Abstract
SARS-CoV-2 is a newly emerged coronavirus that has been widely transmitted since late 2019. It has caused a pandemic and infected roughly 450 million people globally.Hitherto, there is no approved anti-COVID-19 treatment, and vaccination is the only experienced preventive strategy. It mainly promotes the immune system, which is vital as a barrier against COVID-19. Humoral immunity (antibody-mediated immunity), among the various functions of the immune system against the coronavirus, plays an outstanding role in preventing infection. Consequently, we intended to assess IgG and IgM antibodies, 3 and 6 months after infection, to trend their titer and see how long COVID-19 antibodies remained in the human body. According to the research-designed criteria, only 98 patients out of 4500 suspected cases of SARS-CoV-2 infection remained for analysis. Blood samples were taken in three time periods (Day Zero (T0), 3 and 6 months post-infection) and examined for COVID-19’s IgG and IgM antibodies titration using the ELISA platform. Though both IgG and IgM were still detectable for some subjects at the end of the period, the decline in their levels (from 14.45 ± 5.88 to 2.52 ± 2.33 for IgG [85% decline of antibody titer] and 8.3 ± 0.99 to 0.37 ± 0.14 for IgM [95.5% decline of antibody titer]) was statistically significant (P value 0.0001). There was no correlation between gender and IgG and IgM levels. Although the levels of both antibodies were overall higher in the senior group (≥ 60 years old), statistical analysis showed a significantly higher level just for IgM in this group (P value: 0.005). Following the results, although anti-SARS-CoV-2 IgM and IgG antibodies can persist in the blood for 6 months post-infection, their levels steeply declined over time. Therefore, relying on humoral immunity as a trustworthy barrier against SARS-CoV-2 infection calls for more extensive research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
COVID-19 is a horrible pandemic spread worldwide, resulting in hundreds of millions of infected cases and millions of fatalities. The disease initially appeared in Wuhan, China, spread quickly, and eventually became a pandemic (Lu et al. 2020; Zhou et al. 2020). Fever, dry cough, and exhaustion are the most common symptoms of this disease. A large number of infected individuals get severe pneumonia, and some develop acute respiratory distress syndrome (ARDS) (Huang et al. 2020; Liu et al. 2020a, b).
A real-time reverse transcription-polymerase chain reaction (qRT-PCR) test utilizing nasopharyngeal and oropharyngeal swabs is currently the standard diagnosis technique for COVID-19. Although qRT-PCR plays an essential role in detecting this condition, it does have certain drawbacks. For example, in a research conducted in Wuhan, China, on 4880 cases with COVID-19 symptoms or who had close contact with patients, the positive qRT-PCR rate for COVID-19 was 38%, with a high false-negative response (Liu et al. 2020a, b).
There is a significant false-negative rate due to the challenges of obtaining good-quality swab samples at different stages of the disease and the qRT-PCR test’s troubleshooting. Regarding these milestones, identifying and quarantining infected individuals and breaking the dissemination cycle of this highly contagious disease will be difficult. Antibodies, on the other hand, are among the most critical elements in diagnosing and treating diseases.
Antibody assays have a significant benefit over qRT-PCR so that they can identify people who had SARS-CoV-2 infection even if they have never undergone testing while acutely ill (Sidiq et al. 2020). Serological tests can be completed more quickly, cheaply, and with fewer steps than molecular approaches (Lee et al. 2020; Wolff et al. 2020).
Nucleic acid amplification tests (NAATs) can be performed in conjunction with serological diagnostics when the viral load of patients is below the detection limit of qRT-PCR assays (Wolff et al. 2020; To et al. 2020).
Furthermore, serological tests can be practical in the following situations: (1) diagnosing patients with negative qRT-PCR results and strong clinical evidence suggesting infection, (2) diagnosis of patients more than 1 week after the onset of symptoms, and (3) figuring out the potential immunity and the likelihood of protection against a reinfection (Rastawicki and Rokosz-Chudziak 2020; GeurtsvanKessel et al. 2020; Lassaunière et al. 2020; Wang et al. 2020).
