Abstract
Pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH) are subtypes of pulmonary hypertension—a rare, life-threatening condition defined by chronically elevated pulmonary artery pressure. Treatment goals include improving patient functioning, symptom management, and delaying disease progression. Traditional therapies have focused on affected physiologic pathways, with endothelin-receptor antagonists and/or phosphodiesterase-5 inhibitors as first-line agents and prostacyclins for more severe disease. Riociguat, a therapeutic agent that stimulates soluble guanylate cyclase via nitric-oxide pathways, was approved in 2013 for treatment of PAH and CTEPH. Riociguat significantly improved exercise capacity, hemodynamic parameters, and other functional endpoints in phase 3 trials. Safety and efficacy were maintained in long-term extension studies. Adverse events (AEs) were generally mild to moderate and self-limiting and did not require treatment discontinuation. To optimize adherence, frequent patient monitoring and management of AEs are recommended. Management strategies include patient education, treatment/prevention of adverse events, and individualized dose adjustment.
Funding: Bayer HealthCare Pharmaceuticals, Whippany, NJ, USA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulmonary hypertension (PH) is a chronic cardiopulmonary disorder affecting more than 25 million people worldwide. The World Health Organization (WHO) has classified PH into five groups based on pathophysiology and hemodynamic characteristics as follows:
-
1.
pulmonary arterial hypertension (PAH),
-
2.
left-heart-related PH,
-
3.
lung/hypoxia-related PH,
-
4.
chronic thromboembolic pulmonary hypertension (CTEPH), and
-
5.
PH with unclear or multifactorial mechanisms [1].
Group 1 PH, or PAH, is a rare, life-threatening condition defined by a mean pulmonary artery pressure ≥25 mmHg at rest and pulmonary capillary wedge pressure ≤15 mmHg [2]. Similarly, group 4 PH, or CTEPH, is characterized by the same pulmonary pressures in the presence of organized emboli in the proximal and/or distal pulmonary arteries [3].
In both conditions, chronically elevated pressure in the pulmonary arteries can result in impaired blood gas exchange and vascular remodeling that ultimately lead to right heart failure [4]. Patients typically present with a constellation of symptoms that impair functional capacity and quality of life. These symptoms may include dyspnea on exertion, fatigue, presyncope/syncope, angina, and edema [5–8]. Treatment goals of PAH are to improve WHO functional class (FC), increase exercise capacity as measured by 6-min walk distance (6MWD), normalize (improve) right ventricular function, delay disease progression, and improve patient survival [4, 9].
In PAH, therapeutic management targets the affected physiologic pathways: the endothelin pathway (bosentan, ambrisentan, macitentan); the nitric oxide (NO) pathway (sildenafil, tadalafil, riociguat), and the prostacyclin pathway (treprostinil, epoprostenol, iloprost, selexipag). Therapy may begin with a single oral drug targeting the endothelin or NO pathway [3, 10] or with an upfront combination [3]. As the disease progresses, patients may be managed with add-on therapies or switched to a different class of treatment. Inhaled or parenteral prostacyclins are typically reserved for more advanced disease [3, 10].
In CTEPH, the definitive treatment is pulmonary thromboendarterectomy (PTE), which can be potentially curative [6]. Several randomized, controlled and open-label trials of 3–6 months’ duration have reported mixed results with drug therapy for patients who have inoperable or residual CTEPH [6]. Indeed, only one approved treatment option, riociguat, a soluble guanylate cyclase (sGC) stimulator, is available for patients in the US with disease that persists or recurs post-PTE or for those patients ineligible for PTE surgery [11–13]. With few exceptions, riociguat has now been approved for the treatment of inoperable or persistent/recurrent CTEPH and for PAH throughout the world, including North and South America, Europe, Russia, Australia, and Japan.
