Abstract
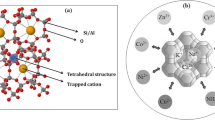
This study examined the feasibility of using zeolite clinoptilolite to removal nitrate, phosphate pollutants, and salt from the agricultural drainage water. To that end, significant pollutant absorption parameters, such as adsorbent particle size, pollutant concentration, salinity, temperature, retention time, pH, and adsorbent concentration, were optimized in the batch condition. Having optimized the parameters, the researchers conducted adsorption experiments on an experimental model, similar to the subsurface drainage systems applied in farms. Adsorption experiments were carried out at the optimized parameter levels on four models, namely a reference model (D0), a model with adsorbents around the drains (D1), a model with adsorbents around the plant roots (D2), and a model with adsorbents on the soil surface (D3). These models were fed with untreated drainage water from the farms in the south of Khuzestan during the fertilization season. In the batch adsorption experiments, the results showed 63% nitrate removal efficiency, 39% phosphate removal efficiency, and 79% salt removal efficiency using 30 g.L-1 of 1000 µm adsorbent particles for a pH of 5, and initial pollutant concentration of 80 mg.L-1 nitrate and 10 mg.L-1 phosphate in 12 dS/m salinity during a 90-min retention time period at 50 °C ambient temperature. These parameter levels led to nitrate, phosphate, and salt removal efficiencies of 59.72, 29.28, and 77.47%, respectively, in the model with clinoptilolite adsorbents around the drains (D1).
Article Highlights
-
Using zeolite clinoptilolite to filter nitrate, phosphate, salt from agricultural drainage water.
-
The results were suggestive of a 76% pollutant and salt-removal efficiency.
-
TThe nitrate -removal efficiency was 59.72% in the model with clinoptilolite adsorbents.
-
The phosphate-removal efficiency was of 29.28% in the model with clinoptilolite adsorbents.
-
The salt-removal efficiency was 77.47% in the model with clinoptilolite adsorbents.
















Similar content being viewed by others
Data Availability
Data will be made available on request.
References
Adinehvand J, Shokuhi Rad A, Tehrani AS (2016) Acid-treated zeolite (clinoptilolite) and its potential to zinc removal from water sample. Int J Environ Sci Technol 13:2705
Afshin S, Rashtbari Y, Vosoughi M, Rehman R, Ramavandi B, Behzad A, Mitu L (2020) Removal of basic blue-41 dye from water by stabilized magnetic iron nanoparticles on clinoptilolite zeolite. Rev Chim 71(2):218–229
Bhatnagar A, Sillanpää M (2011) A review of emerging adsorbents for nitrate removal from water. Chem Eng J 168(2):493–504
Bibiano-Cruz L, Garfias J, Salas-García J (2016) Batch and column test analyses for hardness removal using natural and homoionic clinoptilolite: breakthrough experiments and modeling. Sustain Water Resour Manag 2:183
Bish DL, Boak JM (2001) Clinoptilolite-heulandite nomenclature. Rev Mineral Geochem 45(1):207–216
Dang VBH, Doan HD, Dang-Vu T, Lohi A (2009) Equilibrium and kinetics of biosorption of cadmium (II) and copper (II) ions by wheat straw. Bioresour Technol 100:211–219
EPA (2012) 2012 Edition of the drinking water standards and Health Advisors, EPA822-S-12-001. United States Environmental Protection Agency, USA
Eshraghi F, Nezamzadeh-Ejhieh A (2018) EDTA-functionalized clinoptilolite nanoparticles as an effective adsorbent for Pb(II) removal. Environ Sci Pollut Res 25(14):14043–14056
Farzad N (2017) Using natural zeolite in planting bed to retain water and prevent fertilizer leaching. 1st National Conference on Water Management Approach to Optimize Water Use in Agriculture, Hamedan, Permanent Secretariat. [In Persian]
Golestanifar H, Asadi A, Alinezhad A, Haybati B, Vosoughi M (2016) Isotherm and kinetic studies on the adsorption of nitrate onto nanoalumina and iron-modified pumice. Desalin Water Treat 57(12):5480–5487
Gupta VK, Agarwal Sh, Fakhri AM, Sadeghi AN (2017) Application of response surface methodology to optimize the adsorption performance of a magnetic graphene oxide nanocomposite adsorbent for removal of methadone from the environment. J Colloid Interf Sci 497:193–200
