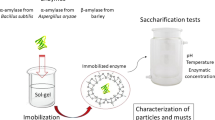
Abstract
A β-amylase extracted from Ecballium elaterium was purified on Q-Sepharose anion exchange chromatography and identified with molecular mass. A successful immobilization by adsorption onto a matrix of titanium dioxide-cellulose acetate butyrate and copolymer of acrylonitrile-acrylamide yielded 32.17%. Thermal stability approved that Ecballium β-amylase retained 89.21% of its initial activity (IA) after 180 min at 70 °C. A promoted catalytic activity was revealed at 70 °C and pH 7.5 with the immobilization procedure. The heterogeneous system based on the process of the substrate conversion degree (α) was studied kinetically via the modified Prout–Tompkins topochemical equation. α increased in accordance with the temperature increase up to 100 min of the reaction time. At 30 °C only 7.6% of optimum conversion degree was lost after 3 h. Power factor (χ) was equal to 1.16. An accomplished application of Ecballium β-amylase proved that it could be a good candidate to produce maltose syrup due to the highest maltose content of 52.9% after 8 h hydrolysis. It will be also very useful during manufacturing processes to minimize starch saccharification time even enzymatic liquefaction costs.





Similar content being viewed by others
Availability of Data and Material
All data generated or analysed during this study are included in this article.
References
Karim A, Bibi Z, Nawaz MA, Aman A, Qader SAU (2021) Thermodynamics, kinetics and optimization of catalytic behavior of polyacrylamide-entrapped carboxymethyl cellulase (CMCase) for prospective industrial use. Bioprocess Biosyst Eng 44:2417–2427
Anigboro AA, Ajoh AI, Avwioroko OJ, Ehwarieme DA, Tonukari NJ (2023) Solid-state fermentation of Cassava (Manihot esculenta) peels using Rhizopus oligosporus: application of the fermented peels in yeast production and characterization of α-amylase enzyme produced in the process. Chemistry Africa 6:1669–1678. https://doi.org/10.1007/s42250-022-00582-3
Cheng W, Sun Y, **a X, Yang L, Fan M, Li Y, Wang L, Qian H (2022) Effects of β-amylase treatment conditions on the gelatinization and retrogradation characteristics of wheat starch. Food Hydrocolloids 124:107286
DiCosimo R, McAuliffe J, Poulose AJ, Bohlmann G (2013) Industrial use of immobilized enzymes. Chem Soc Rev 42:6437–6474
Dos Santos JCS, Bonazza HL, de Matos LJBL, Carneiro EA, Barbosa O, Fernandez-Lafuente R, Gonçalves LRB, de Sant’Ana HB, Santiago-Aguiar RS (2017) Immobilization of CALB on activated chitosan: Application to enzymatic synthesis in supercritical and near-critical carbon dioxide. Biotechnol Rep 14:16–26
Kahraman MV, Bayramoglu G, Kayaman-Apohan N, Gungor A (2007) α-Amylase immobilization on functionalized glass beads by covalent attachment. Food Chem 104:1385–1392
Reshmi R, Sanjay G, Sugunan S (2006) Enhanced activity and stability of α- amylase immobilized on alumina. Cat Comm 7:460–465
Cinar K, Gunes G, Gulec HA (2020) Enzymatic synthesis of prebiotic carbohydrates from lactose: Kinetics and optimization of transgalactosylation activity of β-galactosidase from Aspergillus oryzae. J Food Process Eng 43:e13435. https://doi.org/10.1111/jfpe.13435
Azelee NIW, Nordin N, Illias M, Manas NHA, Ghazali MNFM (2020) Enzyme kinetics study for heterogeneous system of pretreated kenaf hydrolysis. Pertanika J Sci Technol 28:197–216
Valcheva E, Veleva S, Valchev I, Dimitrov I (2000) Kinetic model of xylanase action on kraft pulp. React Kin Cat Lett 71:231–238. https://doi.org/10.1023/A:1010310706612
