Abstract
Implants are frequently administered devices to provide mechanical support to restore the function of diseased tissues and supports the natural healing process. The new concept of coated implants come to exist after 1980s to overcome the limitations related to prosthetic failures. The hurdles such as corrosion, infection and lack of bone integration of implant are the primary culprits for the failures. The present review provides a deep insight about the carbon nanomaterials (CBNs) as potential candidates for implant coatings. Additionally, the document highlights the limitations of pristine materials and discusses the different modalities to resolve the issues. The unique structural, thermal, mechanical and electrical properties of CBNs have been presented in detail to understand the significant utilization of CBNs in biomedical sciences and technology. The review may provide an opportunity for researches to develop novel materials for futuristic applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Joint replacement is major surgical intervention to treat the severe damage of articular surfaces, due to cancer deformities of musculoskeletal tissues and distortions by various mechanical interferences. The procedure involves the replacement of total joint or part of joint by the prosthesis. The most common articular damage is osteoarthritis and it is mainly characterized by the degradation of bone, pain and deformity [128]. The first successful joint replacement attempt is recorded in 1948 at New York orthopedic hospital. About 2.2 million bone surgeries are taken place every year to get rid of the condition [81]. The articular joint prosthesis is a device, which restores the function of the joint by providing mechanical support or complete replacement of skeletal tissue. Generally, the devices are made up of metals, ceramics, plastics/polymers and composites. Traditionally, various metals have been practiced in healthcare particularly on orthopaedics, such as lead, silver, aluminum, iron, copper and zinc etc. Despite their adverse effects towards the biological system, due to lack of technology advancement. In modern medical technology, several bio-friendly materials/metals are successfully employed in complex surgeries. The metals like stainless steel, zirconium, titanium and their alloys are largely used in orthopaedics, since the late 1930s [33]. Predominantly, stainless steel, cobalt chrome alloy, titanium and their alloys are mostly utilized for musculoskeletal applications, due to their good biocompatibility, high corrosion resistance, extraordinary mechanical strength and low cost [93].
Regardless of the great progress of medical implants technology and it is suffered from a set of limitations to restrict the success rate up to some extent. Frequent issues related to orthopedic implants like corrosion, loosening of the device; due to poor mechanical strength, infection, non- integration of implant with host tissue and wearing are the prime factors of concerns [19, 31, 87]. The far most characteristic of an orthopedic implant is integration to host tissue to boost the success rate by resisting the colony formation of microbes and corrosion [23].
Infections of fracture-fixation devices involve complex treatment with a 6-week course of systemic antibiotics and it requires less time interval (15 days) for superficial infections [114]. In many cases, the infections lead to the failure of the prosthesis and require the revision of surgery [22]. The coating on the implant is come to exist to avoid the infection [101] and corrosion related failures; further, the bioactive coatings largely enhance the integration to the host tissues [34].
The coating is a specific material cover on the surface of the implant system with predefined thickness. In general, the objective of the coating is to protect the implant from corrosion, infection, friction, promote the osteoconductivity and osseointegration. Interaction at the interface of the implant and surrounding tissue is a key factor in the success of implant and where coating plays a vital role. The ideal properties of coating material is non-toxic, non-carcinogenic, non-immunogenic and non-thrombogenic [5] and it is very difficult to find all ideal characteristics in a single coating material/formulation. Coatings in dentistry have been reported since 1970’s but orthopedics follows the footsteps quite late in the 1980’s. First coated implantation is taken place at St Thomas Hospital in London in 1981 [106].
Design of coating material is challenging research and it has to full fill certain measures. Firstly, it should be biocompatible and non-immunogenic; second, it must have sufficient mechanical strength and should form a mechanical bond to the host tissue to withstand, when functional stress occurs. Third, it has to be osteoconductive to promote the integration of the host tissue and it should possess osteoinductive to differentiation of recruited stem cells into osteogenic cells from surrounding tissues. Finally, the coating material should hold an antimicrobial activity to avoid the failure of implant system by post-surgery infection. None of the current commercial implants or coatings possess all required properties [128]. Therefore, there is a need for further research on biological coatings, which fulfill the maximum benchmarks and improves the life of the implant.
Adequate research is available on orthopedic coating material such as Hydroxyapatite (HA) [29, 42, 73, 120]. HA attracts more to the researchers as its chemical composition similar to natural bone. Moreover, HA is highly biocompatibility and osteoconductive, hence it is quite suitable for orthopedic applications. Collagen and collagen-based composites are used mostly in regenerative medicine but poor mechanical properties limit their usefulness in hard tissue applications [14, 38, 52, 56, 64]. Bioglass [12, 70, 84, 92] is an attractive alternative, since, it promotes proliferation of bone tissue much faster as compared to healing occur in autogenously bone graft and HA [69]. Furthermore, it has been well documented that the bioglass stimulates the angiogenesis and offer antibacterial effect [121]. Despite, its benefits, bioactive glass is still in limited usage by its high elastic modulus. Polymers [78] are used for coatings, such as polycaprolactone (PCL) [48, 117], poly-D L lactide [27, 119], polyurethane [126], polyaniline and polyimide, which shows high anti-corrosive and good cell differentiation properties. Apart from composites of synthetic polymers, the composites of natural polymer like cellulose, starch and acetate with HA were well demonstrated for enhanced osseointegration [74, 88, 89, 116]. Antibacterial and osteoconductive drug molecules are also used for various coatings [96, 111]. Particularly, drugs like simvastatin are used to enhance cell growth and callus formation, whereas antibiotics like gentamicin and ciprofloxacin are used to inhibit post-surgical infections [96]. The key role of the coating to provide control release of the drug, else it leads to drug-related toxicity from uncontrolled release [51, 119]. In alternative, growth factors such as Bone-Morphogenetic Protein (BMP-2), Platelet-derived Growth Factor (PDGF), Transforming growth factor (TGF) and Vascular Endothelial Growth Factor (VEGF) are generally administrated through polymers to enhancing the bioactivity of the coating, however, these limits by uncontrolled growth of tissues and high cost [51, 75].
The contemporary researchers are trying to avoid all adverse effects of implants by adopting nanotechnology and explore new-engineered materials of a natural source. Carbon is an extraordinary material for various applications and it is available in various forms with a range of properties. It has been being used extensively in aerospace, electrical, electronics and energy generation applications, but limited in biology. Carbon is discovered in prehistory and has been known since the ancient period. Naturally occurred carbon source such as amorphous carbon, graphite and diamonds are in allotropic in nature. Engineered carbon structures like nanotubes (CNT) and fullerenes are also in allotropic of carbons. Sumio ljima et al. reported fiber form of carbon structure in 1991, known as CNT and it possesses significant mechanical, thermal and electrical properties [39]. Whereas, fullerene is much stable carbon structure, since its graphite-like bonds [24]. The sheet-like graphene is single layer carbon source and it is the building block of all allotropes, when it is stacked to 3D it forms 3D graphite, rolled to form 1D CNT and wrapped to form 3D fullerene [3]. Significantly, nano-sized carbon sources were well demonstrated in augmented properties in various aspects such as mechanical, electrical, thermal and physical properties [2]. The enhanced properties of the nano-sized materials come from its unique structures and atomic alterations [100]. The first study in biomedical on nano-carbon for osteoblast differentiation is reported by Webster and co-workers in 2002. The study was reported with increased osteoblast proliferation, alkaline phosphatase activity, and bone mineral deposition on carbon nanofibers than micron-sized fibers and titanium implants [124]. Carbon is an important material for biological applications by its succeeding motives; it improves the level of osseointegration, since, hybrid of microscale pits in implant surface and layers of sheets or tubes mimic the cellular environment thus enhances the rate of integration. It shows excellent antimicrobial activity: due to its ordered structure and strong mechanical properties, CNTs are stronger than steel. Even more the graphene is stronger than all carbon derivatives including diamond [86]. Though, graphene is the strongest material, it is lightest and thinnest material ever found [23]. Fullerenes are commonly known as ‘Buckyball’ (C60). It is a spherical cage-like structure which is made up of sixty sp2 carbons and discovered in 1985 [54]. Further, it shows unique electronic properties from its stemming symmetrical structure. Exploring various applications of CBN starts with the discovery of C60. Fullerene is not much accepted in orthopedics because of the discovery of more practical carbon-based materials like CNT and graphene. However, it shows anti-HIV activity [9].