This review will focus on the use of riociguat in clinical practice. Recommendations for managing adverse events (AEs) are based on the expertise and experience with riociguat of PH allied health professionals. This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Riociguat: a Novel Oral Agent for the Treatment of PAH and Inoperable or Persistent/Recurrent CTEPH
As understanding of the pathophysiology—including the role of endothelial-cell derived NO—of PAH has evolved, so has the development of new therapeutic agents. In healthy individuals, NO induces vasodilation by increasing production of cyclic guanosine monophosphate (cGMP) via activation of the intracellular enzyme sGC [14]. In patients with PAH, production of NO is impaired, resulting in reduction of sGC activity and reduced intracellular cGMP levels, which can lead to pulmonary vasoconstriction, vascular remodeling, and a prothrombotic state [15, 16].
In 2013, riociguat, an oral agent and the first member of a novel class of therapeutics known as sGC stimulators, was introduced [16–20]. Riociguat has a dual mode of action: it sensitizes sGC to endogenous NO by stabilizing NO-sGC binding and it directly stimulates sGC independently of NO (Fig. 1) [16, 19]. Through these mechanisms, riociguat restores the NO-sGC-cGMP pathway and leads to increased generation of cGMP [16, 19, 21].
Riociguat mechanism of action. Riociguat has a dual mechanism. In the presence (a) of nitric oxide (NO), riociguat sensitizes soluble guanylate cyclase (sGC) to endogenous NO by stabilizing sGC–NO binding. Additionally, riociguat directly stimulates sGC independently (b) of NO via a different binding site. Both actions result in increased generation (c) of cyclic guanosine monophosphate, which in turn results in subsequent vasodilation (d) and regulation of fibrosis, proliferation, and inflammation (e)
Riociguat is the first drug approved by the US Food and Drug Administration for the treatment of both PAH and inoperable or persistent/recurrent CTEPH [17, 22, 23]. In the treatment of inoperable CTEPH, riociguat is the only drug to consistently demonstrate clinical efficacy in a placebo-controlled study. Improvements were observed in exercise capacity and pulmonary hemodynamics, as well as a range of clinically relevant secondary endpoints [12, 23]. In a pivotal PAH trial, riociguat significantly improved exercise capacity in treatment-native patients with symptomatic PAH, as well as in those pretreated with endothelin-receptor antagonists (ERAs) or prostanoids [22]. Riociguat was also shown to significantly improve time-to-clinical-worsening (TTCW) events (defined as all-cause mortality; heart/lung transplantation; atrial septostomy; initiation of new PAH-specific therapy; and worsening of 6MWD, WHO FC, or clinical status) as well as hemodynamic parameters and other secondary endpoints in PAH (Table 1) [22].
Clinical Profile of Riociguat
Absorption and Distribution
Riociguat is readily absorbed, with an absolute bioavailability of approximately 94% and with peak plasma concentrations achieved within 1.5 h after administration. Riociguat also shows complete oral absorption with no relevant food effects [17, 24, 25]. Systemic exposure to riociguat is dose proportional from 0.5 to 2.5 mg, with an area-under-the-curve variability across all doses of approximately 60% and intrasubject variability of approximately 30%. Dosing strategy is, therefore, targeted to individualized dose optimization with avoidance of hypotension [17, 24, 25].
The bioavailability of crushed preparations of riociguat has also been studied. Exposure was similar between whole and crushed-tablet preparations; although minor food effects were observed, the bioavailability of the formulations are interchangeable. Thus, riociguat is still an option for patients who are unable to swallow tablets [26].
Metabolism and Excretion
More than 90% of riociguat is eliminated in the urine and feces, largely as metabolites. Elimination half-life in patients with PAH is approximately 12 vs 7 h in healthy subjects. Likewise, average systemic clearance is 1.8 L/h in patients with PAH—approximately half that seen in healthy subjects (3.4 L/h) [17]. Plasma concentrations in smokers are reduced by 50–60% compared with nonsmokers. Based on pharmacokinetic modeling, for patients who are smokers, doses higher than 2.5 mg three times a day (TID) may be considered to match exposure seen in nonsmoking patients. The safety and efficacy of dosages greater than 2.5 mg TID have not been established. A subsequent dose reduction may be required in a circumstance where a patient quits or was required to abstain from smoking while on therapy.