IgM is the earliest immunoglobulin raised against viral invasion. Then IgG levels begin to grow, with higher specificity and viral neutralizing activity (Dispinseri et al. 2021). The antibody assay is a confirmed test to detect the presence and trend of COVID-19 disease. Li et al. (2020) developed a 15-min point-of-care lateral flow immunoassay to detect IgM and IgG in human blood. According to their primary research, the total testing sensitivity and specificity were 88.66, and 90.63%, respectively (Li et al. 2020).
Some studies have revealed that IgM and IgG antibodies against SARS-CoV-2 rise nearly four days after the appearance of the COVID-19 symptoms and peak one month later (Song et al. 2021; **e et al. 2020). However, there is a lack of concepts regarding how long these antibodies are sustainable. This study intended to evaluate serum levels’ trend of anti-SARS-CoV-2 IgM/IgG over 6 months.
2 Methods
2.1 Patient Enrollment
Between May 1, 2020, and June 1, 2021, all referring cases to the laboratory for COVID-19 were asked to sign the consent form for study and publication. Patients have the right to refuse to sign the consent form; refusal to sign this form will not affect their care in any way.
Passing the exclusion/inclusion criteria, all individuals with qRT-PCR-confirmed COVID-19 disease participated in this longitudinal observational study. Blood samples were taken from patients as part of the routine protocol. The baseline of IgM and IgG immunoglobulins against COVID-19-specific proteins was tested for all included patients for the first time (Day Zero (T0)), and IgM and IgG levels were again tested three and 6 months later.
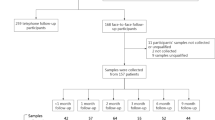
Regarding three steps of exclusion with reasons (vaccinated individuals, pregnant patients, those with reinfection, patients with comorbidities, consumption of medications impacting the immune system, such as cancer and chemotherapy, etc.), the data of 98 individuals remained and qualified to be analyzed (Fig. 1. Flowchart of patients’ enrolment). The Biomedical Research Ethics Committee of Baqiyatallah University of Medical Sciences, Tehran, Iran, approved the study under the code IR.BMSU.REC.1399.068 (https://ethics.research.ac.ir).
Flowchart of patient's enrolment, follow-up, and analysis. Excluded cases with reason are observed in the right boxes. Considering the exclusion criteria, the data of 98 individuals were qualified for analysis. The first step of exclusion with reason referred to individuals with reinfections, immunological comorbidities, getting vaccinated, suspected IgG titer (IgG ≤ 1.1), etc. The second step of exclusion with reason referred to the participants who were reinfected by SARS-CoV-2, vaccine receivers, pregnant females, and people who decided not to follow the investigation. An individual was excluded for a reason specified by the out-layer data after retest (suspected of latent comorbidity)
2.2 Analysis and Instrumentation
Samples were evaluated using the ELISA platform to explore the anti-SARS-CoV-2 IgG (indirect ELISA) and IgM (capture antibody) antibodies following the manufacturer manual (PT-SARS-CoV-2, Pishtaz Teb Co.) (Fig. 2). Reported sensitivity and specificity of this kit claimed to be 94.1% and 98.3% for IgG and 85.4% and 99.4% for IgM, respectively. According to the kit manual, index values higher than 1.1 were considered positive, and below 0.9 were considered negative cases for both IgG and IgM. Cases whose index values were between 0.9 and 1.1 were considered suspicious.
Serological assays of patients’ blood samples with confirmed COVID-19. To test IgG titer (upper array), anti-SARS-CoV-2 IgG antibodies in the plasma of COVID-19 patients bind to the SARS-CoV-2 N antigen-coated at the bottom of the wells. This complex is detected by antihuman IgG antibody-HRP. A capture antibody approach is recruited through an enzymatic color reaction For IgM assay (lower array). Antihuman IgM antibodies coated at the bottom of the wells capture anti-SARS-CoV-2 IgM antibodies. This complex is detected by SARS-CoV-2 N/S antigen-HRP through an enzymatic color reaction. Figure has been created with BioRender.com
Additionally, the CRP latex test was created to quickly and accurately detect and measure C-reactive protein (CRP). It is a fast slide agglutination technique based on a serum modification of the latex fixing method. Accordingly, the increase in the CRP levels above the upper limit of the reference was indicated by the presence of an apparent agglutination in the samples and patients’ CRP levels were shown to be a marker of COVID-19 severity (Fig. 3).