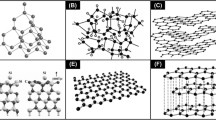
Carbon nanotubes are rolled up structures of single and multi-sheets of graphene which gives single-walled CNTs (SWCNT) and multi-walled CNTs (MWCNT), respectively [61]. CNTs are characterized by a diameter, band gap and chiral angle. SWCNT has a diameter in the range of 0.5–1.5 nm and MWCNTs have a diameter ≥ 100 nm [45]. CNTs have a high aspect ratio (length:diameter), approximately 132,000,000:1 [23]. About 15 years ago, Thomson et al. cultured mouse fibroblast on tissue culture plates coated with carbon. They found that, no adverse effect on cells at a moderate dose and thereafter they investigate the merits as a coating for biomedical use [112]. CNTs are highly useful for biomedical applications, since its properties such as good biocompatibility, high electrical conduction, mechanical and surface properties [15, 79]. They show excellent packing density and ability to deposit uniformly on a metal substrate [61]. The production capacity of CNT is continuously growing every year to an exponential degree and decreases price to use the CNTs in various applications [18]. CNTs are well established in various fields like electronics (to increase the conductivity of electrodes in lead-acid batteries), [30] used as fillers in various matrices especially in the polymer to improve the mechanical property [62], energy storage devices [99] and biomedical applications [18]. Figure 1 represents the structures of various carbon materials.
However, some challenges limit the use of CNTs in real-world applications. Primarily, the cytotoxicity is the major hurdle, due to residual impurities during synthesis [61]. Impurities like amorphous carbon, residuals of catalytic particles are highly disturbed the living environment. Even more, the purification strategies also introduce cytotoxic substances in certain cases (e.g. sodium dodecyl sulfate) [46]. Kaiser et al. have identified high oxidative stress generated when CNTs interact with cells and initiates the oxidative stress-responsive pathway, which releases pro-inflammatory cytokines such as IL. Most of the in vivo studies reveal that, a high concentration of CNT causes changes in cell and tissue morphology [4, 118]. Beside, CNT causes acute toxicity [45]. Therefore, proper modification or alteration of pristine materials are highly necessary to reduce the adverse effects in biological use.
As mentioned, CNTs toxicity is mainly caused by synthesis residues such as heavy metals like nickel, cobalt, nickel-aluminum alloy [58]. Alternatively, graphene can be synthesized in relatively pure form and thereby decreases the risk of toxicity [68]. Graphene was first isolated by Novoselov and Geim in 2004 and it is a one-atom thin sheet of carbons and arranged in a 2D honeycomb-like structure [25]. The aromatic structure of graphene contains a crowd of free π-electrons and presence at each atom. The unique structural features of graphene possesses, excellent properties like large specific surface area, high Young’s modulus (1TPa), high electron mobility, and high thermal conductivity and impermeability to gases [28]. Geim and Novoselov reported a simple method for the extraction of graphene from graphite via chemical exfoliation and research on graphene is still in an infancy stage particularly for the biomedical application [9].
Graphene oxide (GO) is an oxidized derivative of graphene. GO has a number of reactive oxygen functional group like epoxy, carboxylic and hydroxylic group [16]. Because of its derivatization GO become more biocompatible, hydrophilic in character and interacts easily with protein via covalent, hydrogen and electrostatic bonding [28]. The GO is more suitable for medical application than graphene, since its low toxicity, better dispersion in an aqueous medium, the presence of a large number of functional groups enables it react with various substrates and GO has a wide range of physical properties than pristine graphene [9].
Another pure form of carbon is Nano-diamond. It is well known for its anti-corrosive, anti-wear properties and significantly it is more bio-compatible than CNT and fullerenes [102]. Nano-diamond has also exhibited excellent osteogenesis feature [94]. Figure 2 shows the various applications of CBNs in orthopedic implant coatings.
Hamed et al. reported a study on morphological change and its effect on mechanical properties of graphite carbon nanoparticles (GCNs) embedded epoxy resin composite. The study was carried out with variable concentrations of GCNs from 1 to 5 wt% in epoxy resin. The team reported that relatively short and agglomerated morphology in 5 wt% as compared to 2 wt%. Interestingly the 2 wt% composite was shown enhanced hardness (12.5%) and modulus (8%) as compared to the pristine epoxy, while the 5 wt% composite shows nearly same properties as pristine epoxy. The same trend was reported in tensile and compressive properties for 2 and 5 wt% composites. The suggested reason is stress concentration is higher in an epoxy matrix surrounded by a rod-shaped particle as opposed to round or elliptical particles and stress concentration effect rising from 1 to 5 wt% composites. Therefore the concentration optimization of CBN is a key parameter to achieve enhanced properties in coating technology [82].
2 CBNs as orthopaedic coatings
2.1 Osteoconductive coatings
From last decade, CBNs are greatly exploring in the biomedical arena, due to its significant characters and keenly monitoring its toxicity. CNT accelerates the precipitation of calcium phosphate (CaP), due to the presence of more nucleation centers for crystal nucleation and growth [126]. The phenomenon is known for, when bone is subjected stress, it generates an electrical signal to bone remodeling thereby regulates the bone regeneration and bone healing [41, 77, 104]. Therefore, CNT contained scaffold can be used for stimulating the cell growth and tissue regeneration by facilitating the physioelectrical signal transfer as CNTs are good electric and thermal conductor [61]. Further, to fasten the bone healing and to improve the surface bioactivity Ning CAO reinforces the CNT in HA and enhanced osteoconductive was observed against pristine carbon fibers. The study was carried out in goat model for 18 months, calcium and phosphorus ions leached from HA to provide suitable biological mineralization which accelerates the osteoblast differentiation and collagen synthesis.
The CNT reinforced coatings are promising materials for a high load-bearing orthopedic applications such as hip, knee and shoulder joints as they provide mechanical strength and nurture the precipitation of HA. Sharma et al. developed PMMA bone cement reinforced with amine functionalized GO. The in vivo studies in rabbit model shows enhanced osseointegration in NH2 functionalized graphene as compared to pristine PMMA [105]. Figure 3a–d shows the X-ray images of rabbit tibia implanted with bone cement and hybrid of bone cement-amine functionalized graphene at zero and twentieth day of post-surgery.
