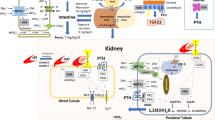
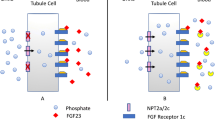
Abstract
Purpose of review
The purpose of this article is to review (1) the molecular mechanisms and hormonal factors involved in phosphate handling and regulation, (2) how to appropriately interpret serum and urine phosphate measurements in pediatric patients, (3) the pathophysiology of hypophosphatemic and hyperphosphatemic conditions, and (4) current strategies for treatment of hypophosphatemia and hyperphosphatemia in pediatric patients.
Recent findings
For decades, treatment of some hypophosphatemic conditions like X-linked hypophosphatemic rickets (XLH), characterized by a primary increase in fibroblast growth factor 23 (FGF23) activity, consisted of non-specific therapy with phosphate supplementation and calcitriol administration. However, in the past few years, burosumab, a targeted anti-FGF23 antibody, has been developed, representing a promising new medication for the treatment of pediatric XLH patients. The treatment of hyperphosphatemic conditions like chronic kidney disease (CKD) consists of dietary phosphate restriction and enteral phosphate binders; however, the development of new binders and inhibitors of cellular phosphate transporters may offer additional treatment options in the future.
Summary
The evaluation and treatment of disorders of phosphate balance in children is complex, as numerous interrelated mechanisms and hormones are involved in phosphate handling and regulation. Knowledge of the pathophysiology of hypophosphatemic and hyperphosphatemic conditions informs optimal diagnostic and treatment strategies.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Ruppe MD. Jan de Beur SM. Disorders of phosphate homeostasis. In: Bilezikian JP, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. 9th ed. American Society for Bone and Mineral Research: Hoboken, NJ; 2019. p. 674–83.
Goretti Penido M, Alon US. Phosphate homeostasis and its role in bone health. Pediatr Nephrol. 2012;27(11):2039–48. https://doi.org/10.1007/s00467-012-2175-z.
Bacchetta J, Salusky IB. Evaluation of hypophosphatemia: lessons from patients with genetic disorders. Am J Kidney Dis. 2012;59(1):152–9. https://doi.org/10.1053/j.ajkd.2011.08.035.
K/DOQI clinical practice guidelines for nutrition in children with chronic kidney disease. Am J Kidney Dis. 2009;53:S1–123.
• Hernando N, Wagner CA. Mechanisms and regulation of intestinal phosphate absorption. Compr Physiol. 2018;8(3):1065–90. https://doi.org/10.1002/cphy.c170024. This review provides a comprehensive summary of the mechanisms underlying phosphate transport and regulation.
Gattineni J, Baum M. Genetic disorders of phosphate regulation. Pediatr Nephrol. 2012;27(9):1477–87. https://doi.org/10.1007/s00467-012-2103-2.
Hernando N, Gagnon KB, Lederer ED. Phosphate transport in epithelial and nonepithelial tissue. Physiol Rev. 2020. https://doi.org/10.1152/physrev.00008.2019.
Jubiz W, Canterbury JM, Reiss E, Tyler FH. Circadian rhythm in serum parathyroid hormone concentration in human subjects: correlation with serum calcium, phosphate, albumin, and growth hormone levels. J Clin Invest. 1972;51(8):2040–6. https://doi.org/10.1172/jci107010.
Portale AA, Halloran BP, Morris RC Jr. Dietary intake of phosphorus modulates the circadian rhythm in serum concentration of phosphorus. Implications for the renal production of 1,25-dihydroxyvitamin D. J Clin Invest. 1987;80(4):1147–54. https://doi.org/10.1172/jci113172.
Isakova T, **e H, Barchi-Chung A, Smith K, Sowden N, Epstein M, et al. Daily variability in mineral metabolites in CKD and effects of dietary calcium and calcitriol. Clin J Am Soc Nephrol. 2012;7(5):820–8. https://doi.org/10.2215/cjn.11721111.
Becker GJ, Walker RG, Hewitson TD, Pedagogos E. Phosphate levels--time for a rethink? Nephrol Dial Transplant. 2009;24(8):2321–4. https://doi.org/10.1093/ndt/gfp220.
Lippi G, Salvagno GL, Montagnana M, Brocco G, Guidi GC. Influence of hemolysis on routine clinical chemistry testing. Clin Chem Lab Med. 2006;44(3):311–6. https://doi.org/10.1515/cclm.2006.054.
Perović A, Dolčić M. Influence of hemolysis on clinical chemistry parameters determined with Beckman Coulter tests - detection of clinically significant interference. Scand J Clin Lab Invest. 2019;79(3):154–9. https://doi.org/10.1080/00365513.2019.1576099.
Ball CL, Tobler K, Ross BC, Connors MR, Lyon ME. Spurious hyperphosphatemia due to sample contamination with heparinized saline from an indwelling catheter. Clin Chem Lab Med. 2004;42(1):107–8. https://doi.org/10.1515/cclm.2004.021.
Cachat F, Bardy D, Durussel C, Di Paolo E. Spurious hyperphosphatemia in a patient with alteplase-locked central venous catheter. Pediatr Nephrol. 2006;21(2):301–2. https://doi.org/10.1007/s00467-005-2088-1.
Schiller B, Virk B, Blair M, Wong A, Moran J. Spurious hyperphosphatemia in patients on hemodialysis with catheters. Am J Kidney Dis. 2008;52(3):617–20. https://doi.org/10.1053/j.ajkd.2008.03.033.
Isakova T, Gutierrez O, Shah A, Castaldo L, Holmes J, Lee H, et al. Postprandial mineral metabolism and secondary hyperparathyroidism in early CKD. J Am Soc Nephrol. 2008;19(3):615–23. https://doi.org/10.1681/asn.2007060673.
