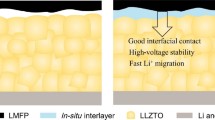
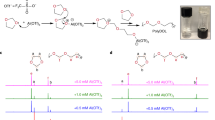
Abstract
Solid polymer electrolyte (SPE) is a potential material for the next-generation safe battery system. However, the inability of SPEs to maintain mechanical strength and ionic conductivity is a bottleneck in further research. Here, a poly(ether-thioether) electrolyte with a thiol-ene crosslinked network was prepared by in situ click polymerization and supported on an electro-spun polyimide (PI) mat to provide an ultrathin membrane (19 µm in thickness). Combining a thiol-ene crosslinked network and the reinforcement of the electro-spun PI mat, this SPE membrane obtains high storage modulus (135 MPa), high ionic conductivity (2.24 × 10−4 S cm−1), and wide electrochemical window (up to 4.0 V) at 60°C. In addition, the Li-Li symmetrical cell based on the as-prepared electrolyte demonstrates stable cycling performance during Li plating/strip**, lasting more than 800 h at 0.1 mA cm−2. Such LiFePO4/Li cells with an ultrathin thiol-ene network SPE membrane achieve over 250-cycle stability at 0.5 C and 60°C. This work develops a new ultrathin polymer electrolyte for stable solid-state and high-performance lithium metal batteries.

摘要
固态聚合物电解质(SPE)是下一代安全电池系统的潜在材料, 但 SPE不能同时保持较高的机械**度和离子传导率, 使下一步研究进入瓶 颈. 在此, 我们通过原位点击反应在静电纺丝聚酰亚胺(PI)膜上制备了 一种具有交联结构的聚醚硫醚电解质, 厚度仅为19 μm. 由于烯-巯网络 的交联结构和静电纺丝PI膜的增**作用, 该SPE膜在60°C下具有 135 MPa的储能模量, 2.24 × 10−4 S cm−1的离子传导率和4.0 V的电化学 稳定窗口, 并且锂-锂对称电池在0.1 mA cm−2下循环超过800 h, 展现出 了优异的循环稳定性. 用该超薄聚合物电解质膜组装的LiFePO4/Li电池 在60°C, 0.5 C下能够循环超过250圈. 这项工作开发了一种用于固态高 性能锂金属电池的新型聚合物电解质.
Similar content being viewed by others
References
Zhou Q, Ma J, Dong S, et al. Intermolecular chemistry in solid polymer electrolytes for high-energy-density lithium batteries. Adv Mater, 2019, 31: 1902029
Cui Y, Wan J, Ye Y, et al. A fireproof, lightweight, polymer-polymer solid-state electrolyte for safe lithium batteries. Nano Lett, 2020, 20: 1686–1692
Zhou B, Yang M, Zuo C, et al. Flexible, self-healing, and fire-resistant polymer electrolytes fabricated via photopolymerization for all-solid-state lithium metal batteries. ACS Macro Lett, 2020, 9: 525–532
Song Y, Liu X, Ren D, et al. Simultaneously blocking chemical crosstalk and internal short circuit via gel-stretching derived nanoporous non-shrinkage separator for safe lithium-ion batteries. Adv Mater, 2022, 34: 2106335
Vishnugopi BS, Kazyak E, Lewis JA, et al. Challenges and opportunities for fast charging of solid-state lithium metal batteries. ACS Energy Lett, 2021, 6: 3734–3749
** G, **ao M, Wang S, et al. Polymer-based solid electrolytes: Material selection, design, and application. Adv Funct Mater, 2021, 31: 2007598
Tan DHS, Banerjee A, Chen Z, et al. From nanoscale interface characterization to sustainable energy storage using all-solid-state batteries. Nat Nanotechnol, 2020, 15: 170–180
Liu H, Cheng XB, Huang JQ, et al. Controlling dendrite growth in solid-state electrolytes. ACS Energy Lett, 2020, 5: 833–843
Cui G, Tominaga Y. Polymer electrolytes toward next-generation batteries. Macro Chem Phys, 2022, 223: 2200013
Zhao Q, Stalin S, Zhao CZ, et al. Designing solid-state electrolytes for safe, energy-dense batteries. Nat Rev Mater, 2020, 5: 229–252
Famprikis T, Canepa P, Dawson JA, et al. Fundamentals of inorganic solid-state electrolytes for batteries. Nat Mater, 2019, 18: 1278–1291
Balaish M, Gonzalez-Rosillo JC, Kim KJ, et al. Processing thin but robust electrolytes for solid-state batteries. Nat Energy, 2021, 6: 227–239
Fan L, Wei S, Li S, et al. Recent progress of the solid-state electrolytes for high-energy metal-based batteries. Adv Energy Mater, 2018, 8: 1702657
Li S, Zhang S, Shen L, et al. Progress and perspective of ceramic/polymer composite solid electrolytes for lithium batteries. Adv Sci, 2020, 7: 1903088
Xue Z, He D, **e X. Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J Mater Chem A, 2015, 3: 19218–19253
**ao Y, Wang Y, Bo SH, et al. Understanding interface stability in solid-state batteries. Nat Rev Mater, 2019, 5: 105–126
Cheng J, Hou G, Sun Q, et al. A novel coral-like garnet for high-performance PEO-based all solid-state batteries. Sci China Mater, 2022, 65: 364–372