X-ray images of rabbit tibia implanted with pristine bone cement and hybrid of bone cement-amine functionalized graphene. A, B show the cavity filled with pristine bone cement and bone cement hybrid at zero days, respectively and C, D represents the twentieth day of post-surgery. Fluorescent images of human osteoblast cells incubated for 48 h in E graphene, F SiO2 substrate (actin filaments appear in green color and nuclei is in blue). Osteoblasts are homogeneously spreader with high density in graphene, whereas low density with island-like clumps are observed in SiO2 substrate after a 48 h incubation
Several polymers are well bioactive but inferior in mechanical properties limit its use in tissue engineering. X. Shi. et al. fabricated on SWCNT reinforced poly (propylene fumarate) (PPF) nanocomposite. Significantly, the functionalized CNTs are shown improved dispersion in PPF over pristine CNTs [107]. Titanium (Ti) is commonly used implant material for dental applications because of its biocompatibility and high Young’s modulus [17]. Various surface modification (e.g. apatite coat, anodization) were employed over titanium to achieve early integration of bone tissues. Saori Inoue and co-workers investigated on the MWCNT based coating on anodized titanium implant. The Human osteosarcoma cells (SaOS2) are cultured with CNT-Ti disks and reported high proliferation rate as compared to HA. Even more, the DNA content of cells on CNT-Ti is significantly higher than simple HA coating after 7 days of incubation. Furthermore, enhanced bone contact ratio with CNT-Ti against HA is reported in an animal model [40].
The 3D graphene foam provides a suitable environment to hMSC cells for better attachment, proliferation and osteoconductivity [13]. Kalbacova et al. investigated cellular activities simultaneously on graphene and silicon dioxide. The results show enhanced cell adhesion and proliferation in graphene then silicon dioxide and qualitative information is presented in Fig. 3e, f [28, 46]. Focal adhesions (FA) are large protein complexes helps in transmission of mechanical force and regulatory signals between the extracellular matrix (ECM) and an interacting cell. Kim et al. suggested that the unique structure of the GO film promotes the formation of HA [53]. The study of Misra and research group was revealed that, the higher surface area of graphene and its ripples and wrinkles like morphology is largely promoted the cell adhesion and proliferation [76]. Similarly, Murugan et al. developed a bioactive coating of PCL reinforced with HA and GO and the coated Ti alloy implant was examined in a rabbit model. Figure 4a shows the surface morphology (SEM) of the coating. Interestingly, the coating shows better antibacterial activity (Fig. 4b) and induces rapid bone formation as compared to pristine HA-PCL coated implant (Fig. 4c) [80].
A SEM images of HA–GO–PCL composite coated Ti alloy implant B Antibacterial activity of HA–GO–PCL coating shows zone of inhibition against S. aureus and E.coli C In-vivo histological analysis of bone formation without GO reinforcement in HA-PCL composite at day 14(c1) and day 28(c2) with GO of HA–PCL composite on day 14 and 28 presented in c3 and c4, respectively
In similar fashion Suo et al. developed a composite coating of chitosan with GO and HA. Electrophoretic deposition technique was employed to coat the composite on Ti implant. The in vitro study reveals the enhanced osteogenic activity and in vivo examination in rat model shows improved bone regeneration. Figure 5a, b shows the micro-CT and histopathological examination of rat tibia. The study was concluded that GO reinforced composite has extensively enhanced the bone formation (a4), thereby higher mineralization (b4) as compared to pristine HA cation (a1 and b1), GO–HA (a2 and b2) and chitosan (a3 and b3) [109].
A Micro-CT images of a transverse section of rats tibia A1 HA coated Ti implant, A2 GO–HA coated implant A3 chitosan coated implant A4 GO–chitosan–HA coated implant. B depicts the histopathological images of B1 HA coated Ti implant B2 GO–HA coated implant B3 chitosan coated implant B4 GO–chitosan–HA coated implant
Paula and co-workers extensively worked on poly (l-lactic acid) (PLLA) applications in medicine, since, its biocompatibility and biodegradability. Use of such materials eliminates the need for further surgery as they got degraded and eliminated from the body, but the limitation of PLLA involves lack of mechanical strength and osteoconductivity. Therefore, Paula et al. used HA and GO as filler material in PLLA and results suggest that PLLA/HA/GO nanocomposite has shown improved mechanical and osteoconduction properties with the addition of 1% (w/w) of GO [72]. Wan and Chen are investigated the mechanical properties and bioactivity of nanofibers from GO reinforced poly (ε-caprolactone) (PCL). Nanofibrous GO/PCL composite is prepared by electrospinning method. The study shows that the presence of 0.3% wt. GO is increased tensile strength, Young’s modulus and energy at break of PCL composite membrane by 95%, 66% and 416%, respectively. Bioactivity is also improved and porosity is maintained over 94% [117]. Graphene is a very excellent reinforcing component for composite formation. Kim et al. reinforced the graphene and GO into calcium carbonate crystals. GO/graphene-CaCO3 hybrid was exhibited enhanced apatite (HA) formation in simulated body fluid over the pristine graphene and GO. In vitro studies suggest that graphene/GO–HA composite enhance the proliferation of osteoblasts [53]. Recently, Li et al. reported an in vivo study for enhanced osteoconductivity for graphene coated implant in a rabbit model. The study reveals that rapid bone formation in GO coated implant as compared to pristine Ti implant. Moreover, the GO coated implant inhibits the fibrous scar formation. Figure 6a shows the histopathological analysis at different time intervals, Fig. 6b explains the mineral deposition and 6C presents the X-ray imaging for qualitative analysis for bone healing [60]. Table 1 shows some effective formulations for osteoconductive coatings for various biomedical applications.
A Hard tissue histological analysis by Van Gieson (VG) staining, group 1 represents the animal with GO coated Ti implant and group-2 for pristine Ti implant after 4, 12 and 24 weeks of implantation. The red color represents the newly formed bone, black indicates implant and blue is for fibrous scar formation. B Quantitative analysis of the mineral deposition in (pink bar) implant without the coating and (blue bar) with GO coating. C X-ray image of the surgical site reveals better healing in GO coated implant
Reduced graphene oxide (rGO) obtained by various methods of UV, thermal and chemical treatment of GO under the reducing condition with reducing agents like hydrazine [28]. Izumi and co-workers have done a comparative study of the bioactivity of GO and rGO. GO and rGO are coated onto collagen scaffold, implanted into rats back and characterized by SEM. DNA content and cell ingrowths of the implanted scaffold are measured after 10 days of surgery. The compressive strength of GO and rGO coated collagen scaffold is increased by 1.7 fold and 2.7 fold greater than the non-coated scaffold. Tissue growth rate is 39% in rGO and 20% in GO coated scaffolds. Result clearly indicates that rGO is more bioactive than GO [49]. rGO–HA composite is found to be enhanced mechanical property along with the improved proliferation and alkaline phosphatase (ALP) activity of human osteoblastic cells. HA-rGO nanocomposite is synthesized by liquid precipitation method [68]. In these way various carbon forms play a role as osteoconductive and hence recently researcher attracted more towards carbon nanomaterials for biomedical applications.