The safety and efficacy of riociguat have not been demonstrated in patients with renal impairment (creatinine clearance <15 mL/min or on dialysis) or in patients with severe hepatic impairment (i.e., Child Pugh class C). Riociguat is not recommended for use in such patients [17, 27, 28].
Drug–Drug Interactions
Riociguat is extensively metabolized through the cytochrome P (CYP)450 enzyme system and is a substrate of the efflux transporters P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP). Coadministration with broad spectrum CYP450 inhibitors, such as P-gp/BCRP inhibitors (e.g., ketoconazole, itraconazole), or human immunodeficiency virus protease inhibitors (e.g., ritonavir) may increase riociguat exposure, possibly resulting in hypotension; dose reduction should be considered in patients taking these medications concomitantly [17].
Riociguat has no pharmacokinetic interactions with prostanoids that merit dose adjustment. However, the exposure of riociguat is reduced when coadministered with ERAs that induce CYP3A4 (e.g., bosentan) [22]. Additionally, riociguat has no clinically relevant drug–drug interactions with warfarin and aspirin, common background therapies in both CTEPH and PAH [17, 29].
Contraindications
Coadministration of riociguat with nitrates or NO donors (such as amyl nitrite) in any form is contraindicated. Concomitant administration of riociguat with specific PDE-5is (such as sildenafil, tadalafil, vardenafil) or nonspecific PDE inhibitors (such as dipyridamole or theophylline) is contraindicated. Riociguat should not be administered within 24 h of sildenafil or 24 h before or within 48 h after tadalafil. Riociguat is contraindicated in patients with pulmonary hypertension associated with idiopathic interstitial pneumonias. [17]
Riociguat is contraindicated during pregnancy (pregnancy category X). For this reason, female patients in the US, regardless of reproductive potential, can only receive riociguat through a risk evaluation and mitigation strategy (REMS) restricted-distribution program. Male patients are not enrolled in the REMS program. Female patients of reproductive potential must have a negative pregnancy test prior to starting treatment with riociguat, monthly during treatment, and 1 month after discontinuation of treatment with riociguat. They must also use acceptable methods of contraception during treatment with riociguat and for 1 month after cessation of treatment with riociguat. Patients may choose one highly effective form of contraception (intrauterine device, contraceptive implant, or tubal sterilization) or a combination of methods (hormone method with a single or dual barrier method).
To prescribe riociguat to female patients, US healthcare providers must attain certification by enrolling in the REMS program and completing mandatory training. Patients should be counseled on pregnancy planning and prevention (including emergency contraception) and advised to contact their healthcare provider if they become pregnant or suspect they may be pregnant. Pharmacies must also be certified with the REMS program and may only dispense riociguat to patients who are authorized to receive it [17]. Clinicians may use criteria listed in Table 2 when selecting patients most likely to benefit clinically from riociguat.
Dosing of Riociguat
Riociguat is available in five dose strengths (0.5, 1.0, 1.5, 2.0, 2.5 mg), allowing for individualized dosing based on clinical response. In patients with systolic blood pressure (SBP) ≥95 mmHg, the recommended starting dosage of riociguat is 1.0 mg TID. If the patient exhibits no signs of hypotension, the dose should be increased by 0.5 mg at 2 to 4-week intervals to an optimized patient dosage or a maximum of 2.5 mg TID (Fig. 2) [17].