2.3 Statistical Analysis
SPSS Statistics V22.0 was used to analyze the collected data. The difference between IgM and IgG positive levels was assessed using the Chi-square test. The data were presented using the mean and standard deviation. For normally distributed data, the t-test was employed, and for data with a non-normal distribution, the Mann–Whitney U test was utilized. To compare qualitative variables, a Chi-square test was used. The statistical significance criterion was chosen at a P value of less than 0.05 (statistical abbreviations; Df, degrees of freedom; F, F value; P: P value).
3 Results
3.1 Demographic Data and CRP Levels
Over the study time, 4500 patients referred to the laboratory for COVID-19 testing. After 3 months, 1254 cases remained meeting the inclusion criteria. At the end of the follow-up, the research comprised a total of 98 individuals. In this study, more than 1000 cases got vaccinated and were ruled out of the study (Fig. 1). The patients varied in age from 4 to 83 years old, with an average of 49.67 ± 14.6 years. Males accounted for 61.2% of the cases, while females accounted for 38.8%. The CRP testing was negative in 27.55% while strongly positive in 13.27% of the subjects (Fig. 3).
3.2 Comparison of IgG Levels at Different Time Intervals
According to the findings of repeated observational analysis, the mean IgG level over time was significantly different (Df 2.96, F 544.59, P = 0.001). So, after data correction for patients’ age and gender, the difference in IgG levels at different time intervals was statistically significant. The positive rate dropped down to 99% at 3 and 66.3% at 6 months post-infection (P = 0.001). (Tables 1, 2; Fig. 4). Furthermore, no significant association between gender and IgG levels was seen at various time intervals. Additionally, there was no significant association between age and average IgG levels throughout the time intervals (Df 1, F 2.208, P 0.141). In other words, there was no noticeable impact of patient age on IgG levels at different time intervals, as seen in Fig. 5.
Comparison of IgG levels at different time intervals. The overall trend was significantly descending. At the initial phase (day zero (T0)), the highest amount of IgG was recorded (14.455). After 3 months, this amount dropped to 7.49, and eventually, after 6 months, it decreased to 2.52. #Significant value in comparison with third period. *Significant value in comparison with other two periods
The effect of age on the mean IgG levels at different time intervals. T0 stands for the Day Zero of testing. < 60 refers to individuals under 60 years old. ≥ 60 relates to individuals 60 or over 60 years old. The total mean and trend didn't demonstrate any significant interaction between age and IgG levels
3.3 Comparison of IgM Levels at Different Time Intervals
The average level of IgM over the study time was significantly different (Df 2.96, F 84.34, P = 0.001). In light of the results, after data correction for the patients of different ages and genders, the diversity in IgM levels at different time intervals was statistically significant. So that the positive rate dropped down to 1% at 3 and 6 months post-infection (P = 0.001) (Tables 3, 4; Fig. 6). Meanwhile, at different periods, a significant association between age and IgM levels was found (Df 1, F 8.18, P 0.005). In other words, there was a noticeable impact of patient age on IgM levels at different time intervals. According to the graph, patients aged over 60 had a higher starting IgM level, whereas those under 60 had a higher average IgM level in the second and third periods (Fig. 7).
Comparison of IgM levels at different time intervals. The highest average level of IgM was recorded at the beginning of the study (T0: 8.30). After 3 and 6 months, the IgM level decreased to 0.43 and 0.37, respectively. The overall trend shows a significant decline in IgM levels. #Significant value in comparison with the third period. *Significant value in comparison with the other two periods
4 Discussion
Antibody responses to SARS-CoV-2 in 1–3 months have been extensively investigated, while long-term (6 months or more) are still little studied. The durability of IgG and IgM antibody levels against SARS-CoV-2 at a 6-month interval was investigated in this study. Likewise, the manner of age (over and less than 60) was then evaluated. In this regard, tough exclusion criteria such as reinfection, immunologic comorbidities, and vaccination were considered reaching precise results. Moreover, suspicious cases (IgG ≤ 1.1), follow-up failing, and pregnancy were considered exclusion criteria.