Habibollahi S, Hajializadeh A (2014) TDS reduction of industrial effluent using natural zeolite. 1st National Conference on the Environment, Dehaghan, Payame Nour University of Dehaghan. [In Persian]
He K, Chen Y, Tang Z, Hu Y (2016) Removal of heavy metal ions from aqueous solution by zeolite synthesized from fly ash. Environ Sci Pollut Res 23(3):2778–2788
Hii SL, Yong SY, Wong CL (2009) Removal of rhodamine B from aqueous solution by sorption on Turbinaria conoides (Phaeophyta). J Appl Phycol 21:625–631
Kabuba J (2020) Physico-chemical treatment of clinoptilolite by chitosan for the removal of nitrate from wastewater. In: Ao SI, Kim H, Amouzegar M (eds) Transactions on engineering technologies. WCECS 2018. Springer, Singapore
Kashefi Asl M, Hasani A, Naserkhaki E (2017) Removal of nitrate anion from water by surfactant modified carbonate cancrinite zeolite. In: International Symposium on chemical engineering and materials research, Tehran. Farzin Sustainable Scientific and Industry Development Center (in Persian)
Mazeikiene A, Valentukeviciene M, Jankauskas J (2010) Laboratory study of ammonium ion removal by using zeolite (clinoptilolite) to treat drinking water. J Environ Eng Landsc Manag 18(1):54–61
Mikhak A, Sohrabi A, Kassaee MZ (2017) Removal of nitrate and phosphate from water by clinoptilolite-supported iron hydroxide nanoparticle. Arab J Sci Eng 42:2433–2439
Mitrogiannis D, Psychoyou M, Kornaros ME (2020) Calcium-modified clinoptilolite as a recovery medium of phosphate and potassium from anaerobically digested olive mill wastewater. Environ Sci Pollut Res 27:2977–2991
Niri MV, Mahvi AH, Alimohammadi M, Shirmardi M, Golastanifar H, Mohammadi MJ, Naeimabadi A, Khishdost M (2015) Removal of natural organic matter (NOM) from an aqueous solution by NaCl and surfactant-modified clinoptilolite. J Water Health 13(2):394–405
Pandey PK, Sharma SK, Sambi SS (2015) Removal of lead (II) from waste water on zeolite–NaX. J Environ Chem Eng 3:2604–2610
Physical and chemical properties of drinking water, ISIRI 1503 Standard (2010) (in Persian)
Saadat M, Nezamzadeh-Ejhieh A (2016) Clinoptilolite nanoparticles containing HDTMA and Arsenazo III as a sensitive carbon paste electrode modifier for indirect voltammetric measurement of Cesium ions. Electrochim Acta 217:163–170
Sharma SK, Sobti RC (2012) Nitrate removal from ground water: a review. J Chem 9(4):1667–1675
Spark KM, Johnson BB, Wells JD (1995) Charactrizing heavy-metal adsorption on oxides and oxyhydroxides. Eur J Soil Sci 116:621–631
Taghdisian H, Tasharrofi S, Hosseinnia A (2018) Nitrate reduction using zeolite clinoptilolite: kinetic investigation and evaluation of effective process parameters. 5th International Conference on Applied Research in Chemistry and Chemical Engineering with an emphasis on indigenous technology in Iran, Tehran. The Society of Indigenous Technologies of Iran. [In Persian]
Yargholi B, Azarneshan S (2014) Water and wastewater sampling and analysis instructions. Agricultural Technical and Engineering Research Institute, Agricultural Research, Education and Extension Organization, Ministry of Jihad Agriculture. [In Persian]
Acknowledgements
We are grateful to the Research Council of Shahid Chamran University of Ahvaz for financial support (GN: SCU.WI98.280).
Funding
This study was funded by “Shahid Chamran University of Ahvaz”.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by SS, MA, MG, and SB. The first draft of the manuscript was written by SS, MA, and MG, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there are no competing interests.
Ethical Approval
Not applicable.
Consent to Participate
Consent was obtained from all individual participants included in the study.
Consent to Publish
The participant has consented to the submission of the case report to the journal.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sadeghi, S., Albaji, M., Golabi, M. et al. Using Modified Clinoptilolite Zeolite to Remove Pollutants and Salt from Agricultural Drainage Water in a Model Drainage System. Int J Environ Res 15, 859–873 (2021). https://doi.org/10.1007/s41742-021-00359-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-021-00359-5