Riociguat recommended dosing schedule. The recommended starting dosage of riociguat is 1.0 mg three times daily (TID). Patients who may be at risk of hypotension can initiate treatment with riociguat at 0.5 mg TID. Dosage should be titrated up in 0.5-mg increments, no sooner than 2 weeks apart, up to a maximum of 2.5 mg TID. Asterisk indicates that if systolic blood pressure remains >95 mmHg and the patient has no signs or symptoms of hypotension, the dosage should be uptitrated by 0.5 mg TID. If at any time, the patient has symptoms of hypotension, decrease the dosage by 0.5 mg TID
In specific patients, a starting dose of riociguat 0.5 mg TID may be warranted. Because riociguat is extensively metabolized through the CYP450 enzyme system and is a substrate of the efflux transporters P-gp and BCRP, patients on strong P-gp and BCRP inhibitors should be considered for a lower 0.5-mg TID starting dosage. Similarly, patients who, based on a clinician’s assessment, may not tolerate hypotensive effects may be started on 0.5 mg TID [17].
Transitioning to and from Riociguat
Concomitant administration of riociguat with PDE-5i’s is contraindicated and patients transitioning to or from riociguat must undergo a washout period. Sildenafil should be discontinued at least 24 h prior to administering riociguat and tadalafil should be discontinued at least 48 h prior to administering riociguat. In patients switching from tadalafil who are at risk of hypotension, a starting dose of riociguat 0.5 mg may be considered. It is recommended that these patients be monitored for signs and symptoms of hypotension when starting riociguat. Riociguat should be discontinued for at least 24 h before transitioning to a PDE-5i and it is recommended that patients be monitored for signs and symptoms of hypotension once PDE-5i therapy is initiated.
Dosing with Riociguat at Experienced PH Centers
Clinicians specializing in the management of CTEPH and/or PAH may take a more individualized approach to dose adjustment. Additionally, although it is recommended that doses be adjusted in 2-week increments, this represents the minimum acceptable dose-adjustment window to achieve maximal dose and benefit as quickly possible [17]. If patients are not tolerating uptitration, their dosage can be decreased to the previous dosage and maintained for an additional 2 weeks before attempting uptitration again. Management strategies should be tailored to personal treatment goals and tolerability.
Management of Adverse Events
Low Systemic Blood Pressure
Through its reduction of systemic and pulmonary vascular resistance, riociguat may lower SBP. Risk of hypotension is increased in patients with PH who already have resting hypotension, are hypovolemic, or have severe left ventricular outflow obstruction [17].
Hypotension was observed in approximately 10% of patients taking riociguat TID in the phase 3 CHEST-1 and PATENT-1 registration trials. Severe drug-related hypotension that qualified as a serious adverse event (SAE) was observed in only two riociguat-treated patients, one each in CHEST-1 and PATENT-1, and led to one discontinuation in PATENT-1 [22, 23].
Hypotension in patients being treated with riociguat at either a stable dosage or during dose adjustment is managed by first assessing clinical symptoms of hypotension and concomitant cardiovascular medications. Systemic antihypertensive medications may be discontinued or their dosages reduced to allow for continuation or uptitration of riociguat. A staggered-interval dose-adjustment approach can be helpful in reaching the target dosage of riociguat.
In patients with blood pressure that remains low but who are otherwise asymptomatic, maintaining the current dosing of riociguat without adjustment is reasonable. Per label guidance, if SBP is ≤95 mmHg with any associated symptoms, and a patient is not currently taking concomitant antihypertensive medications or medications that lower blood pressure, the dosage of riociguat should be decreased by 0.5 mg. Reassess the patient’s blood pressure and clinical status in 2 weeks and again in 4 weeks, for the possibility of further downtitration of riociguat when dose adjustment is limited by hypotension [17].
Headache and Nasopharyngitis
Headache and nasopharyngitis are among the most common AEs observed during therapy with riociguat. They are likely related to activation of the NO-cGMP pathway, which causes a cascade of signaling events resulting in vasodilation of arterioles in the nasal and throat passages, as well as the meninges and brain [30].