Lahmar I, Radeva G, Marinkova D, Velitchkova M, Belghith H, Ben abdallahYotovaBelghith FLK (2018) Immobilization and topochemical mechanism of a new β-amylase extracted from Pergularia tomentosa. Proc Biochem 64:143–151
Lahmar I, Radeva G, Marinkova D, Velitchkova M, Belghith H, Ben Abdallah F, Yotova L, Belghith K (2018) Valorization of a plant β-amylase: immobilization and dataset on the kinetic process. Data Brief 16:386–391. https://doi.org/10.1016/j.dib.2017.11.071
Lahmar I, Radeva G, Marinkova D, Velitchkova M, Lyubov Y (2023) Exponential equation describing kinetic process of immobilized β-Amylase extracted from Glycine max seeds. Chemistry Africa. https://doi.org/10.1007/s42250-023-00721-4
Li W, Bai Y, Mousaa SAS, Zhang Q, Shen Q (2011) Effect of high hydrostatic pressure on physicochemical and structural properties of rice starch. Food BiopTech 5:2233–2241. https://doi.org/10.1007/s11947-011-0542-6
Lahmar I, Ben Nasri-Ayachi M, Belghith K (2022) Laticifer identification, rubber characterization, phenolic content, and antioxidant activity of Pergularia tomentosa latex extract. BioMed Res Int 2022:1–8. https://doi.org/10.1155/2022/7158905
Chan KT, Meng FY, Li Q, Ho CY, Lam TS, To Y, Lee WH, Li M, Chu KH, Toh M (2010) Cucurbitacin B induces apoptosis and S phase cell cycle arrest in BEL-7402 human hepatocellular carcinoma cells and is effective via oral administration. Cancer Lett 249:118–124
Sakat S, Jukevar AR, Gambhire MN (2010) In vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata Linn. Int J Pharm Pharm Sci 2:146–155
Marinkova D, Yotova L, Ringeard JM, Griesmar P (2014) Influence of Ni2+ on urease activity produced by biofilms of Arthocbacter oxydans 1388. Biotech Biotechn Equip 28:226–270
Marinkova D, Danalev D, Serfaty S, Yotova L, Caplain E, Griemar P (2012) Characterization of new titanium oxide polymer hybrid membranes for biofilm formation. Phos Sulph Sil Rel Elem 187:926–936
Lahmar I, El Abed H, Khemakhem B, Belghith H, Ben Abdallah F, Belghith K (2017) Optimization, purification, and starch stain wash application of two new α-amylases extracted from leaves and stems of Pergularia tomentosa. BioMed Res Int 2017:1–9. https://doi.org/10.1155/2017/6712742
Lahmar I, Yotova L (2016) Investigation of different enzyme activities from Pergularia tomentosa L. and Ecballium elaterium L. J Chem Techn Metal 51:263–270
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Yotova L, Ivanov I (2000) Kinetic studies and analytical application of cholesterol oxidase and peroxidase immobilized to synthetic polymer. Appl Biochem Biotech 87:141–151
Tolbert NE (1973) Activation of polyphenol oxidase of chloroplasts. Plant Physiol 51:234–244
Bernfeld P (1955) Amylases α and β. In: Colowick SP, Kaplan NO (eds) Methods in enzymology. Academic Press, New York
Valcheva E, Valchev I, Yotova L (2011) Kinetics of enzyme action of Cartazyme NS-10 prior to bleaching of kraft pulp. Bioch Eng J 7:223–226
Singh K, Ahmad F, Singh VK, Kayastha K, Kayastha AM (2017) Purification, biochemical characterization and Insilico modeling of α-amylase from Vicia faba. J Mol Liq 234:133–141
Singh K, Kayastha AM (2014) α-amylase from wheat (Triticum aestivum) seeds: Its purification, biochemical attributes and active site studies. Food Chem 162:1–9
Sottirattanapan P, Nantachai K, Daduang S, Funahashi T, Yamad M (2017) Purification and characterization of amylase from roots of Paederia foetida Linn. Biocat Agric Biotech 10:329–335