The outstanding highlight of the present research is that such a study will be difficult to undertake in the future due to the near-impossibility of finding the target population since the target population should be infected for the first time, have not been vaccinated, and should not be reinfected throughout the study, in addition to fulfilling general admission criteria. As a result, given the worldwide scope of the COVID-19 pandemic and the wide range of available vaccines, locating a qualified population will be difficult.
During this study, in addition to measuring the IgG/IgM level, the CRP level was also determined. CRP levels may predict the severity of the disease and lung damage at the beginning of COVID-19. According to Liu et al. (2020a, b), patients with COVID-19 had a 65% increase in CRP. According to the studies with COVID-19 axis, CRP levels are significantly different between the deceased party and the recovered population, which could serve as a predictive indicator of disease severity (Ruan et al. 2020; Agache et al. 2021). CRP is an exemplary assay for evaluating patient conditions in conjunction with other clinical findings because it indicates a strong correlation between patient severity and COVID-19 patient symptoms. In the present study, 72.45% of patients showed positive CRP, which is nearly consistent with other reports.
Our findings showed that the IgG levels for 66.3% of patients were positive even after 6 months but eventually decreased to one-sixth of their beginning levels (from 14.45 ± 5.88 to 2.52 ± 2.33 for IgG [85% decline in antibody titer]). These are consistent with Luo's research, which looked at average IgG/IgM antibody titers against SARS-CoV-2 antibodies from symptom onset to 210 days. They discovered that typical titers peaked within 30 days of symptom onset and then steadily decreased to one-fifth at 180 and 210 days (Luo et al. 2021).
In the present study, the positive rate for IgG dropped to 99% at 3 and 66.3% at 6 months post-infection. It was more dramatic for IgM, so its positive rate dropped down to 1% at 3 and 6 months post-infection. Maine et al. (2020) found that after 3 months of the onset of symptoms, the positive rate of IgM drops to 30.8%. On the other hand, IgG levels maintained positive in 92.3% of patients 3–6 months after the beginning of symptoms (Maine et al. 2020), which is consistent with the findings of the current investigation.
Another study conducted on 54 SARS-CoV-2-positive people reported that after reaching a peak in IgG titers on day 60, they steadily declined to 50% on day 180. There was no correlation between IgG titer and age, sex, or comorbidities in their research, although patients with acute and severe symptoms had much higher IgG levels (De Donno et al. 2021), while we observed a steeper decline in IgG titer and discovered a significant correlation between age and IgM but not with IgG antibody levels. Since antibody titers and plasma neutralization activity have been shown to correlate with disease severity, the rapid decline in antibody rate in our cohort may be related to the mild to moderate symptoms verbally reported by individuals. On the other side, Luo et al. (2021) showed a significant correlation between IgG and IgM antibody levels with age and illness severity but not with patient sex.
One of the elements influencing the humoral immune response is antigen persistence in the patients’ bodies (Rezaie et al. 2019). The median length of viral persistence is generally greater in older individuals and patients with severe symptoms. Because of this phenomenon, people exposed to SARS-CoV-2 antigens for prolonged periods, maintain higher antibody levels. As previously shown, the initial IgM titer in over 65-year-old participants is higher than others, but the decay rate is quicker (Luo et al. 2021; Menon et al. 2021); we have also seen this trend for IgM in our investigations.
It’s debatable, but there’s been evidence of a correlation between age, fever, upper respiratory symptoms, gastrointestinal (GI) symptoms, symptoms duration, and elevated anti-Spike titers (Boonyaratanakornkit et al. 2020). Menon et al. discovered a correlation between the total number of symptoms, fever, GI manifestations, and IgG decay rate. Patients with the symptomatic disease had a greater antibody response and a slower decay rate than others (Menon et al. 2021). Here, we had only the data for the age and gender of the subjects. Therefore addressing different aspects of antibody decay rates was not possible. However, there is no guarantee that a greater antibody level or a slower degradation rate would result in stronger neutralizing effects. The neutralizing antibodies (NAbs) rate against SARS-CoV-2 is decisive and determines the strength of the immunological defence against COVID-19. The lack of neutralizing antibody levels measurement is likely the most important defect of the present study, which calls for more exclusive research.