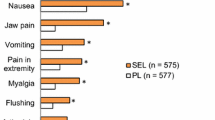
Headache occurred in 25% of riociguat-treated patients in CHEST-1 compared with 14% who received placebo. In PATENT-1, 27% of patients receiving 2.5 mg TID and 32% of patients receiving 1.5 mg TID experienced headache compared with 20% of patients receiving placebo. Nasopharyngitis occurred in 10–15% of riociguat-treated patients compared with 9–11% of placebo-treated patients [22, 23]. An earlier phase 2 safety and tolerability study found few SAEs and mild AEs, which had all resolved by the time of study completion [31].
In patients experiencing nasopharyngitis or headache, over-the counter (OTC) medications should be used as needed or with a preventive approach. Patients may be offered therapy with analgesics or antihistamines. We have found that 500 mg of acetaminophen 45 min prior to dosing is effective for mitigating headache symptoms. Nasal hydration/irrigation with saline may also provide symptomatic relief. Treatment with nonsteroidal anti-inflammatory medications should be approved by the provider prior to use due to risks associated with fluid retention and renal insufficiency. Patients should be encouraged to make use of OTC therapies in anticipation of side effects during uptitration before side effects become severe. Those with persistent, intolerable side effects of headache or nasopharyngitis may be managed with a riociguat dose reduction.
Gastrointestinal Adverse Events
Gastrointestinal (GI) AEs are associated with many classes of medication and can have a negative impact on adherence if not properly managed. The most commonly observed GI AEs with riociguat in phase 3 trials were dyspepsia (21%), nausea (14%), vomiting (10%), and diarrhea (12%), and these occurred in the placebo group as well (dyspepsia 8%; nausea 11%; vomiting 7%; and diarrhea 8%) [22, 23]. Patients should be assessed to determine whether feelings of nausea correlate with low blood pressure.
Generally, GI AEs in patients taking riociguat are mild to moderate. One patient in CHEST-1 experienced diarrhea that was considered a serious AE and <1% of patients in both CHEST-1 and PATENT-1 discontinued therapy due to GI AEs [17].
Management of GI AEs should be proactive and begin with an inventory of any current medications that may contribute to stomach upset, vomiting, or diarrhea. For example, many patients with PH are on a background regimen that includes calcium channel blockers (which may cause nausea and constipation) and/or aspirin (a known GI irritant) [9]. Consider dose reduction or discontinuation of nonessential medications that may exacerbate GI symptoms in patients taking riociguat.
As GI AEs are well established, they may also be prophylactically managed by initiating therapy with a proton pump inhibitor (PPI) or an H2-antagonist. In light of recent concerns surrounding PPIs and Clostridium difficile, H2-antagonists may pose less risk to patients [32]. If the patient is started on PPI therapy, they should be educated on the signs and symptoms of C. difficile infection, which include fever, chills, and persistent diarrhea with a distinct foul odor. If diarrhea develops, a stool sample and complete blood count should be obtained. Patients currently on therapy with riociguat should be offered OTC treatment with antacids, antidiarrheals, or antinausea agents to be taken as needed. However, patients should be counseled that antacids, such as aluminum hydroxide/magnesium, decrease absorption of riociguat and should be taken either 1 h before or 1 h after dose administration [17].
Peripheral Edema
Peripheral edema is a common side effect of pharmacotherapies that produce a vasodilatory effect. Peripheral edema was observed in 17% of patients taking riociguat 2.5 mg TID in PATENT-1 compared with 11% of patients taking placebo [22]. It should be noted that peripheral edema is a symptom of PAH due to right ventricular failure. Peripheral edema is also a symptom of conditions that can be comorbid with PAH, such as chronic deep venous insufficiency and kidney or liver disease [15]. In CHEST-1, 16% of patients taking riociguat and 20% of patients taking placebo experienced peripheral edema [23].
Peripheral edema can often be addressed with lifestyle modifications. Dietary adjustments, such as reducing sodium and fluid intake, may be helpful. Compression stockings may provide relief and are available in most pharmacies [33]. Body positioning can also improve peripheral edema. Patients should be counseled to elevate their legs above their heart for 30 min 3–4 times daily to reduce swelling [33, 34].