Another potent carbon contender for osteoconductive coatings is nano-diamond. The Recent in vivo study has been confirmed that, the nano-crystalline diamond (NCD) promotes intense bone growth, than micro-crystalline after 4 and 8 weeks. The nano-diamond also possess antioxidant, anti-inflammatory, anticancer and anti-allergic properties [124]. Though, a mechanism for osteoconductivity is not clear, but it is assumed that the osteoblast filopodial extension and cytoskeleton spreading are mechanically affected due to geometry and size of the surface features on NCD which lead to elevated cell proliferation and differentiation [47].
2.2 Antibacterial coatings
Post-surgery infection management is a vital aspect of arthroplasty. Sterilization of the implant system and the surgical site is minimum criteria to minimize the condition. Generally, infections lead to inflammation and more critically leads to a revision of surgery and some cases it leads to patient mortality [7]. Nanomaterials by their particular ordered structure possess the antimicrobial property. However, the general approach is loading of anti-microbial drugs on nanomaterials intended for infection resistance. Due to large surface area nanomaterial offers enhanced drug loading capacity [115]. “Antibacterial activity” of material defined as an ability to resist the colonization of bacteria [130]. SWCNT has been widely studied for its antimicrobial properties and Fig. 7c, d shows SEM images of E. coli morphology incubated with and without SWCNT. Interestingly, cells are highly intact and maintained their membrane structure without SWCNT, whereas the destructed membrane structure and loss of integrity was observed in SWCNT presence. Pristine CNT produces cell lysis and it is essential to make a surface modification with the suitable molecule to avoid the condition [50]. Similarly Liu et al. studied the antibacterial activity of SWCNT on the E.coli and Bacillus subtilis. The study reveals that after 120 min. complete bacterial cell lysis was taken place Fig. 7a, b Shows the AFM images of the bacterial cell [65].
AFM images of the A E. coli A1 untreated cell A2 SWCNT treatment after 10 min. A3 after 60 min. A4 after 120 min complete cell lysis B Bacillus subtilis B1 untreated cell B2 the cell after 10 min of treatment B2 cells after 60 min and B4 the cell after 120 min. C SEM images of the E. coli C1 incubated without SWCNT for 60 min. C2 Incubated with SWCNT
Hybrid CNTs with polymers and different metal ions (e.g. Copper, Silver) find to be more effective than pristine CNT to inhibit the microbial attachment and colony formation [66]. Interestingly, Kang et al. [51] reported that SWCNT is more toxic than MWCNT to the bacteria as the diameter of CNT plays a major role in anti-bacterial activity. S. Liu and co-workers studied the effect of pristine SWCNT dispersion in media. The homogeneously dispersed CNTs have shown greater antibacterial activity than in aggregate form and the effect is examined by colony formation count and viability assay method. Furthermore, the study reveals that the dispersed SWCNT shows higher antibacterial property towards gram-positive bacteria than gram-negative [66]. However, purification process of CNT is very tedious and the residual fragments are primarily responsible for cell toxicity. Therefore, research focuses more on functionalization or surface medication to reduce the toxicity of pristine CNTs. From the bingeing, silver (Ag) is well established for its anti-bacterial applications. The action comes from its strong interaction with protein sulfhydryl group, which founds in cell wall and cytoplasm thereby affects the metabolic activity [22]. M. Prodana and co-workers enhance the anti-microbial activity of functionalized MWCNT by using silver nanoparticle. Silver nanoparticles are attached to the surface of functionalized MWCNT (MWCNT-COOAg) by simple electrostatic adsorption. The result shows that CNT enhances percentage inhibition of E. Coli growth from 19 to 34% and it is better than a pristine silver nanoparticle. Further, MWCNT-COOAg enhances the percentage inhibition from 34 to 68% [95]. A similar study was reported by A** Niu and group, in which CNTs are wrapped with silver nanoparticles. The research shows that, CBN exhibit good antibacterial property against E-coli and inhibits bacterial growth higher than 0.5% [83]. Copper (Cu) is ranked second after Ag in terms of antibacterial property and it is stable and non-toxic in a physiological environment. Pandey et al. [90] reported that CNT with ceria and silver nanoparticle incorporated HA coating on the Ti-implant enhances the cell adhesion as well as significantly inhibit the growth of E-coli. The most common infection at the site of implant prosthesis is Staphylococcus epidermidis. Hirschfeld et al. [35] demonstrated MW-CNT impregnated with the rifampicin coated on the TiAl6V4 implant study reveals that the MW-CNT coated implant synergistically inhibit the biofilm formation as well as it helps in controlling the release pattern of rifampicin. Liu et al. [67] reported that Cu coated CNT has higher antibacterial activity than the Cu coated pyrolytic carbon and the suggested reason is CNT offers a higher surface area for Cu deposition than pyrolytic carbon. Wenbing Hu et al. synthesized antibacterial GO sheet by modified Hummer method with low cytotoxicity than CNT. GO demonstrates the excellent antibacterial activity and significantly suppressed the viability of E-coli up to 98.5%. Reduced graphene oxide is also showed antibacterial activity and decreases the metabolic activity of E-coli about 24% [36]. Ghosh et al. synthesized a GO-Para Amino Benzoic Acid (PABA) nanosheets by modified chemical exfoliation of graphite. The GO-PABA-Tetracycline hybrid nanosheet shows good biocompatibility, high drug loading efficiency and good antibacterial activity [26]. Pandey et al. rise the antibacterial effect of graphene nanosheet by loading of gentamicin sulfate. The study has revealed that graphene is a good matrix to achieve controlled release and diffusion dominated release mechanism [91].
Hydrogenated diamond-like carbon (DLC) contains the mixture of Graphite and Diamond. It attracts the scientist because of a wide range of structural variation. The study is carried out on pure DLC, metal doped DLC and carbide doped DLC by various scientists. The studies have been revealed that the increased copper content in DLC enhances the antibacterial activity against E. coli and concludes that DLC films are highly suitable for protective material in biomedical field [10].