Silva JNCA, Miranda S, Bolina ICA, Silva WC, Hirata DB, Castro HF, Mendes AA (2014) Immobilization of porcine pancreatic lipase on poly-hydroxybutyrate particles for the production of ethyl esters from macaw palm oils and pineapple flavor. Biochem Eng J 82:139–149
Das R, Talat M, Srivastava ON, Kayastha AM (2018) Covalent immobilization of peanut β-amylase for producing industrial nanobiocatalysts: a comparative study of kinetics, stability and reusability of the immobilized enzyme. Food Chem 245:488–499
Lage FAP, Bassi JJ, Corradini MCC, Todero LM, Luiz JHH, Mendes AA (2016) Preparation of a biocatalyst via physical adsorption of lipase from Thermomyces lanuginosus on hydrophobic support to catalyze biolubricant synthesis by esterification reaction in a solvent-free system. Enz Microb Techn 84:56–67
Sagu ST, Nso EJ, Homann T, Kapseu C, Rawel HM (2015) Extraction and purification of β-amylase from by three phase partitioning. Food Chem 183:144–153
Pal A, Khanum F (2011) Covalent immobilization of xylanase on glutaraldehyde activated alginate beads using response surface methodology: characterization of immobilized enzyme. Proc Biochem 46:1315–1322
Naidu MA, Saranraj P (2013) Bacterial amylase: a review. Int J Phar Biol Archiv 4:274–287
Samanta S, Das A, Halder SK, Jana A, Kar S, Mohapatra PKD, Pati B, Mondal KC (2014) Thermodynamic and kinetic characteristics of an α-amylase from Bacillus licheniformis SKB4. Acta Biol Szeged 58:147–156
Wang H, Wenz G (2013) Topochemical control of the photodimerization of aromatic compounds by y-cyclodextrin thioethers aqueous solution. Beilstein J Org Chem 9:1858–1866
Radeva G, Veleva S, Valcheva E (2008) A Correlation between the kinetic and thermodynamic adsorption characteristics of an optical brightener on a pulp surface. Ads Sci Techn 26:515–521
Duan X, Zhu Q, Zhang X, Huang Y (2021) Expression, biochemical and structural characterization of high-specific-activity β-amylase from Bacillus aryabhattai GEL-09 for application in starch hydrolysis. Microb Cell Fact 20:1–14. https://doi.org/10.1186/s12934-021-01649-5
Mihajlovski KR, Radovanović NR, Veljović ĐN, Šiler-Marinković SS, Dimitrijević-Branković SI (2016) Improved beta-amylase production on molasses and sugar beet pulp by a novel strain Paenibacillus chitinolyticus CKS1. Ind Crops Prod 80:115–121
Sun Y, Duan X, Wang L, Wu J (2016) Enhanced maltose production through mutagenesis of acceptor binding subsite + 2 in Bacillus stearothermophilus maltogenic amylase. J Biotechnol 217:53–61
Li S, Zuo ZR, Niu DD, Singh S, Permaul K, Prior BA, Shi GY, Wang ZX (2011) Gene cloning, heterologous expression, and characterization of a high maltose-producing α-amylase of Rhizopus oryzae. Appl Biochem Biotech 164:581–592
Wang YC, Zhao N, Ma JW, Yan QJ, Jiang ZQ (2019) High-level expression of a novel α-amylase from Thermomyces dupontii in Pichia pastoris and its application in maltose syrup production. Int J Bio Macro 127:683–692. https://doi.org/10.1016/j.ijbiomac.2019.01.162
Funding
The authors do not receive any funding from an external source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest associated with this publication. All authors have read and agreed to the publication of the manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lahmar, I., Velitchkova, M., Radeva, G. et al. β-Amylase Squirting Cucumber Between Immobilization, Kinetic Modeling and Application for Maltose Syrup Production. Chemistry Africa 7, 1293–1301 (2024). https://doi.org/10.1007/s42250-023-00833-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-023-00833-x