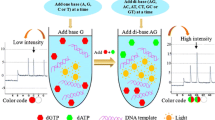
Abstract
Major technological challenges in point-of-care diagnostics are in the development of simple, fast, and inexpensive methods for high-throughput and multiplexed genoty** of single-nucleotide polymorphisms (SNPs). Herein, we develop a facile SNP detection platform based on platinum nanoparticles–induced etching of gold nanorods (AuNRs) by H2O2. The IVS-II-1 (G>A) β-thalassemia mutation, as one of the most prevalent mutations in the Middle East, was used as a model disease. In the presence of H2O2, ferrous ion (Fe2+) triggers a Fenton reaction with the catalytic decomposition of H2O2 into highly reactive hydroxyl (HO·) and hydroperoxyl (HOO·) radicals. These species etch AuNRs along the longitudinal axes to short AuNRs or even Au nanoparticles. For signaling SNPs, monobase-modified platinum nanoparticles (M-PtNPs) are hybridized to mutated sites of the duplex DNA. PtNPs catalyze the decomposition of H2O2 to water and oxygen, thus reducing the amount of H2O2 available for oxidative etching of AuNRs, and generating a series of distinct colors depending on the frequency of SNP in the target DNA. The frequency of SNP can be detected with the naked-eye or with UV-vis spectroscopy. The naked-eye detection limits of G–T and A–C mismatches are 17 and 15 pM, whereas UV-vis method responds linearly to these mismatches in the ranges from 10 to 200 pM and 5 to 120 pM with detection limits of 4 and 2 pM (3σ/slope), respectively. The present genosensor demonstrates a straightforward and easy-to-interpret method for naked-eye discrimination between PCR products of normal, heterozygous, and homozygous β-thalassemia-related mutation of β-hemoglobin.

Graphical abstract






Similar content being viewed by others
References
H. Jónsson, P. Sulem, B. Kehr, S. Kristmundsdottir, F. Zink, E. Hjartarson, M.T. Hardarson, K.E. Hjorleifsson, H.P. Eggertsson, S.A. Gudjonsson, L.D. Ward, G.A. Arnadottir, E.A. Helgason, H. Helgason, A. Gylfason, A. Jonasdottir, A. Jonasdottir, T. Rafnar, M. Frigge, S.N. Stacey, O.T. Magnusson, U. Thorsteinsdottir, G. Masson, A. Kong, B.V. Halldorsson, A. Helgason, D.F. Gudbjartsson, K. Stefansson, Parental influence on human germline de novo mutations in 1,548 trios from Iceland. Nature 549(7673), 519–522 (2017)
L. Zhang, Q. Cai, R.S. Wiederkehr, M. Fauvart, P. Fiorini, B. Majeed, M. Tsukuda, T. Matsuno, T. Stakenborg, Multiplex SNP genoty** in whole blood using an integrated microfluidic lab-on-a-chip. Lab Chip 16(20), 4012–4019 (2016)
D. Paquet, D. Kwart, A. Chen, A. Sproul, S. Jacob, S. Teo, K.M. Olsen, A. Gregg, S. Noggle, M. Tessier-Lavigne, Efficient introduction of specific homozygous and heterozygous mutations using CRISPR/Cas9. Nature 533(7601), 125–129 (2016)
N. Moradi, A. Noori, M.A. Mehrgardi, M.F. Mousavi, Scanning electrochemical microscopy for electrochemical detection of single-base mismatches by tagging ferrocenecarboxylic acid as a redox probe to DNA. Electroanalysis 28(4), 823–832 (2016)
K. Ghanbari, S.Z. Bathaie, M.F. Mousavi, Electrochemically fabricated polypyrrole nanofiber-modified electrode as a new electrochemical DNA biosensor. Biosens. Bioelectron. 23(12), 1825–1831 (2008)