2.3 Anticorrosive coatings
Corrosion is a crucial problem associated with metallic implants and it is characterized by oxidization of metal surfaces by chemical or electrochemical interactions. CBN has many remarkable properties as being chemically inert and they can be used as anticorrosive coatings in biomedical applications. The suggested mechanism is that, CNT act as physical barrier for chemical interaction by filling inside the crevices, gaps and holes on the surface of Nickel (Ni) [2.4 Advanced material coating: carbon quantum dots The three-dimensionally confined semiconductor pseudo atoms called quantum dots emerge as cutting-edge materials for next-generation research and applications. The quantum dot is nanocrystal with less than 10 nm particle size and able to behave like an individual atom. The carbon quantum dot possesses size dependent unique optical properties and offers low toxicity for medical applications. Carbon quantum dots are extensively used in biosensors, bioimaging, electronics, drug delivery and Optronics, due to its versatile fluorescence and photo luminousness properties [59, 63]. Carbon quantum dots not yet exploring much for orthopedic applications. Recently, Jichuan Qiu et al. reported the effect of the graphene quantum dot on the bone marrow-derived mesenchymal stem cells. The study finds that the extensive cell uptake of quantum dots along with homogenous cytoplasmic distribution. Besides, it up-regulates the phenotypic gens like Runx2, osteopontin (OPN), and osteocalcin (OCN) responsible for osteogenesis. Figure 8a shows the immunofluorescent images of MSCs with the enhanced activity of the OPN and OCN with proportionate to the concentration of GO quantum dots. Further the study presents quantitatively the expression of early phenotypic marker ALP, the gene RUNX2 are responsible for osteogenic differentiation. Also the study observes the expression of bone extracellular matrix proteins OPN and OPL with respect concentration and the incubation period (Fig. 8b) The study reveals that increased expression of the factors with increasing incubation time and concentration, except in OPN, where after 10 days of incubation the expression significantly decreases [97]. A Immunofluorescent images of the bone extracellular protein marker OPN and the OCN expressed in MSCs incubated with the different concentration of the graphene quantum dots, Blue color depicts the nuclei stained with DAPI and green color represents the graphene quantum dots. B Quantitative estimation of the expression of the different biomarker against concentration and time
3 Adverse biological effect of CBN coated implants
Although CBN coated material is reported for antibacterial, anticorrosive and osteoconductive applications. Nonetheless, the CBN coatings are prone to adverse biological events, when the coating is worn by mechanical failure and their toxicity. The structural aspect of CBN is highly influenced the biological activity. The nanodimond is well reported for its antibacterial activity, but it is also toxic for normal cells, when the ratio of SP3 to SP2 hybridization decreases [8, 71]. The presence of hydrogen atom on the film results in weaken the chemical inertness and thus decline the bactericidal activity of implant. Similarly, Taeger et al. the clinical performance of DLC coated implant. Results suggest that the coated implant shows four times more aseptic loosing compared to ceramic coatings. The retrieved implants were examined for failure and observed the delamination of coating and pitting as shown in Fig. 9 [110]. The study suggests that the delamination is occurred due to poor adhesion and interaction of the implant (Ti and Co) surface with DLC [1].
As CNT is the most promising material for coatings, but the toxicity limits its applications in biomedical applications. The toxicological profile of CNT was well reviewed by Constantine et al. The CNT induces the oxidative stress and results in DNA damage in organs like liver, lungs and spleen [21]. Nevertheless, very little information is available on the effect of wear debris of CNT. Lahiri et al. carried out wear debris activity of HA-CNT composite coating. The study divulges that debris of HA-CNT composite is not evident for any adverse effect on osteoblast, moreover the wear particles show normal activation of macrophages [57]. While, the graphene demonstrates diverse toxicological profiles and it was well emphasised by Yang et al. The in vitro studies suggest the pristine graphene exhibit poor cell penetration and good biocompatibility. Although some reports advocate that graphene shows a negative effect on cell via inducing oxidative stress, ROS generation and alteration of expression of key genes and proteins [122]. However, no productive report was found to understand the effect of wear debris and leaching of graphene coated implants.
4 Conclusion
The scientific community is reporting extensive work on CBN coatings for orthopedic applications. The current review introduced deep insight of various applications of CBNs in orthopedic coatings, which can also use in other biomedical coatings. The document presents the special glimpse on the novel carbon material like Carbon quantum dots for hard tissue applications. CBN’s offer versatile property like biocompatibility, unique surface, electrical, chemical, mechanical and thermal properties of the other advanced materials. Hence, CBNs can expect to see a continuous rise in utilization in the biomedical field. Mostly, CBNs can be used potentially for medical coatings, drug delivery, medical device fabrication and diagnosis. Graphene and its derivatives still need to explore for their exceptional properties, functionalization and long-term effects.
References
Affatato S, Frigo M, Toni A (2000) An in vitro investigation of diamond-like carbon as a femoral head coating. J Biomed Mater Res Off J Soc Biomater Jpn Soc Biomater Aust Soc Biomater Korean Soc Biomater 53:221–226
Ajayan PM (1999) Nanotubes from carbon. Chem Rev 99:1787–1800
Allen MJ, Tung VC, Kaner RB (2009) Honeycomb carbon: a review of graphene. Chem Rev 110:132–145
Bishop L et al (2017) An in-vivo toxicity assessment of occupational components of the carbon nanotube life cycle to provide context to potential health effects. ACS Nano 11:8849–8863
Bosco R, Van Den Beucken J, Leeuwenburgh S, Jansen J (2012) Surface engineering for bone implants: a trend from passive to active surfaces. Coatings 2:95–119
Branzoi IV, Iordoc M, Branzoi F, Vasilescu-Mirea R, Sbarcea G (2010) Influence of diamond-like carbon coating on the corrosion resistance of the NITINOL shape memory alloy. Surf Interface Anal 42:502–509
Burke JP (2003) Infection control—a problem for patient safety. N Engl J Med 348:651
Capote G, Bonetti L, Santos L, Trava-Airoldi V, Corat E (2008) Adherent amorphous hydrogenated carbon films on metals deposited by plasma enhanced chemical vapor deposition. Thin Solid Films 516:4011–4017
