordes M-F, de Cointet P, Savi P, Bernat A, Lalé A, Petitou M, Maffrand J-P, Herbert J-M (1997) New orally active non-peptide fibrinogen receptor (GpIIb-IIIa) antagonists: identification of ethyl 3-[N-[4-[4-[amino [(ethoxycarbonyl) imino] methyl] phenyl]-1, 3-thiazol-2-yl]-N-[1-[(ethoxycarbonyl) methyl] piperid-4-yl] amino] propionate (SR 121787) as a potent and long-acting antithrombotic agent. J Med Chem 40(21):3393–3401
Tapia RA, Prieto Y, Pautet F, Walchshofer N, Fillion H, Fenet B, Sarciron M-E (2003) Synthesis and antiprotozoal evaluation of benzothiazolopyrroloquinoxalinones, analogues of kuanoniamine A. Bioorg Med Chem 11(16):3407–3412
Srivastava S, Yadav R, Srivastava S (2004) Synthesis and biological activity of 4-oxothiazolidines and their 5-arylidenes. Indian J Chem 43B:399–405
Siddiqui HL, Zia-Ur-Rehman M, Ahmad N, Weaver GW, Lucas PD (2007) Synthesis and antibacterial activity of Bis [2-amino-4-phenyl-5-thiazolyl] Disulfides. Chem Pharmaceut Bull 55(7):1014–1017
Kayagil I, Demirayak S (2009) Synthesis and anticancer activities of some thiazole derivatives. Phosphorus Sulfur Silicon 184(9):2197–2207
Misra RN, **ao H-y, Williams DK, Kim KS, Lu S, Keller KA, Mulheron JG, Batorsky R, Tokarski JS, Sack JS (2004) Synthesis and biological activity of N-aryl-2-aminothiazoles: potent pan inhibitors of cyclin-dependent kinases. Bioorg Med Chem Lett 14(11):2973–2977
El-Subbagh HI, Abadi AH, Lehmann J (1999) Synthesis and antitumor activity of ethyl 2-substituted-aminothiazole-4-carboxylate analogs. Archiv der Pharmazie Int J Pharmaceut Med Chem 332(4):137–142
Gorczynski MJ, Leal RM, Mooberry SL, Bushweller JH, Brown ML (2004) Synthesis and evaluation of substituted 4-aryloxy-and 4-arylsulfanyl-phenyl-2-aminothiazoles as inhibitors of human breast cancer cell proliferation. Bioorg Med Chem 12(5):1029–1036
Schrauzer GN, Kohnle J (1964) Coenzym B12-modelle. Chem Ber 97(11):3056–3064
Çukurovali A, Taş E, Ahmedzade M (1997) The synthesis and some transition metal complexes of tolyl n-imidazolylmethyl ketoxime. Synth React Inorg Metal Chem Org 27(8):1115–1166
Cie S (2002) X-area (version 1.18) and X-red32 (version 1.04). Darmstadt, Germany
Sheldrick GM (2015) SHELXT–integrated space-group and crystal-structure determination. Acta Crystallogr Sect A Found Adv 71(1):3–8
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallogr Sect C Struct Chem 71(1):3–8
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JA, Puschmann H (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Crystallogr 42(2):339–341
Frisch M, Trucks G, Schlegel HB, Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Mennucci B, Petersson G (2009) Gaussian 09, revision A. 02, Gaussian. Inc., Wallingford, CT 200
Hohenberg P, Kohn W (1964) Inhomogeneous electron gas. Phys Rev 136(3B):B864
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37(2):785
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98(7):5648–5652
Foresman JB, Frisch A (1996) Exploring chemistry with electronic structure methods, 2nd edn. Gaussian Inc., Pittsburg
Dennington R, Keith T, Millam J, Eppinnett K, Hovell WL, Gilliland R (2009) GaussView, Version
Giannozzi P, Baroni S, Bonini N, Calandra M, Car R, Cavazzoni C, Ceresoli D, Chiarotti GL, Cococcioni M, Dabo I (2009) QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J Phys Condens Matt 21(39):395502
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77(18):3865
Perdew JP, Zunger A (1981) Self-interaction correction to density-functional approximations for many-electron systems. Phys Rev B 23(10):5048
Sun Y-X (2006) 4-(5-Chloro-2-hydroxybenzylideneamino)-1, 5-dimethyl-2-phenyl-1H-pyrazol-3 (2H)-one. Acta Crystallogr Sect E Struct Rep Online 62(12):o5858–o5859