M. Yousef Elahi, S.Z. Bathaie, S.H. Kazemi, M.F. Mousavi, DNA immobilization on a polypyrrole nanofiber modified electrode and its interaction with salicylic acid/aspirin. Anal. Biochem. 411(2), 176–184 (2011)
A.A. Gorodetsky, A. Ebrahim, J.K. Barton, Electrical detection of TATA binding protein at DNA-modified microelectrodes. J. Am. Chem. Soc. 130(10), 2924–2925 (2008)
B. Wei, T. Zhang, X. Ou, X. Li, X. Lou, F. **a, Stereochemistry-guided DNA probe for single nucleotide polymorphisms analysis. ACS Appl. Mater. Interfaces 8(25), 15911–15916 (2016)
A. Bonanni, C.K. Chua, G. Zhao, Z. Sofer, M. Pumera, Inherently electroactive graphene oxide nanoplatelets as labels for single nucleotide polymorphism detection. ACS Nano 6(10), 8546–8551 (2012)
F. Patolsky, A. Lichtenstein, I. Willner, Detection of single-base DNA mutations by enzyme-amplified electronic transduction. Nat. Biotechnol. 19(3), 253–257 (2001)
G. Liu, T.M. Lee, J. Wang, Nanocrystal-based bioelectronic coding of single nucleotide polymorphisms. J. Am. Chem. Soc. 127(1), 38–39 (2005)
M. Ye, Y. Zhang, H. Li, Y. Zhang, P. Tan, H. Tang, S. Yao, A novel method for the detection of point mutation in DNA using single-base-coded CdS nanoprobes. Biosens. Bioelectron. 24(8), 2339–2345 (2009)
K. Kerman, M. Saito, Y. Morita, Y. Takamura, M. Ozsoz, E. Tamiya, Electrochemical coding of single-nucleotide polymorphisms by monobase-modified gold nanoparticles. Anal. Chem. 76(7), 1877–1884 (2004)
S.M. Khoshfetrat, M.A. Mehrgardi, Amplified electrochemical genoty** of single-nucleotide polymorphisms using a graphene–gold nanoparticles modified glassy carbon platform. RSC Adv. 5(37), 29285–29293 (2015)
S.M. Khoshfetrat, M.A. Mehrgardi, Electrochemical genoty** of single-nucleotide polymorphisms by using monobase-conjugated modified nanoparticles. ChemElectroChem 1(4), 779–786 (2014)
S.M. Khoshfetrat, M. Ranjbari, M. Shayan, M.A. Mehrgardi, A. Kiani, Wireless electrochemiluminescence bipolar electrode array for visualized genoty** of single nucleotide polymorphism. Anal. Chem. 87(16), 8123–8131 (2015)
G. Liu, Y. Lin, Electrochemical quantification of single-nucleotide polymorphisms using nanoparticle probes. J. Am. Chem. Soc. 129(34), 10394–10401 (2007)
A. Abbaspour, A. Noori, Electrochemical detection of individual single nucleotide polymorphisms using monobase-modified apoferritin-encapsulated nanoparticles. Biosens. Bioelectron. 37(1), 11–18 (2012)
I. Willner, F. Patolsky, Y. Weizmann, B. Willner, Amplified detection of single-base mismatches in DNA using microgravimetric quartz-crystal-microbalance transduction. Talanta 56(5), 847–856 (2002)
F. **a, X. Zuo, R. Yang, Y. **ao, D. Kang, A. Vallée-Bélisle, X. Gong, J.D. Yuen, B.B.Y. Hsu, A.J. Heeger, K.W. Plaxco, Colorimetric detection of DNA, small molecules, proteins, and ions using unmodified gold nanoparticles and conjugated polyelectrolytes. Proc. Natl. Acad. Sci. 107(24), 10837–10841 (2010)