Cha C, Shin SR, Annabi N, Dokmeci MR, Khademhosseini A (2013) Carbon-based nanomaterials: multifunctional materials for biomedical engineering. ACS Nano 7:2891–2897
Chan Y-H, Huang C-F, Ou K-L, Peng P-W (2011) Mechanical properties and antibacterial activity of copper doped diamond-like carbon films. Surf Coat Technol 206:1037–1040
Chang KC et al (2014) Advanced anticorrosive coatings prepared from electroactive polyimide/graphene nanocomposites with synergistic effects of redox catalytic capability and gas barrier properties. Express Polym Lett 8:243–255
Chen XH, Chen CS, **ao HN, Cheng FQ, Zhang G, Yi GJ (2005) Corrosion behavior of carbon nanotubes—Ni composite coating. Surf Coat Technol 191:351–356
Crowder SW, Prasai D, Rath R, Balikov DA, Bae H, Bolotin KI, Sung H-J (2013) Three-dimensional graphene foams promote osteogenic differentiation of human mesenchymal stem cells. Nanoscale 5:4171–4176
Cunniffe GM, O’Brien FJ (2011) Collagen scaffolds for orthopedic regenerative medicine. JOM 63:66
Damaraju SM et al (2017) Three-dimensional piezoelectric fibrous scaffolds selectively promote mesenchymal stem cell differentiation. Biomaterials 149:51–62
Dreyer DR, Park S, Bielawski CW, Ruoff RS (2010) The chemistry of graphene oxide. Chem Soc Rev 39:228–240
Elias CN, Lima JHC, Valiev R, Meyers MA (2008) Biomedical applications of titanium and its alloys. JOM 60:46–49
Endo M, Hayashi T, Kim YA, Muramatsu H (2006) Development and application of carbon nanotubes. Jpn J Appl Phys 45:4883
Engelhart S, Segal RJ (2017) Allergic reaction to vanadium causes a diffuse eczematous eruption and titanium alloy orthopedic implant failure. Cutis 99:245
Fan H, Wang L, Zhao K, Li N, Shi Z, Ge Z, ** Z (2010) Fabrication, mechanical properties, and biocompatibility of graphene-reinforced chitosan composites. Biomacromolecules 11:2345–2351
Firme CP III, Bandaru PR (2010) Toxicity issues in the application of carbon nanotubes to biological systems. Nanomed Nanotechnol Biol Med 6:245–256
Fordham WR et al (2014) Silver as a bactericidal coating for biomedical implants. Surf Coat Technol 253:52–57
Ganguly D, Shahbazian-Yassar R, Shokuhfar T (2014) Recent advances in nanotubes for orthopedic implants. J Nanotechnol Smart Mater 1:201
Gay T, Kaufman J, McGuigan M (2005) Stronger than steel: carbon nanotubes. Boston University, Boston
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6:183–191
Ghosh D, Chandra S, Chakraborty A, Ghosh SK, Pramanik P (2010) A novel graphene oxide-para amino benzoic acid nanosheet as effective drug delivery system to treat drug resistant bacteria. Int J Pharm Sci Drug Res 2:127–133
Gollwitzer H, Ibrahim K, Meyer H, Mittelmeier W, Busch R, Stemberger A (2003) Antibacterial poly (d, l-lactic acid) coating of medical implants using a biodegradable drug delivery technology. J Antimicrob Chemother 51:585–591
Gu M, Liu Y, Chen T, Du F, Zhao X, **ong C, Zhou Y (2014) Is graphene a promising nano-material for promoting surface modification of implants or scaffold materials in bone tissue engineering? Tissue Eng Part B Rev 20:477–491
Habibovic P, Barrere F, Blitterswijk CA, Groot K, Layrolle P (2002) Biomimetic hydroxyapatite coating on metal implants. J Am Ceram Soc 85:517–522
Habisreutinger SN, Nicholas RJ, Snaith HJ (2017) Carbon nanotubes in perovskite solar cells. Adv Energy Mater 7:1601839
Hallab NJ, Jacobs JJ (2017) Chemokines associated with pathologic responses to orthopedic implant debris. Front Endocrinol 8:5
Hammer P, dos Santos FC, Cerrutti BM, Pulcinelli SH, Santilli CV (2012) Corrosion resistant coatings based on organic–inorganic hybrids reinforced by carbon nanotubes. In: Razavi RS (ed) Recent researches in corrosion evaluation and protection. InTech, Rijeka
Hang TTX, Truc TA, Nam TH, Oanh VK, Jorcin J-B, Pébère N (2007) Corrosion protection of carbon steel by an epoxy resin containing organically modified clay. Surf Coat Technol 201:7408–7415
Hang TTX, Truc TA, Olivier M-G, Vandermiers C, Guérit N, Pébère N (2010) Corrosion protection mechanisms of carbon steel by an epoxy resin containing indole-3 butyric acid modified clay. Prog Org Coat 69:410–416
Hirschfeld J et al (2017) Long-term release of antibiotics by carbon nanotube-coated titanium alloy surfaces diminish biofilm formation by Staphylococcus epidermidis. Nanomed Nanotechnol Biol Med 13:1587–1593
Hu W et al (2010) Graphene-based antibacterial paper. ACS Nano 4:4317–4323
Huang X, Qi X, Boey F, Zhang H (2012) Graphene-based composites. Chem Soc Rev 41:666–686
Ibara A et al (2013) Osteoconductivity and biodegradability of collagen scaffold coated with nano-β-TCP and fibroblast growth factor 2. J Nanomater 2013:46
Iijima S (2002) Carbon nanotubes: past, present, and future. Physica B 323:1–5
Inoue S (2014) Multiwalled carbon nanotubes coating accelerates osteoconductivity of anodized titanium [an abstract of dissertation and a summary of dissertation review]
Jacob J, More N, Kalia K, Kapusetti G (2018) Piezoelectric smart biomaterials for bone and cartilage tissue engineering. Inflamm Regen 38:2
Jaffe WL, Scott DF (1999) Total hip arthroplasty with hydroxyapatite-coated prostheses. In: Imura S, Wada M, Omori H (eds) Joint arthroplasty. Springer, Berlin, pp 159–187
Ji X et al (2012) Sol–gel-derived hydroxyapatite-carbon nanotube/titania coatings on titanium substrates. Int J Mol Sci 13:5242–5253
Jiang B, Tian C, Song G, Chang W, Wang G, Wu Q, Fu H (2013) A novel Ag/graphene composite: facile fabrication and enhanced antibacterial properties. J Mater Sci 48:1980–1985
Kaiser J-P, Krug HF, Wick P (2009) Nanomaterial cell interactions: how do carbon nanotubes affect cell physiology? Future Medicine 4:57–63
Kalbacova M, Broz A, Kong J, Kalbac M (2010) Graphene substrates promote adherence of human osteoblasts and mesenchymal stromal cells. Carbon 48:4323–4329
Kalbacova M, Rezek B, Baresova V, Wolf-Brandstetter C, Kromka A (2009) Nanoscale topography of nanocrystalline diamonds promotes differentiation of osteoblasts. Acta Biomater 5:3076–3085
Kamath MS, Ahmed SSSJ, Dhanasekaran M, Santosh SW (2014) Polycaprolactone scaffold engineered for sustained release of resveratrol: therapeutic enhancement in bone tissue engineering. Int J Nanomed 9:183
Kanayama I et al (2014) Comparative study of bioactivity of collagen scaffolds coated with graphene oxide and reduced graphene oxide. Int J Nanomed 9:3363
Kang S, Pinault M, Pfefferle LD, Elimelech M (2007) Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir 23:8670–8673
Kang SM, Kong B, Oh E, Choi JS, Choi IS (2010) Osteoconductive conjugation of bone morphogenetic protein-2 onto titanium/titanium oxide surfaces coated with non-biofouling poly (poly (ethylene glycol) methacrylate). Colloids Surf B 75:385–389
Kawakami T, Antoh M, Hasegawa H, Yamagishi T, Ito M, Eda S (1992) Experimental study on osteoconductive properties of a chitosan-bonded hydroxyapatite self-hardening paste. Biomaterials 13:759–763
Kim S, Ku SH, Lim SY, Kim JH, Park CB (2011) Graphene–biomineral hybrid materials. Adv Mater 23:2009–2014