If self-care measures do not improve symptoms, addition or dose increase of diuretic therapy may be warranted. When initiating diuretic therapy, patients should be educated about daily weights and provided strict patient-specific parameters on when to contact the office with weight gain/loss. Diuretics can be effective in managing peripheral edema when regular monitoring of renal function and electrolytes is performed [34]. There is insufficient evidence to recommend loop vs potassium-sparing diuretics, and in some cases, the synergistic effect of both may be needed. If diuretics are ineffective, the evolution of edema should be further evaluated, as it may represent disease progression.
Concomitant Administration with Other PH-Specific Drugs
In addition to the general AEs associated with riociguat, certain AEs are more likely to occur in patients taking other PAH-drugs in combination with riociguat. Use of ERAs, for example, increases the risk of peripheral edema due to reduced renal excretion of sodium and water secondary to endothelin (ET)A/ETB blockade [35]. Patients who take ERAs with riociguat will require more extensive counseling on lifestyle management strategies for peripheral edema and may be appropriate candidates for early initiation or adjustment of diuretics.
Prostacyclin therapy in patients with PH is also associated with a well-established pattern of AEs, including headache and GI disturbances (nausea, vomiting, diarrhea) [36]. As these AEs are similar to those commonly associated with riociguat, patients taking both therapies should be educated on self-care and treatment strategies. Pretreatment with antiemetic or antidiarrheal therapies should be carefully considered in patients taking prostacyclins with riociguat.
Adverse Event Management: the Key to Patient Adherence
Patient support and education are critical factors in promoting adherence to treatment. An effective patient-adherence strategy begins with disease awareness. Research has shown that patients are more motivated to adhere to a prescribed treatment if they recognize its importance [37]. On the other hand, adherence can be negatively impacted by disbelief or general feelings of hopelessness regarding diagnosis [38]. It is, therefore, imperative to engage the patient in dialogue on diagnosis and the rationale for therapy with riociguat. Patients should be counseled that CTEPH and PAH are complex and serious conditions, but innovations in therapy are hel** many patients live longer, more active lives [9, 39, 40].
Setting expectations for therapy, including potential AEs and effective management of same, is another important aspect of patient counseling. Of note, medication AEs themselves have been shown to account for only 5–10% of cases of patient nonadherence; lack of proper medication counseling and poor communication around AEs are more important determinants of nonadherence [37].
Patients started on riociguat should have a clear understanding of the potential AEs they may experience. Providers should discuss strategies to manage potential AEs along with self-care measures. Patients should also be counseled on serious AEs that need be reported to healthcare providers immediately or that require urgent evaluation (e.g., hypotension with syncope, bleeding) [37].
Conclusion
CTEPH and PAH are rare, life-threatening diseases that require chronic management. Recently, riociguat, a novel therapeutic agent that stimulates the sGC enzyme, was approved for the treatment of both conditions [17]. Upon binding, sGC catalyzes conversion of cGMP, which promotes vasodilation, and inhibits smooth-muscle proliferation, platelet aggregation, and vascular remodeling [16, 19].
In large-scale clinical trials, riociguat has been shown to significantly improve exercise capacity as well as hemodynamic parameters in patients with PAH and CTEPH [22, 23]. These clinical benefits were sustained in long-term follow-up studies [39, 40].
Riociguat is generally safe and well tolerated [22, 23]. AEs are both predictable and manageable based on the vasodilatory mechanism of action. The most common AEs observed in clinical trials include headache, nasopharyngitis, GI disturbances, and peripheral edema. Increased rates of hypotension have also been observed. Patients should be reassured that the majority of these AEs are of mild to moderate severity, occur early in the course of treatment, and dissipate over the first few months of therapy. Patients at high risk for certain AEs may be offered preventive treatment or dose adjustment.