5 Conclusion
The tracking of COVID-19 antibodies durability in eligible volunteers after infection demonstrated that their levels declined significantly over 6 months. It was also concluded that IgG and IgM levels do not depend on gender. However, older individuals (over 60 years old) had somewhat higher levels of IgM levels at the beginning of infection and lower levels 6 months after infection.
References
Agache I, Song Y, Posso M, Alonso-Coello P, Rocha C, Solà I, Beltran J, Akdis CA, Akdis M, Brockow K (2021) Efficacy and safety of dupilumab for moderate-to-severe atopic dermatitis: a systematic review for the EAACI Biologicals Guidelines. Allergy 76:45–58
Boonyaratanakornkit J, Morishima C, Selke S, Zamora D, McGuffin S, Shapiro AE, Campbell VL, McClurkan CL, **g L, Gross R (2020) Clinical, laboratory, and temporal predictors of neutralizing antibodies to SARS-CoV-2 after COVID-19. MedRxiv. https://doi.org/10.1101/2020.10.06.20207472
De Donno A, Lobreglio G, Panico A, Grassi T, Bagordo F, Bozzetti MP, Massari S, Siculella L, Damiano F, Guerra F (2021) IgM and IgG profiles reveal peculiar features of humoral immunity response to SARS-CoV-2 infection. Int J Environ Res Public Health 18:1318
Dispinseri S, Secchi M, Pirillo MF, Tolazzi M, Borghi M, Brigatti C, De Angelis ML, Baratella M, Bazzigaluppi E, Venturi G (2021) Neutralizing antibody responses to SARS-CoV-2 in symptomatic COVID-19 is persistent and critical for survival. Nat Commun 12:1–12
GeurtsvanKessel CH, Okba N, Igloi Z, Bogers S, Embregts CW, Laksono BM, Leijten L, Rokx C, Rijnders B, Rahamat-Langendoen J (2020) An evaluation of COVID-19 serological assays informs future diagnostics and exposure assessment. Nat Commun 11:1–5
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, **a J, Wei Y, Wu W, **e X, Yin W, Li H, Liu M, **ao Y, Gao H, Guo L, **e J, Wang G, Jiang R, Gao Z, ** Q, Wang J, Cao B (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506. https://doi.org/10.1016/s0140-6736(20)30183-5
Lassaunière R, Frische A, Harboe ZB, Nielsen AC, Fomsgaard A, Krogfelt KA, Jørgensen CS (2020) Evaluation of nine commercial SARS-CoV-2 immunoassays. MedRxiv
Lee CY-P, Lin RT, Renia L, Ng LF (2020) Serological approaches for COVID-19: epidemiologic perspective on surveillance and control. Front Immunol 11:879
Li Z, Yi Y, Luo X, **ong N, Liu Y, Li S, Sun R, Wang Y, Hu B, Chen W, Zhang Y, Wang J, Huang B, Lin Y, Yang J, Cai W, Wang X, Cheng J, Chen Z, Sun K, Pan W, Zhan Z, Chen L, Ye F (2020) Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol 92:1518–1524. https://doi.org/10.1002/jmv.25727
Liu R, Han H, Liu F, Lv Z, Wu K, Liu Y, Feng Y, Zhu C (2020a) Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin Chim Acta 505:172–175. https://doi.org/10.1016/j.cca.2020.03.009
Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, Wang Z, Li J, Li J, Feng C, Zhang Z, Wang L, Peng L, Chen L, Qin Y, Zhao D, Tan S, Yin L, Xu J, Zhou C, Jiang C, Liu L (2020b) Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 63:364–374. https://doi.org/10.1007/s11427-020-1643-8
Lu H, Stratton CW, Tang YW (2020) Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol 92:401–402. https://doi.org/10.1002/jmv.25678
Luo C, Liu M, Li Q, Zheng X, Ai W, Gong F, Fan J, Liu S, Wang X, Luo J (2021) Dynamic changes and prevalence of SARS-CoV-2 IgG/IgM antibodies: analysis of multiple factors. Int J Infect Dis 108:57–62