Kroto HW, Heath JR, O’Brien SC, Curl RF, Smalley RE (1985) C60: buckminsterfullerene. Nature 318:162–163
Kumar S, Pawar SP, Chatterjee K, Bose S (2014) Antimicrobial and conducting polymer substrate derived from hybrid structures of silver nanoparticles and multiwall carbon nanotubes. Mater Technol 29:B59–B63
Kuroda K, Moriyama M, Ichino R, Okido M, Seki A (2009) Formation and osteoconductivity of hydroxyapatite/collagen composite films using a thermal substrate method in aqueous solutions. Mater Trans 50:1190–1195
Lahiri D, Benaduce AP, Rouzaud F, Solomon J, Keshri AK, Kos L, Agarwal A (2011) Wear behavior and in vitro cytotoxicity of wear debris generated from hydroxyapatite–carbon nanotube composite coating. J Biomed Mater Res Part A 96:1–12
Lam C-W, James JT, McCluskey R, Arepalli S, Hunter RL (2006) A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit Rev Toxicol 36:189–217
Lee EJ et al (2014) Graphene oxide-decorated PLGA/collagen hybrid fiber sheets for application to tissue engineering scaffolds. Biomater Res 18:18–24
Li K et al (2018) Evaluation of the osteogenesis and osseointegration of titanium alloys coated with graphene: an in vivo study. Sci Rep 8:1843
Li X, Liu X, Huang J, Fan Y, Cui F-Z (2011) Biomedical investigation of CNT based coatings. Surf Coat Technol 206:759–766
Li Z, Tang M, Dai J, Wang T, Bai R (2016) Effect of multiwalled carbon nanotube-grafted polymer brushes on the mechanical and swelling properties of polyacrylamide composite hydrogels. Polymer 85:67–76
Lim SY, Shen W, Gao Z (2015) Carbon quantum dots and their applications. Chem Soc Rev 44:362–381
Liu L-S, Thompson AY, Heidaran MA, Poser JW, Spiro RC (1999) An osteoconductive collagen/hyaluronate matrix for bone regeneration. Biomaterials 20:1097–1108
Liu S, Ng AK, Xu R, Wei J, Tan CM, Yang Y, Chen Y (2010) Antibacterial action of dispersed single-walled carbon nanotubes on Escherichia coli and Bacillus subtilis investigated by atomic force microscopy. Nanoscale 2:2744–2750
Liu S et al (2009) Sharper and faster “nano darts” kill more bacteria: a study of antibacterial activity of individually dispersed pristine single-walled carbon nanotube. ACS Nano 3:3891–3902
Liu T, Tang H, Zhao J, Li D, Li R, Sun X (2007) A study on the bactericidal properties of Cu-coated carbon nanotubes. Front Mater Sci Chin 1:147–150
Liu Y, Huang J, Li H (2013) Synthesis of hydroxyapatite–reduced graphite oxide nanocomposites for biomedical applications: oriented nucleation and epitaxial growth of hydroxyapatite. J Mater Chem B 1:1826–1834
Low KL, Tan SH, Zein SHS, Roether JA, Mouriño V, Boccaccini AR (2010) Calcium phosphate-based composites as injectable bone substitute materials. J Biomed Mater Res Part B Appl Biomater 94:273–286
Macedo NLd, Matuda FdS, Macedo LGSd, Gonzales MB, Ouchi SM, Carvalho YR (2004) Bone defect regeneration with bioactive glass implantation in rats. J Appl Oral Sci 12:137–143
Marciano F, Bonetti L, Santos L, Da-Silva N, Corat E, Trava-Airoldi V (2009) Antibacterial activity of DLC and Ag-DLC films produced by PECVD technique. Diam Relat Mater 18:1010–1014
Marques PAAP, Gonçalves G, Singh MK, Grácio J (2012) Graphene oxide and hydroxyapatite as fillers of polylactic acid nanocomposites: preparation and characterization. J Nanosci Nanotechnol 12:6686–6692
Melin Svanborg L, Meirelles L, Franke Stenport V, Kjellin P, Currie F, Andersson M, Wennerberg A (2014) Evaluation of bone healing on sandblasted and acid etched implants coated with nanocrystalline hydroxyapatite: an in vivo study in rabbit femur. Int J Dent 2014:1–7
Miculescu F, Maidaniuc A, Voicu SI, Thakur VK, Stan GE, Ciocan L (2017) Progress in hydroxyapatite–starch based sustainable biomaterials for biomedical bone substitution applications. ACS Sustain Chem Eng 5:8491–8512
Minagawa T, Tabata Y, Oyama A, Furukawa H, Yamao T, Yamamoto Y (2014) Controlled release of granulocyte colony-stimulating factor enhances osteoconductive and biodegradable properties of Beta-tricalcium phosphate in a rat calvarial defect model. Int J Biomater 2014:134521
Misra RDK, Chaudhari PM (2013) Cellular interactions and stimulated biological functions mediated by nanostructured carbon for tissue reconstruction and tracheal tubes and sutures. J Biomed Mater Res Part A 101:528–536
More N, Kapusetti G (2017) Piezoelectric material—a promising approach for bone and cartilage regeneration. Med Hypotheses 108:10–16
Morrison C, Macnair R, MacDonald C, Wykman A, Goldie I, Grant MH (1995) In vitro biocompatibility testing of polymers for orthopaedic implants using cultured fibroblasts and osteoblasts. Biomaterials 16:987–992
Muhulet A, Miculescu F, Voicu SI, Schütt F, Thakur VK, Mishra YK (2018) Fundamentals and scopes of doped carbon nanotubes towards energy and biosensing applications. Mater Today Energy 9:154–186
Murugan N, Murugan C, Sundramoorthy AK (2018) In vitro and in vivo characterization of mineralized hydroxyapatite/polycaprolactone–graphene oxide based bioactive multifunctional coating on Ti alloy for bone implant applications. Arab J Chem 11:959–969
Nardecchia S, Serrano MC, Gutiérrez MC, Portolés MT, Ferrer ML, del Monte F (2012) Osteoconductive performance of carbon nanotube scaffolds homogeneously mineralized by flow-through electrodeposition. Adv Funct Mater 22:4411–4420
Nezhad H, Thakur V (2018) Effect of morphological changes due to increasing carbon nanoparticles content on the quasi-static mechanical response of epoxy resin. Polymers 10:1106
Niu A, Han Y, Wu J, Yu N, Xu Q (2010) Synthesis of one-dimensional carbon nanomaterials wrapped by silver nanoparticles and their antibacterial behavior. J Phys Chem C 114:12728–12735
Ordikhani F, Tamjid E, Simchi A (2014) Characterization and antibacterial performance of electrodeposited chitosan–vancomycin composite coatings for prevention of implant-associated infections. Mater Sci Eng C 41:240–248
Ou J, Wang J, Liu S, Mu B, Ren J, Wang H, Yang S (2010) Tribology study of reduced graphene oxide sheets on silicon substrate synthesized via covalent assembly. Langmuir 26:15830–15836
Overney G, Zhong W, Tomanek D (1993) Structural rigidity and low frequency vibrational modes of long carbon tubules. Zeitschrift für Physik D Atoms Mol Clust 27:93–96
Panagiotopoulou VC et al (2017) Assessment of corrosion in retrieved spine implants. J Biomed Mater Res B Appl Biomater 106:632–638
Pandele A, Comanici F, Carp C, Miculescu F, Voicu S, Thakur V, Serban B (2017) Synthesis and characterization of cellulose acetate-hydroxyapatite micro and nano composites membranes for water purification and biomedical applications. Vacuum 146:599–605
Pandele A et al (2018) Cellulose acetate membranes functionalized with resveratrol by covalent immobilization for improved osseointegration. Appl Surf Sci 438:2–13
Pandey A et al (2018) Enhanced tribological and bacterial resistance of carbon nanotube with ceria-and silver-incorporated hydroxyapatite biocoating. Nanomaterials 8:363
