Abstract
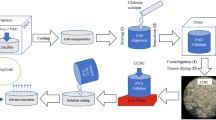
PVA, Chitosan film containing metal oxide nanocomposites (MONC) or chalcone moiety, prepared with a thickness of around 0.03 cm. Different molecular weights of MONC are used for the preparation of different polymer composite films. FT-IR, XRD, XPS, EDX, and TGA techniques were used to characterise the prepared film. Semicrystalline (40–150 nm) behaviour is observed for prepared film, which is confirmed by XRD, and the film undergoes multistage decomposition on heating, confirmed by TGA. SEM analysis concludes the film has a blooming flower like structure on the surface. Different elements of the polymer and dopant were confirmed using XPS and EDX analysis. FT-IR analysis suggests that there is no chemical interaction between the polymer and the hosting materials. The film shows a AC conductivity value of 1.36 × 10−5 Scm−1, and it shows higher dielectric properties.












Similar content being viewed by others
References
Burger N, Laachachi A, Ferriol M, Lut M, Toniazzo V, Ruch D (2016) Review of thermal conductivity in composites: mechanisms, parameters and theory. Prog Polym Sci 61:1–28. https://doi.org/10.1016/j.progpolymsci.2016.05.001
Pang H, Xu L, Yan DX, Li ZM (2014) Conductive polymer composites with segregated structures. Prog Polym Sci 39:1908–1933. https://doi.org/10.1016/j.progpolymsci.2014.07.007
Deng H, Lin L, Ji M, Zhang S, Yang M, Fu Q (2014) Progress on the morphological control of conductive network in conductive polymer composites and the use as electroactive multifunctional materials. Prog Polym Sci 39:627–655. https://doi.org/10.1016/j.progpolymsci.2013.07.007
Liu X, Li C, Pan Y, Schubert DW, Liu C (2019) Shear-induced rheological and electrical properties of molten poly(methyl methacrylate)/carbon black nanocomposites. Compos Part B Eng 164:37–44. https://doi.org/10.1016/j.compositesb.2018.11.054
Liu X, Pan Y, Zheng G, Schubert DW (2016) Rheological and electrical behavior of poly(methyl methacrylate)/carbon black composites as investigated by creep recovery in shear. Compos Sci Technol 128:1–7. https://doi.org/10.1016/j.compscitech.2016.03.011
Li Y, Zhou B, Zheng G, Liu X, Li T, Yan C, Cheng C, Dai K, Liu C, Shen C, Guo Z (2018) Continuously prepared highly conductive and stretchable SWNT/MWNT synergistically composited electrospun thermoplastic polyurethane yarns for wearable sensing. J Mater Chem C 6:2258–2269. https://doi.org/10.1039/c7tc04959e
Sha L, Chen Z, Chen Z, Zhang A, Yang Z (2016) Polylactic acid based nanocomposites: promising safe and biodegradable materials in biomedical field. Int J Polym Sci 2016:1–11. https://doi.org/10.1155/2016/6869154
Azwa Z, Yousif B, Manalo A, Karunasena W (2013) A review on the degradability of polymeric composites based on natural fibres. Mater Des 47:424–442. https://doi.org/10.1016/j.matdes.2012.11.025
Pandey JK, Singh RP (2005) Green nanocomposites from renewable resources: effect of plasticizer on the structure and material properties of clay-filled starch. Starch-Stärke 57:8–15. https://doi.org/10.1002/star.200400313
Tourillon G, Garnier F (1983) Effect of dopant on the physicochemical and electrical properties of organic conducting polymers. J Phys Chem 87(13):2289–2292. https://doi.org/10.1021/j100236a010
Gardner J, Bartlett P (1995) Application of conducting polymer technology in microsystems. Sens Actuat A Phys A 51:57–66. https://doi.org/10.1016/0924-4247(95)85004-X
Karthik S, Suresh J, Saravanan P, Arun A (2020) Highly conducting solid electrolytefilms based on PVA and iron alum: synthesis, characterization and electrical properties. J Sci Adv Mater Devices 5:400–408. https://doi.org/10.1016/j.jsamd.2020.05.006
Thangaraj V, Yogapriya M, Thirumalai K, Swaminathan M, Sundaramurthy A, Nandhakumar R, Suresh S, Vakees E, Araichimani A (2018) Sol−Gel synthesis of Ce4−xSr1+xFe5−xZnxO14+δ [0 ≤ x ≤ 0.45] superparamagnetic oxide systems and its magnetic, dielectric, and drug delivery properties. ACS Omega 3:16509–16518. https://doi.org/10.1021/acsomega.8b02817