Sarapas JM, Tew GN. Poly(ether-thioethers) by thiol-ene click and their oxidized analogues as lithium polymer electrolytes. Macromolecules, 2016, 49: 1154–1162
Yue L, Ma J, Zhang J, et al. All solid-state polymer electrolytes for high-performance lithium ion batteries. Energy Storage Mater, 2016, 5: 139–164
Zhong L, Wang S, **ao M, et al. Addressing interface elimination: Boosting comprehensive performance of all-solid-state Li-S battery. Energy Storage Mater, 2021, 41: 563–570
Lv Z, Zhou Q, Zhang S, et al. Cyano-reinforced in-situ polymer electrolyte enabling long-life cycling for high-voltage lithium metal batteries. Energy Storage Mater, 2021, 37: 215–223
Xu R, **ao B, Xuan C, et al. Facile and powerful in situ polymerization strategy for sulfur-based all-solid polymer electrolytes in lithium batteries. ACS Appl Mater Interfaces, 2021, 13: 34274–34281
Liu Y, Zhao Y, Lu W, et al. PEO based polymer in plastic crystal electrolytes for room temperature high-voltage lithium metal batteries. Nano Energy, 2021, 88: 106205
Fan LZ, He H, Nan CW. Tailoring inorganic-polymer composites for the mass production of solid-state batteries. Nat Rev Mater, 2021, 6: 1003–1019
Zhang B, Zhang Y, Zhang N, et al. Synthesis and interface stability of polystyrene-poly(ethylene glycol)-polystyrene triblock copolymer as solid-state electrolyte for lithium-metal batteries. J Power Sources, 2019, 428: 93–104
Meabe L, Huynh TV, Mantione D, et al. UV-cross-linked poly(ethylene oxide carbonate) as free standing solid polymer electrolyte for lithium batteries. Electrochim Acta, 2019, 302: 414–421
Zhou B, Jiang J, Zhang F, et al. Crosslinked poly(ethylene oxide)-based membrane electrolyte consisting of polyhedral oligomeric silsesquioxane nanocages for all-solid-state lithium ion batteries. J Power Sources, 2020, 449: 227541
Wang Q, Dong T, Zhou Q, et al. An in-situ generated composite solid-state electrolyte towards high-voltage lithium metal batteries. Sci China Chem, 2022, 65: 934–942
Wen P, Zhao Y, Wang Z, et al. Solvent-free synthesis of the polymer electrolyte via photo-controlled radical polymerization: Toward ultra-fast in-built fabrication of solid-state batteries under visible light. ACS Appl Mater Interfaces, 2021, 13: 8426–8434
Zhang Y, Lu W, Cong L, et al. Cross-linking network based on poly (ethylene oxide): Solid polymer electrolyte for room temperature lithium battery. J Power Sources, 2019, 420: 63–72
He F, Tang W, Zhang X, et al. High energy density solid state lithium metal batteries enabled by sub-5 µm solid polymer electrolytes. Adv Mater, 2021, 33: 2105329
Wu J, Rao Z, Cheng Z, et al. Ultrathin, flexible polymer electrolyte for cost-effective fabrication of all-solid-state lithium metal batteries. Adv Energy Mater, 2019, 9: 1902767
Wu J, Yuan L, Zhang W, et al. Reducing the thickness of solid-state electrolyte membranes for high-energy lithium batteries. Energy Environ Sci, 2021, 14: 12–36
Wang Z, Shen L, Deng S, et al. 10 µm-thick high-strength solid polymer electrolytes with excellent interface compatibility for flexible all-solid-state lithium-metal batteries. Adv Mater, 2021, 33: 2100353
Wan J, **e J, Kong X, et al. Ultrathin, flexible, solid polymer composite electrolyte enabled with aligned nanoporous host for lithium batteries. Nat Nanotechnol, 2019, 14: 705–711
Xu Y, Zhang S, Liang T, et al. Porous polyamide skeleton-reinforced solid-state electrolyte: Enhanced flexibility, safety, and electrochemical performance. ACS Appl Mater Interfaces, 2021, 13: 11018–11025
Lu J, Zhou J, Chen R, et al. 4.2 V poly(ethylene oxide)-based all-solid-state lithium batteries with superior cycle and safety performance. Energy Storage Mater, 2020, 32: 191–198
Mindemark J, Lacey MJ, Bowden T, et al. Beyond PEO—Alternative host materials for Li+-conducting solid polymer electrolytes. Prog Polym Sci, 2018, 81: 114–143
Wang H, Sheng L, Yasin G, et al. Reviewing the current status and development of polymer electrolytes for solid-state lithium batteries. Energy Storage Mater, 2020, 33: 188–215