Pandey H, Parashar V, Parashar R, Prakash R, Ramteke PW, Pandey AC (2011) Controlled drug release characteristics and enhanced antibacterial effect of graphene nanosheets containing gentamicin sulfate. Nanoscale 3:4104–4108
Peitl Filho O, Latorre GP, Hench L (1996) Effect of crystallization on apatite-layer formation of bioactive glass 45%. J Biomed Mater Res 30:509–514
Peivandi MT (2013) Exploring the reasons for orthopedic implant failure in traumatic fractures of the lower limb. Arch Iran Med 16:478
Pramatarova L et al (2007) Artificial bones through nanodiamonds. J Optoelectron Adv Mater 9:236
Prodana M, Ionita D, Ungureanu C, Bo** D, Demetrescu I (2011) Enhancing antibacterial effect of multiwalled carbon nanotubes using silver nanoparticles. Microscopy 6:549–556
Pullisaar H, Reseland JE, Haugen HJ, Brinchmann JE, Østrup E (2014) Simvastatin coating of TiO2 scaffold induces osteogenic differentiation of human adipose tissue-derived mesenchymal stem cells. Biochem Biophys Res Commun 447:139–144
Qiu J et al (2016) Effects of graphene quantum dots on the self-renewal and differentiation of mesenchymal stem cells. Adv Healthc Mater 5:702–710
Raman RKS et al (2012) Protecting copper from electrochemical degradation by graphene coating. Carbon 50:4040–4045
Rana M, Asim S, Hao B, Yang S, Ma PC (2017) Carbon nanotubes on highly interconnected carbonized cotton for flexible and light-weight energy storage. Adv Sustain Syst 1:10700022
Rao CNR, Seshadri R, Govindaraj A, Sen R (1995) Fullerenes, nanotubes, onions and related carbon structures. Mater Sci Eng R Rep 15:209–262
Schmidmaier G, Lucke M, Wildemann B, Haas NP, Raschke M (2006) Prophylaxis and treatment of implant-related infections by antibiotic-coated implants: a review. Injury 37:S105–S112
Schrand AM, Dai L, Schlager JJ, Hussain SM, Osawa E (2007) Differential biocompatibility of carbon nanotubes and nanodiamonds. Diam Relat Mater 16:2118–2123
Sedaghat S, Mehraji M (2013) Novel hybrid of silver nono particles deposited on to the different carbon substrates and evaluation of antibacterial effects. J Appl Sci Eng Technol 3:219–222
Shamos MH, Lavine LS, Shamos MI (1963) Piezoelectric effect in bone. Nature 197:81
Sharma R et al (2017) Osteoconductive amine-functionalized graphene–poly (methyl methacrylate) bone cement composite with controlled exothermic polymerization. Bioconjug Chem 28:2254–2265
Shepperd JAN, Apthorp H (2005) A contemporary snapshot of the use of hydroxyapatite coating in orthopaedic surgery. Bone Joint J 87:1046–1049
Shi X et al (2007) Fabrication of porous ultra-short single-walled carbon nanotube nanocomposite scaffolds for bone tissue engineering. Biomaterials 28:4078–4090
Show Y, Nakashima T, Fukami Y (2013) Anticorrosion coating of carbon nanotube/polytetrafluoroethylene composite film on the stainless steel bipolar plate for proton exchange membrane fuel cells. J Nanomater 2013:2
Suo L et al. (2018) The enhancement of osseointegration using a graphene oxide/chitosan/hydroxyapatite composite coating on titanium fabricated by electrophoretic deposition. J Biomed Mater Res Part B Appl Biomater 106:1–11
Taeger G, Podleska L, Schmidt B, Ziegler M, Nast-Kolb D (2003) Comparison of diamond-like-carbon and alumina-oxide articulating with polyethylene in total hip arthroplasty. Materialwissenschaft und Werkstofftechnik: Entwicklung Fertigung, Prüfung, Eigenschaften und Anwendungen technischer Werkstoffe 34:1094–1100
Tai IC, Fu Y-C, Wang C-K, Chang J-K, Ho M-L (2013) Local delivery of controlled-release simvastatin/PLGA/HAp microspheres enhances bone repair. Int J Nanomed 8:3895
Thomson LA, Law FC, Rushton N, Franks J (1991) Biocompatibility of diamond-like carbon coating. Biomaterials 12:37–40
Tong Y, Bohm S, Song M (2013) Graphene based materials and their composites as coatings. Austin J Nanomed Nanotechnol 1:1003
Trampuz A, Zimmerli W (2006) Diagnosis and treatment of infections associated with fracture-fixation devices. Injury 37:S59–S66
Vermisoglou EC, Pilatos G, Romanos GE, Devlin E, Kanellopoulos NK, Karanikolos GN (2011) Magnetic carbon nanotubes with particle-free surfaces and high drug loading capacity. Nanotechnology 22:355602
Voicu SI et al (2016) Sericin covalent immobilization onto cellulose acetate membrane for biomedical applications. ACS Sustain Chem Eng 4:1765–1774
Wan C, Chen B (2011) Poly (ε-caprolactone)/graphene oxide biocomposites: mechanical properties and bioactivity. Biomed Mater 6:055010
Wang L et al (2010) Direct fibrogenic effects of dispersed single-walled carbon nanotubes on human lung fibroblasts. J Toxicol Environ Health Part A 73:410–422
Wildemann B et al (2005) Short term in vivo biocompatibility testing of biodegradable poly (D, L-lactide)—growth factor coating for orthopaedic implants. Biomaterials 26:4035–4040
Woodard JR et al (2007) The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 28:45–54
Wu C, Zhou Y, Xu M, Han P, Chen L, Chang J, **ao Y (2013) Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials 34:422–433
Yang K, Li Y, Tan X, Peng R, Liu Z (2013) Behavior and toxicity of graphene and its functionalized derivatives in biological systems. Small 9:1492–1503
Yang L, Chinthapenta V, Li Q, Stout D, Liang A, Sheldon BW, Webster TJ (2011) Understanding osteoblast responses to stiff nanotopographies through experiments and computational simulations. J Biomed Mater Res Part A 97:375–382
Yang L, Zhang L, Webster TJ (2011) Carbon nanostructures for orthopedic medical applications. Nanomedicine 6:1231–1244
Yang Z, Xu H, Li M-K, Shi Y-L, Huang Y, Li H-L (2004) Preparation and properties of Ni/P/single-walled carbon nanotubes composite coatings by means of electroless plating. Thin Solid Films 466:86–91
Zawadzak E et al (2008) Polyurethane foams electrophoretically coated with carbon nanotubes for tissue engineering scaffolds. Biomed Mater 4:015008
Zhang B, Kwok CT, Cheng FT, Man HC (2011) Fabrication of nano-structured HA/CNT coatings on Ti6Al4V by electrophoretic deposition for biomedical applications. J Nanosci Nanotechnol 11:10740–10745
Zhang BGX, Myers DE, Wallace GG, Brandt M, Choong PFM (2014) Bioactive coatings for orthopaedic implants—recent trends in development of implant coatings. Int J Mol Sci 15:11878–11921
Zhang Z et al (2015) Mechanical and anticorrosive properties of graphene/epoxy resin composites coating prepared by in situ method. Int J Mol Sci 16:2239–2251
Zhou H, Xu L, Ogino A, Nagatsu M (2008) Investigation into the antibacterial property of carbon films. Diam Relat Mater 17:1416–1419
Zogbi MM, Saito E, Zanin H, Marciano FR, Lobo AO (2014) Hydrothermal–electrochemical synthesis of nano-hydroxyapatite crystals on superhydrophilic vertically aligned carbon nanotubes. Mater Lett 132:70–74
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Bhong, S.Y., More, N., Choppadandi, M. et al. Review on carbon nanomaterials as typical candidates for orthopaedic coatings. SN Appl. Sci. 1, 76 (2019). https://doi.org/10.1007/s42452-018-0082-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-018-0082-z