P. Teengam, W. Siangproh, A. Tuantranont, T. Vilaivan, O. Chailapakul, C.S. Henry, Multiplex paper-based colorimetric dna sensor using pyrrolidinyl peptide nucleic acid-induced AgNPs aggregation for detecting MERS-CoV, MTB, and HPV oligonucleotides. Anal. Chem. 89(10), 5428–5435 (2017)
M. Sabela, S. Balme, M. Bechelany, J.-M. Janot, and K. Bisetty, Adv. Eng. Mater., 1700270
Z. Li, M. Fang, M.K. LaGasse, J.R. Askim, K.S. Suslick, Colorimetric recognition of aldehydes and ketones. Angew. Chem. Int. Ed. 56(33), 9860–9863 (2017)
P. Valentini, R. Fiammengo, S. Sabella, M. Gariboldi, G. Maiorano, R. Cingolani, P.P. Pompa, Gold-nanoparticle-based colorimetric discrimination of cancer-related point mutations with picomolar sensitivity. ACS Nano 7(6), 5530–5538 (2013)
Z. Gao, Z. Qiu, M. Lu, J. Shu, D. Tang, Biosens. Bioelectron. 89(Part 2), 1006 (2017)
X. Wei, Y. Wang, Y. Zhao, Z. Chen, Biosens. Bioelectron 97(Supplement C), 332 (2017)
S. Lu, T. Hu, S. Wang, J. Sun, X. Yang, Ultra-sensitive colorimetric assay system based on the hybridization chain reaction-triggered enzyme cascade amplification. ACS Appl. Mater. Interfaces 9(1), 167–175 (2017)
H. Wu, Y. Liu, H. Wang, J. Wu, F. Zhu, P. Zou, Biosens. Bioelectron 81, 303(Supplement C) (2016)
Y. Song, W. Zhang, Y. An, L. Cui, C. Yu, Z. Zhu, C.J. Yang, Chem. Commun. 48(4), 576 (2012)
N. Ding, N. Yan, C. Ren, X. Chen, Colorimetric determination of melamine in dairy products by Fe3O4 magnetic nanoparticles−H2O2−ABTS detection system. Anal. Chem. 82(13), 5897–5899 (2010)
M. Liang, K. Fan, Y. Pan, H. Jiang, F. Wang, D. Yang, D. Lu, J. Feng, J. Zhao, L. Yang, Anal. Chem. 85(1), 308 (2012)
L.Y. Chau, Q. He, A. Qin, S.P. Yip, T.M. Lee, Platinum nanoparticles on reduced graphene oxide as peroxidase mimetics for the colorimetric detection of specific DNA sequence. J. Mater. Chem. B 4(23), 4076–4083 (2016)
P. Ni, Y. Sun, H. Dai, S. Jiang, W. Lu, Y. Wang, Z. Li, Z. Li, Sens. Actuators, B 226(Supplement C), 104 (2016)
L. **, Z. Meng, Y. Zhang, S. Cai, Z. Zhang, C. Li, L. Shang, Y. Shen, Ultrasmall Pt nanoclusters as robust peroxidase mimics for colorimetric detection of glucose in human serum. ACS Appl. Mater. Interfaces 9(11), 10027–10033 (2017)
Y. Zhou, Z. Ma, Sens. Actuators, B 249, 53 (2017)
S. Singh, P. Tripathi, N. Kumar, S. Nara, Biosens. Bioelectron. 92(Supplement C), 280 (2017)
X. Ma, Z. Chen, P. Kannan, Z. Lin, B. Qiu, L. Guo, Gold nanorods as colorful chromogenic substrates for semiquantitative detection of nucleic acids, proteins, and small molecules with the naked eye. Anal. Chem. 88(6), 3227–3234 (2016)
J. Chen, A.A. Jackson, V.M. Rotello, S.R. Nugen, Colorimetric detection of Escherichia coli based on the enzyme-induced metallization of gold nanorods. Small 12(18), 2469–2475 (2016)
R. de la Rica, M.M. Stevens, Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye. Nat Nano 7(12), 821–824 (2012)
L. Tang, J. Li, Plasmon-based colorimetric nanosensors for ultrasensitive molecular diagnostics. ACS Sensors 2(7), 857–875 (2017)
Z. Zhang, Z. Chen, F. Cheng, Y. Zhang, and L. Chen, Biosens. Bioelectron. 89 (Part 2), 932 (2017)
X. Ma, Y. Lin, L. Guo, B. Qiu, G. Chen, H.-h. Yang, and Z. Lin, Biosens. Bioelectron. 87 (Supplement C), 122 (2017)