Evans J, Vincent CA, Bruce PG. Electrochemical measurement of transference numbers in polymer electrolytes. Polymer, 1987, 28: 2324–2328
Xuan C, Gao S, Wang Y, et al. In-situ generation of high performance thiol-conjugated solid polymer electrolytes via reliable thiol-acrylate click chemistry. J Power Sources, 2020, 456: 228024
Wang H, Wang Q, Cao X, et al. Thiol-branched solid polymer electrolyte featuring high strength, toughness, and lithium ionic conductivity for lithium-metal batteries. Adv Mater, 2020, 32: 2001259
Homann G, Stolz L, Nair J, et al. Poly(ethylene oxide)-based electrolyte for solid-state-lithium-batteries with high voltage positive electrodes: Evaluating the role of electrolyte oxidation in rapid cell failure. Sci Rep, 2020, 10: 4390
Yang X, Jiang M, Gao X, et al. Determining the limiting factor of the electrochemical stability window for PEO-based solid polymer electrolytes: Main chain or terminal −OH group? Energy Environ Sci, 2020, 13: 1318–1325
Deng K, Guan T, Liang F, et al. Flame-retardant single-ion conducting polymer electrolytes based on anion acceptors for high-safety lithium metal batteries. J Mater Chem A, 2021, 9: 7692–7702
Deng K, Zhou S, Xu Z, et al. A high ion-conducting, self-healing and nonflammable polymer electrolyte with dynamic imine bonds for dendrite-free lithium metal batteries. Chem Eng J, 2022, 428: 131224
Lu Y, Tu Z, Archer LA. Stable lithium electrodeposition in liquid and nanoporous solid electrolytes. Nat Mater, 2014, 13: 961–969
Acknowledgements
This work was supported by the National Key Research and Development Program (2019YFA0705701), the National Natural Science Foundation of China (22179149, 22075329, 22008267, 51573215 and 21978332), the Basic and Applied Basic Research Foundation of Guangdong province (2021A0505030022, 2019A1515010803 and 2020A1515011445), and Guangzhou Scientific and Technological Planning Project (201804020025 and 201904010271).
Author information
Authors and Affiliations
Contributions
Li Z and Wang T carried out the project and wrote the original draft; Zhong L and Meng Y refined the draft and supervised the project; **ao M, Han D, Wang S, Zhang S, and Huang S contributed to the data analysis and discussion. All authors contributed to the general discussion.
Corresponding authors
Additional information
Zhifeng Li is currently a ME candidate at the School of Material Science and Engineering, Sun Yat-sen University. He received his BE degree from Sun Yat-sen University in 2020. His research interest is solid polymer electrolytes and their applications in LIBs.
Tianyi Wang received his BE degree from the School of Materials Science and Engineering, Sun Yat-sen University in 2016. He is studying for a doctorate degree under the supervision of Prof. Yuezhong Meng at Sun Yat-sen University.
Lei Zhong received her PhD degree from the School of Material Science and Engineering, Sun Yat-sen University in 2018. She became a postdoctoral and associate research fellow at the School of Materials Science and Engineering, Sun Yat-sen university in 2018 and 2020, respectively. Her research interest includes solid electrolytes and their applications in energy devices, such as lithium sulfur batteries.
Yuezhong Meng received his BSc, MSc, and PhD degrees from Dalian University of Technology. He worked at the City University of Hong Kong, McGill University, Nanyang Technological University and the National University of Singapore for more than 8 years. He became a “Hundred Talents” member of Chinese Academy of Sciences in 1998. Now he is a Pearl-River professor at Sun Yat-sen University and the director of the Key Laboratory of Low-carbon Chemistry and Energy Conservation of Guangdong Province.
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary information
Supporting data are available in the online version of the paper.
Rights and permissions
About this article
Cite this article
Li, Z., Wang, T., Zhong, L. et al. Ultrathin thiol-ene crosslinked polymeric electrolyte for solid-state and high-performance lithium metal batteries. Sci. China Mater. 66, 1332–1340 (2023). https://doi.org/10.1007/s40843-022-2259-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-022-2259-3