Patient education and support help ensure patients are able to achieve the full benefit of therapy with riociguat. Clinicians should work closely with patients to provide clear explanations of therapy and create treatment plans. AEs should be reviewed with all patients before starting therapy and monitored throughout treatment, with supportive-care strategies offered. Involving family and caregivers in treatment monitoring and connecting patients with outside support resources also increase the likelihood of therapeutic success.
References
Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34–41.
Hoeper MM, Bogaard JH, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 suppl):D42–50.
Gailè N, Humbert M, Vachiery J-L, et al. Joint task force for the diagnosis and treatment of pulmonary hypertension of the European society of cardiology (ESC)and the European respiratory society (ERS). 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2016;37:67–119.
McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension. A report of the American college of cardiology foundation task force on expert consensus documents and the American heart association. Circulation. 2009;119(17):2250–94.
Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL registry. Chest. 2010;137(2):376–87.
Kim NH, Delcroix M, Jenkins DP, et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol. 2013;25(25 suppl D):D92–9.
McNeil K, Dunning J. Chronic thromboembolic pulmonary hypertension (CTEPH). Heart. 2007;93(9):1152–8.
Piazza G, Goldhaber SZ. Chronic thromboembolic pulmonary hypertension. N Engl J Med. 2011;364(4):351–60.
McLaughlin VV, Gaine SP, Howard LS, et al. Treatment goals of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 suppl D):D73–81.
Taichman DB, Ornelas J, Chung L, Klinger JR, Lewis S, Mandel J, Palevsky HI, Rich S, Sood N, Rosenzweig EB, Trow TK, Yng R, Elliott G, Badesch DB. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest. 2014;146(2):449–75.
Tiede H, Hinzmann B, Bawden N, Preston I. Management of chronic thromboembolic pulmonary hypertension: a physician-based perception study. Presented at the European respiratory society annual congress. 2012; Austria. Abstract. P3282.
Mayer E, Jenkins D, Lindner J, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg. 2011;141(3):702–10.
Seyfarth HJ, Halank M, Wilkens H, et al. Standard PAH therapy improves long-term survival in CTEPH patients. Clin Res Cardiol. 2010;99(9):553–6.
Ignarro LJ, Buga GM, Wood KS, et al. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA. 1987;84(24):9265–9.
McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006;114(13):1417–31.
Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation. 2011;123(20):2263–73.
Adempas® [package insert], Whippany, NJ. Bayer HealthCare Pharmaceuticals Inc; 2017.
Schermuly RT, Stasch JP, Pullamsetti SS, et al. Expression and function of soluble guanylate cyclase in pulmonary arterial hypertension. Eur Respir J. 2008;32(4):881–91.
Stasch JP, Evgenov OV. Soluble guanylate cyclase stimulators in pulmonary hypertension. Handb Exp Pharmacol. 2013;218:279–313.
Follmann M, Griebenow N, Hahn MG, et al. The chemistry and biology of soluble guanylate cyclase stimulators and activators. Angew Chem Int Ed Engl. 2013;52(36):9442–62.
Stasch JP, Hobbs AJ. NO-independent, haem-dependent soluble guanylate cyclase stimulators. Handb Exp Pharmacol. 2009;191:277–308.
Ghofrani HA, Galiè N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369(4):330–40.
Ghofrani HA, D’Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369(4):319–29.
Becker C, Frey R, Hesse C, Unger S, Reber M, Mueck W. Absorption behavior of riociguat: bioavailability, food effects, and dose-proportionality. BMC Pharmacol Toxicol. 2013;14(suppl 1):P7.
Khaybullina D, Patel A, Zerilli T. Riociguat (adempas): a novel agent for the treatment of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Pharm Ther. 2014;39(11):749–58.
Saleh S, Frey R, Becker C, Unger S, Wensing G, Mück W. Bioavailability, pharmacokinetics, and safety of riociguat given as an oral suspension or crushed tablet with and without food. Pulm Circ. 2016;6(S1):S66–74.