Y. Lin, M. Zhao, Y. Guo, X. Ma, F. Luo, L. Guo, B. Qiu, G. Chen, Z. Lin, Multicolor colormetric biosensor for the determination of glucose based on the etching of gold nanorods. Sci. Rep. 6, 37879 (2016)
Y. Li, X. Ma, Z. Xu, M. Liu, Z. Lin, B. Qiu, L. Guo, G. Chen, Multicolor ELISA based on alkaline phosphatase-triggered growth of Au nanorods. Analyst 141(10), 2970–2976 (2016)
X. Huang, S. Neretina, M.A. El-Sayed, Gold nanorods: from synthesis and properties to biological and biomedical applications. Adv. Mater. 21(48), 4880–4910 (2009)
Y. Mao, J. Li, J. Yan, Y. Ma, Y. Song, T. Tian, X. Liu, Z. Zhu, L. Zhou, C. Yang, Chem. Commun. 53(47), 6375 (2017)
L. Saa, M. Coronado-Puchau, V. Pavlov, L.M. Liz-Marzan, Enzymatic etching of gold nanorods by horseradish peroxidase and application to blood glucose detection. Nanoscale 6(13), 7405–7409 (2014)
K. Saha, S.S. Agasti, C. Kim, X. Li, V.M. Rotello, Gold nanoparticles in chemical and biological sensing. Chem. Rev. 112(5), 2739–2779 (2012)
J. Deng, P. Yu, Y. Wang, L. Yang, L. Mao, Visualization and quantification of neurochemicals with gold nanoparticles: opportunities and challenges. Adv. Mater. 26(40), 6933–6943 (2014)
Y.-C. Yang, W.-L. Tseng, 1,4-Benzenediboronic-acid-induced aggregation of gold nanoparticles: application to hydrogen peroxide detection and biotin–avidin-mediated immunoassay with naked-eye detection. Anal. Chem. 88(10), 5355–5362 (2016)
L. Saa, R. Grinyte, A. Sánchez-Iglesias, L.M. Liz-Marzán, V. Pavlov, Blocked enzymatic etching of gold nanorods: application to colorimetric detection of acetylcholinesterase activity and its inhibitors. ACS Appl. Mater. Interfaces 8(17), 11139–11146 (2016)
R. Kahyesh-Esfandiary, Z.A. Sadigh, M. Esghaei, M.N. Bastani, T. Donyavi, A. Najafi, A. Fakhim, F. Bokharaei-Salim, Detection of HCV genome in peripheral blood mononuclear cells of Iranian seropositive and HCV RNA negative in plasma of patients with beta-thalassemia major: occult HCV infection. J. Med. Virol. 91(1), 107–114 (2019)
P. Chaweephisal, A. Phusua, K. Fanhchaksai, S. Sirichotiyakul, and P. Charoenkwan, Blood Cells, Mol., Dis. 74, 13 (2019)
A. Cao, R. Galanello, Beta-thalassemia. Genet Med 12(2), 61–76 (2010)
S.H. Sharafdarkolaee, M. Motovali-Bashi, P. Gill, Gene 674, 98 (2018)
M. Ercan, V.C. Ozalp, B.G. Tuna, Anal. Biochem. 537(Supplement C), 78 (2017)
X. Jia, L. Yao, Y. Zhou, H. Han, N. Tang, L. Wang, Y. Li, Detection of three common mutations causing β-thalassemia by using a closed-tube multiplex PCR. Exp. Mol. Pathol. 105(2), 208–212 (2018)
W. Jia, P. Chen, W. Chen, Y. Li, Raman characterizations of red blood cells with β-thalassemia using laser tweezers Raman spectroscopy. Medicine 97(39), e12611 (2018)
M.D. Cappellini, J.B. Porter, V. Viprakasit, A.T. Taher, A paradigm shift on beta-thalassaemia treatment: how will we manage this old disease with new therapies? Blood Rev. 32(4), 300–311 (2018)
J.M. Old, Screening and genetic diagnosis of haemoglobin disorders. Blood Rev. 17(1), 43–53 (2003)