Leu CM, Wu ZW, Wei KH (2002) Synthesis and properties of covalently bonded layered silicates/polyimide (BTDA-ODA) nanocomposites. Chem Mater 14:3016–3021. https://doi.org/10.1021/cm0200240
Wang J, Yang J, **e J, Xu N (2002) A novel conductive polymer-sulfur composite cathode material for rechargeable lithium batteries. Adv Mater 14:963–965. https://doi.org/10.1002/1521-4095(20020705)14:13/14%3C963::AID-ADMA963%3E3.0.CO;2-P
Jiang LH, Leu CM, Wei KH (2002) Layered silicates/fluorinated polyimide nanocomposites for advanced dielectric materials applications. Adv Mater 14:426–429. https://doi.org/10.1002/1521-4095(20020318)14:6%3C426::AID-ADMA426%3E3.0.CO;2-O
Hughes M, Shaffer MS, Renouf PAC, Singh C, Che GZ, Fray DJ, Windle AH (2002) Electrochemical capacitance of nanocomposite films formed by coating aligned arrays of carbon nanotubes with polypyrrole. Adv Mater 14:382–385. https://doi.org/10.1002/1521-4095(20020304)14:5%3C382::AIDADMA382%3E3.0.CO;2-Y
Vossmeyar T, Guse B, Besnard I, Bauer RE, Mullen K, Yasuda A (2002) Gold nanoparticle/polyphenylene dendrimer composite films: preparation and vapor-sensing properties. J Adv Mater 14:238–242. https://doi.org/10.1002/1521-4095(20020205)14:3%3C238::AID-ADMA238%3E3.0.CO;2-%23
Suresh J, Vakees E, Karthik S, Kayalvizhi M, Arun A (2014) Polymeric drug based on acrylates for biological applications: synthesis, characterization, antimicrobia, and drug release study. Des Monomers Polym 17(8):753–761. https://doi.org/10.1080/15685551.2014.918014
Kittur FS, Harish Prashanth KV, Udaya Sankar K, Tharanathan RN (2002) Characterization of chitin, chitosan and their carboxymethyl derivatives by differential scanning calorimetry. Carbohydr Polym 49(2):185–193. https://doi.org/10.1016/S0144-8617(01)00320-4
Yavuz AG, Uygun A, Uygun HK (2011) The effect of synthesis media on the properties of substituted polyaniline/chitosan composites. Carbohydr Res 346(14):2063–2069. https://doi.org/10.1016/j.carres.2011.06.009
Aziz SB (2016) Modifying poly (vinyl alcohol)(PVA) from insulator to small-bandgap polymer: a novel approach for organic solar cells and optoelectronic devices. J Electron Mater 45:736–745. https://doi.org/10.1007/s11664-015-4191-9
Kumar S, Dutta PK, Koh J (2011) A physiocochemical and biological study of novel chitosan- chloroquinoline derivative for biomedical applications. Int J Biol Macromol 49:356–361. https://doi.org/10.1016/j.ijbiomac.2011.05.017
Chandra Shekhara Shetty T, Raghavendra S, Dharmaprakash SM (2016) Optical limiting studies on chalcone doped PMMA polymer film. Mater Today Proc 3(6):2163–2168. https://doi.org/10.1016/j.matpr.2016.04.122
Karthik S, Suresh J, Thangaraj V, Kanagasabai Balaji S, Selvasekarapandian SS, Arun A (2019) Electrical and mechanical property of the polyvinyl alcohol based solid electrolyte film contains alum. SN Appl Sci 1(11):1–10. https://doi.org/10.1007/s42452-019-1432-1
Hind Albalawi, Ebtesam M Alharbi, Ahlam I Al Sulami, Noora Al Qahtani, Mohammed O Farea, Rajeh A (2022) Synthesis and characterization of sodium alginate/polyvinyl alcohol/zinc oxide/iron oxide nanocomposites for electrochemical applications. https://doi.org/10.1002/pc.27203
Faiza MZ, Khattak A, Alahmadi AA, Butt SU (2022) Development and investigation of high performance PVA/NiO and PVA/CuO nanocomposites with improved physical. Dielectr Mech Prop Mater 15:5154. https://doi.org/10.3390/ma15155154
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ayyanar, B., Suresh, J., Thangaraj, V. et al. Polyvinyl Alcohol, Chitosan Polymer Film Containing Chalcone, Metal Oxide Nanocomposite: Synthesis Characterization and Electrical Properties. Chemistry Africa 6, 2071–2085 (2023). https://doi.org/10.1007/s42250-023-00613-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-023-00613-7