Maine GN, Lao KM, Krishnan SM, Afolayan-Oloye O, Fatemi S, Kumar S, VanHorn L, Hurand A, Sykes E, Sun Q (2020) Longitudinal characterization of the IgM and IgG humoral response in symptomatic COVID-19 patients using the Abbott Architect. J Clin Virol 133:104663
Menon V, Shariff MA, Gutierrez VP, Carreño JM, Yu B, Jawed M, Gossai M, Valdez E, Pillai A, Venugopal U (2021) Longitudinal humoral antibody response to SARS-CoV-2 infection among healthcare workers in a New York City hospital. BMJ Open 11:e051045
Rastawicki W, Rokosz-Chudziak N (2020) Characteristics and assessment of the usefulness of serological tests in the diagnostic of infections caused by coronavirus SARS-CoV-2 on the basis of available manufacturer’s data and literature review. Przegl Epidemiol 74:49–68
Rezaie E, Nekoie H, Miri A, Oulad G, Ahmadi A, Saadati M, Bozorgmehr M, Ebrahimi M, Salimian J (2019) Different frequencies of memory B-cells induced by tetanus, botulinum, and heat-labile toxin binding domains. Microb Pathog 127:225–232
Ruan Q, Yang K, Wang W, Jiang L, Song J (2020) Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 46:846–848
Sidiq Z, Hanif M, Dwivedi KK, Chopra K (2020) Benefits and limitations of serological assays in COVID-19 infection. Indian J Tuberculosis 67:S163–S66
Song KH, Kim DM, Lee H, Ham SY, Oh SM, Jeong H, Jung J, Kang CK, Park JY, Kang YM, Kim JY, Park JS, Park KU, Kim ES, Kim HB (2021) Dynamics of viral load and anti-SARS-CoV-2 antibodies in patients with positive RT-PCR results after recovery from COVID-19. Korean J Intern Med 36:11–14. https://doi.org/10.3904/kjim.2020.325
To KK-W, Tsang OT-Y, Leung W-S, Tam AR, Wu T-C, Lung DC, Yip CC-Y, Cai J-P, Chan JM-C, Chik TS-H (2020) Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 20:565–574
Wang H, Ai J, Loeffelholz MJ, Tang Y-W, Zhang W (2020) Meta-analysis of diagnostic performance of serology tests for COVID-19: impact of assay design and post-symptom-onset intervals. Emerg Microbes Infect 9:2200–2211
Wolff F, Dahma H, Duterme C, Van den Wijngaert S, Vandenberg O, Cotton F, Montesinos I (2020) Monitoring antibody response following SARS-CoV-2 infection: diagnostic efficiency of 4 automated immunoassays. Diagn Microbiol Infect Dis 98:115140
**e J, Ding C, Li J, Wang Y, Guo H, Lu Z, Wang J, Zheng C, ** T, Gao Y, He H (2020) Characteristics of patients with coronavirus disease (COVID-19) confirmed using an IgM-IgG antibody test. J Med Virol 92:2004–2010. https://doi.org/10.1002/jmv.25930
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, **ao GF, Shi ZL (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273. https://doi.org/10.1038/s41586-020-2012-7
Acknowledgements
The authors would like to thank the Clinical Research Development Unit of Baqiyatallah Hospital, Tehran, Iran, for guidance and advice.
Funding
The authors received no funds, grants, or other support for this study.
Author information
Authors and Affiliations
Contributions
MH and DG designed and supervised the study. FA, OM, NG, AHK, ER, SM, and RH collected the data and drafted the manuscript; RM analyzed and interpreted the data and edited the manuscript. MB analyzed and interpreted the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghasemi, D., Araeynejad, F., Maghsoud, O. et al. The Trend of IgG and IgM Antibodies During 6-Month Period After the Disease Episode in COVID-19 Patients. Iran J Sci Technol Trans Sci 46, 1555–1562 (2022). https://doi.org/10.1007/s40995-022-01382-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-022-01382-7