N. Kubikova, D. Babariya, J. Sarasa, K. Spath, S. Alfarawati, D. Wells, Clinical application of a protocol based on universal next-generation sequencing for the diagnosis of beta-thalassaemia and sickle cell anaemia in preimplantation embryos. Reprod. BioMed. Online 37(2), 136–144 (2018)
L. Angnes, E.M. Richter, M.A. Augelli, G.H. Kume, Gold electrodes from recordable CDs. Anal. Chem. 72(21), 5503–5506 (2000)
X. Ye, L. **, H. Caglayan, J. Chen, G. **ng, C. Zheng, V. Doan-Nguyen, Y. Kang, N. Engheta, C.R. Kagan, C.B. Murray, Improved size-tunable synthesis of monodisperse gold nanorods through the use of aromatic additives. ACS Nano 6(3), 2804–2817 (2012)
H.-R. Zhang, J.-J. Xu, H.-Y. Chen, Electrochemiluminescence ratiometry: a new approach to DNA biosensing. Anal. Chem. 85(11), 5321–5325 (2013)
A. Abbaspour, A. Khajehzadeh, A. Noori, A simple and selective sensor for the determination of ascorbic acid in vitamin C tablets based on paptode. Anal. Sci. 24(6), 721–725 (2008)
A. Abbaspour, M.A. Mehrgardi, A. Noori, M.A. Kamyabi, A. Khalafi-Nezhad, M.N. Soltani Rad, Speciation of iron(II), iron(III) and full-range pH monitoring using paptode: a simple colorimetric method as an appropriate alternative for optodes. Sensors Actuators B Chem. 113(2), 857–865 (2006)
G. Wang, Y. Akiyama, T. Takarada, M. Maeda, Rapid non-crosslinking aggregation of DNA-functionalized gold nanorods and nanotriangles for colorimetric single-nucleotide discrimination. Chem. Eur. J. 22(1), 258–263 (2016)
E. Brillas, I. Sirés, M.A. Oturan, Electro-fenton process and related electrochemical technologies based on fenton’s reaction chemistry. Chem. Rev. 109(12), 6570–6631 (2009)
S.G. Rhee, T.-S. Chang, W. Jeong, D. Kang, Methods for detection and measurement of hydrogen peroxide inside and outside of cells. Mol. Cells 29(6), 539–549 (2010)
Z. Zhu, Z. Guan, S. Jia, Z. Lei, S. Lin, H. Zhang, Y. Ma, Z.-Q. Tian, C.J. Yang, Au@Pt nanoparticle encapsulated target-responsive hydrogel with volumetric bar-chart chip readout for quantitative point-of-care testing. Angew. Chem. Int. Ed. 53(46), 12503–12507 (2014)
Y. Song, Y. Wang, L. Qin, A multistage volumetric bar chart chip for visualized quantification of DNA. J. Am. Chem. Soc. 135(45), 16785–16788 (2013)
E.M. Boon, D.M. Ceres, T.G. Drummond, M.G. Hill, J.K. Barton, Mutation detection by electrocatalysis at DNA-modified electrodes. Nat. Biotechnol. 18(10), 1096–1100 (2000)
Funding
This work was financially supported by the Tarbiat Modares University Research Council.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
PCR amplification of the gene, synthesis of Au nanorods, fabrication of the gold CDtrode, and characterization of the metal nanoparticles are provided in the Supporting Information. Supplementary data related to this article can be found at https://... (DOCX 771 kb)
Rights and permissions
About this article
Cite this article
Bishkul, H., Khoshfetrat, S.M., Noori, A. et al. Rich-color visual genoty** of single-nucleotide polymorphisms based on platinum nanoparticle–induced etching of gold nanorods. emergent mater. 2, 351–361 (2019). https://doi.org/10.1007/s42247-019-00049-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42247-019-00049-1