Frey R, Becker C, Unger S, et al. Pharmacokinetics of the soluble guanylate cyclase stimulator riociguat in individuals with renal impairment. BMC Pharmacol Toxicol. 2013;14(sSuppl 1):22.
Frey R, Becker C, Unger S, et al. Pharmacokinetics of the soluble guanylate cyclase inhibitor riociguat in individuals with hepatic impairment. BMC Pharmaol Toxicol. 2013;14(suppl 1):P21.
Frey R, Muck W, Kirschbaum N, Kratzschmar J, Weimann G, Wensing G. Riociguat (BAY 63-2521) and warfarin: a pharmacodynamic and pharmacokinetic interaction study. J Clin Pharmacol. 2011;51(7):1051–60.
Bagdy G, Riba P, Kecskeméti V, Chase D, Juhász G. Headache-type adverse effects of NO donors: vasodilation and beyond. Br J Pharmacol. 2010;160(1):20–35.
Ghofrani HA, Hoeper MM, Halank M, et al. Riociguat for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension. A phase II study. Eur Respir J. 2010;36(4):792–9.
McDonald EG, Milligan J, Frenette C, Lee TC. Continuous proton pump inhibitor therapy and the associated risk of Clostridium difficile infection. JAMA Intern Med. 2015;175(5):784–91.
O’Brien JG, Chennubhotla SA, Chennubhotla RV. Treatment of edema. Am Fam Physician. 2005;71(11):2111–7.
Cho S, Atwood JE. Peripheral edema. Am J Med. 2002;113(7):580–6.
Baltatu OC, Iliescu R, Zaugg CE, et al. Antidiuretic effects of the endothelin receptor antagonist avosentan. Front Physiol. 2012;3:1–7.
LeVarge BL. Prostanoid therapies in the management of pulmonary arterial hypertension. Ther Clin Risk Manag. 2015;11:535–47.
Vermeire E, Hearnshaw H, Van Royen P, Denekens J. Patient adherence to treatment: three decades of research. A comprehensive review. J Clin Pharm Ther. 2001;26(5):331–42.
World Health Organization. Adherence to long-term therapies. Evidence for action. Switzerland: World Health Organization; 2003.
Simonneau G, D’Armini A, Ghofrani H-A, et al. Predictors of long-term outcomes in patients treated with riociguat for chronic thromboembolic pulmonary hypertension: data from the CHEST-2 open-label, randomised, long-term extension trial. Lancet Respir Med. 2016;4:372–80.
Ghofrani H-A, Grimminger F, Grünig E, et al. Predictors of long-term outcomes in patients treated with riociguat for pulmonary arterial hypertension: data from the PATENT-2 open-label, randomised, long-term extension trial. Lancet Respir Med. 2016;4:361–71.
Acknowledgements
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. Medical writing and editing assistance were provided by Katrina Rodies, CRNP, and Adelphi communications and were funded, as were article processing charges, by Bayer HealthCare Pharmaceuticals, Whippany, NJ, USA.
Compliance with Ethics Guidelines
This article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors.
Disclosures
Judy Germani serves on advisory boards for Bayer HealthCare and Gilead. Martha Kingman has served as a speaker and advisory board member for Bayer HealthCare outside of the submitted work. Natalie Kitterman reports personal fees from Bayer HealthCare, Gilead, Simply Speaking, and United Therapeutics outside of the submitted work. Traci Stewart reports personal fees from Actelion, Bayer HealthCare, and Gilead outside of the submitted work. Melisa Wilson serves on advisory boards for Bayer HealthCare and United Therapeutics outside of the submitted work. Debra Zupancic has nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/6A87F060195284FA.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Germani, J., Kingman, M., Kitterman, N. et al. Riociguat in PAH and CTEPH: Strategies for Patient Management. Pulm Ther 3, 31–43 (2017). https://doi.org/10.1007/s41030-017-0029-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41030-017-0029-3