Sun Y-X, Zhang R, ** Q-M, Zhi X-J, Lü X-M (2006) 4-(4-Chlorobenzylideneamino)-1, 5-dimethyl-2-phenyl-1H-pyrazol-3 (2H)-one and 4-(2-chlorobenzylideneamino)-1, 5-dimethyl-2-phenyl-1H-pyrazol-3 (2H)-one. Acta Crystallogr Sect C Cryst Struct Commun 62(8):o467–o469
Cukurovali A, Karakurt T (2019) Synthesis, spectroscopic, X-ray diffraction and tautomeric properties of 5-(diethylamino)-2-((2-(5-(3-methyl-3-phenylcyclobutyl)-6H-1, 3, 4-thiadiazin-2yl) hydrazono) methyl) phenol: a combined experimental and theoretical study. J Mol Struct 1189:328–337
Karakurt T, Cukurovali A, Subasi NT, Onaran A, Ece A, Eker S, Kani I (2018) Experimental and theoretical studies on tautomeric structures of a newly synthesized 2, 2′(hydrazine-1, 2-diylidenebis (propan-1-yl-1-ylidene)) diphenol. Chem Phys Lett 693:132–145
Dewar MJ, Zoebisch EG, Healy EF, Stewart JJ (1985) Development and use of quantum mechanical molecular models. 76. AM1: a new general purpose quantum mechanical molecular model. J Am Chem Soc 107(13):3902–3909
Monkhorst HJ, Pack JD (1976) Special points for Brillouin-zone integrations. Phys Rev B 13(12):5188
Rabie UM, Abou-El-Wafa MH, Nassar H (2012) Interaction of thiazolidine-2-thione with 2, 3, 5, 6-tetrabromo-1, 4-benzoquinone: a set of sequential interactions involving redox and substitution reactions after an initial charge transfer complexation. Spectrochim Acta Part A Mol Biomol Spectrosc 86:252–255
Lambert JB, Shurvell HF, Lightner DA, Cooks RG (1987) Introduction to organic spectroscopy. Macmillan Publishing Company, New York
Dabbagh HA, Teimouri A, Chermahini AN, Shahraki M (2008) DFT and ab initio study of structure of dyes derived from 2-hydroxy and 2,4-dihydroxy benzoic acids Spectrochim. Acta Part A Mol Biomol Spectrosc 69:449
Karakurt T, Cukurovali A, Subasi NT, Kani I (2016) Molecular structure and computational studies on 2-((2-(4-(3-(2, 5-dimethylphenyl)-3-methylcyclobutyl) thiazol-2-yl) hydrazono) methyl) phenol monomer and dimer by DFT calculations. J Mol Struct 1125:433–442
Yilmaz I, Çukurovali A (2003) Salicylaldehyde thiazolyl hydrazones as ligands. Heteroat Chem Int J Main Group Elem 14(7):617–621
Ünver H (2001) Synthesis and spectroscopic studies in some new Schiff bases. Spectr Lett 34(6):783–791
Joshi H, Kamounah FS, Gooijer C, van der Zwan G, Antonov L (2002) Excited state intramolecular proton transfer in some tautomeric azo dyes and schiff bases containing an intramolecular hydrogen bond. J Photochem Photobiol A Chem 152(1–3):183–191
Ross PD, Subramanian S (1981) Thermodynamics of protein association reactions: forces contributing to stability. Biochemistry 20(11):3096–3102
Mohamadi M, Faghih-Mirzaei E, Ebrahimipour SY, Sheikhshoaie I, Haase W, Foro S (2017) Synthesis, spectroscopic studies, DFT calculations, electrochemical evaluation, BSA binding and molecular docking of an aroylhydrazone-based cis-dioxido Mo (VI) complex. J Mol Struct 1139:418–429
Acknowledgements
Computing resources used in this work were provided by the National Center for High Performance Computing of Turkey (UHeM) under Grant Number <5005172018>.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karakurt, T., Dinçer, M. & Cukurovali, A. Syntheses, spectral characterization, single crystal X-ray diffraction and computational in gas and solid phases studies on chloro- acetic acid N′-(2- hydroxy-naphthalen- 1-ylmethylene)- N- [4-(3-methyl-3-phenyl-cyclobutyl)-thiazol-2-yl]-hydrazide. SN Appl. Sci. 2, 580 (2020). https://doi.org/10.1007/s42452-020-2374-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2374-3