Slatopolsky E, Bricker NS. The role of phosphorus restriction in the prevention of secondary hyperparathyroidism in chronic renal disease. Kidney Int. 1973;4(2):141–5. https://doi.org/10.1038/ki.1973.92.
Payne RB. Renal tubular reabsorption of phosphate (TmP/GFR): indications and interpretation. Ann Clin Biochem. 1998;35(Pt 2):201–6. https://doi.org/10.1177/000456329803500203.
Diab L, Krebs NF. Vitamin excess and deficiency. Pediatr Rev. 2018;39(4):161–79. https://doi.org/10.1542/pir.2016-0068.
Malloy PJ, Feldman D. Genetic disorders and defects in vitamin d action. Endocrinol Metab Clin North Am. 2010;39(2):333–46, table of contents. https://doi.org/10.1016/j.ecl.2010.02.004.
Liamis G, Milionis HJ, Elisaf M. Medication-induced hypophosphatemia: a review. Qjm. 2010;103(7):449–59. https://doi.org/10.1093/qjmed/hcq039.
Foreman JW. Fanconi syndrome. Pediatr Clin N Am. 2019;66(1):159–67. https://doi.org/10.1016/j.pcl.2018.09.002.
Bergwitz C, Miyamoto KI. Hereditary hypophosphatemic rickets with hypercalciuria: pathophysiology, clinical presentation, diagnosis and therapy. Pflugers Arch. 2019;471(1):149–63. https://doi.org/10.1007/s00424-018-2184-2.
Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26(3):345–8. https://doi.org/10.1038/81664.
Boyce AM, Collins MT. Fibrous dysplasia/McCune-Albright syndrome: a rare, mosaic disease of Gα s activation. Endocr Rev. 2020;41(2):345–70. https://doi.org/10.1210/endrev/bnz011.
Wolf M, Chertow GM, Macdougall IC, Kaper R, Krop J, Strauss W. Randomized trial of intravenous iron-induced hypophosphatemia. JCI Insight. 2018;3(23). https://doi.org/10.1172/jci.insight.124486.
Schouten BJ, Hunt PJ, Livesey JH, Frampton CM, Soule SG. FGF23 elevation and hypophosphatemia after intravenous iron polymaltose: a prospective study. J Clin Endocrinol Metab. 2009;94(7):2332–7. https://doi.org/10.1210/jc.2008-2396.
Yamamoto S, Okada Y, Mori H, Fukumoto S, Tanaka Y. Fibroblast growth factor 23-related osteomalacia caused by the prolonged administration of saccharated ferric oxide. Intern Med. 2012;51(17):2375–8. https://doi.org/10.2169/internalmedicine.51.7450.
Zoller H, Schaefer B, Glodny B. Iron-induced hypophosphatemia: an emerging complication. Curr Opin Nephrol Hypertens. 2017;26(4):266–75. https://doi.org/10.1097/mnh.0000000000000329.
Marinella MA. Refeeding syndrome and hypophosphatemia. J Intensive Care Med. 2005;20(3):155–9. https://doi.org/10.1177/0885066605275326.
Jain N, Reilly RF. Hungry bone syndrome. Curr Opin Nephrol Hypertens. 2017;26(4):250–5. https://doi.org/10.1097/mnh.0000000000000327.
• Schindeler A, Biggin A, Munns CF. Clinical evidence for the benefits of Burosumab therapy for X-linked hypophosphatemia (XLH) and other conditions in adults and children. Front Endocrinol (Lausanne). 2020;11:338. https://doi.org/10.3389/fendo.2020.00338. This review summarizes the clinical trial data to date regarding burosumab treatment in adults and children with XLH.
• Imel EA, Glorieux FH, Whyte MP, Munns CF, Ward LM, Nilsson O, et al. Burosumab versus conventional therapy in children with X-linked hypophosphataemia: a randomised, active-controlled, open-label, phase 3 trial. Lancet. 2019;393(10189):2416–27. https://doi.org/10.1016/s0140-6736(19)30654-3. This study is a randomized, active-controlled, open-label, phase 3 trial of burosumab vs. conventional therapy (oral phosphate and calcitriol) in 61 children with XLH aged 1-12 years, with a primary endpoint of change in rickets severity.
Haffner D, Emma F, Eastwood DM, Duplan MB, Bacchetta J, Schnabel D, et al. Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nat Rev Nephrol. 2019;15(7):435–55. https://doi.org/10.1038/s41581-019-0152-5.
Ladenhauf HN, Stundner O, Spreitzhofer F, Deluggi S. Severe hyperphosphatemia after administration of sodium-phosphate containing laxatives in children: case series and systematic review of literature. Pediatr Surg Int. 2012;28(8):805–14. https://doi.org/10.1007/s00383-012-3124-4.
Becknell B, Smoyer WE, O'Brien NF. Hemodialysis for near-fatal sodium phosphate toxicity in a child receiving sodium phosphate enemas. Pediatr Emerg Care. 2014;30(11):814–7. https://doi.org/10.1097/pec.0000000000000267.
McNamara S, Galt DJ. Regarding near-fatal sodium phosphate toxicity in a child receiving sodium phosphate enemas. Pediatr Emerg Care. 2015;31(8):e7. https://doi.org/10.1097/pec.0000000000000523.
Farrow EG, Imel EA, White KE. Miscellaneous non-inflammatory musculoskeletal conditions. Hyperphosphatemic familial tumoral calcinosis (FGF23, GALNT3 and αKlotho). Best Pract Res Clin Rheumatol. 2011;25(5):735–47. https://doi.org/10.1016/j.berh.2011.10.020.
Boyce AM, Lee AE, Roszko KL, Gafni RI. Hyperphosphatemic tumoral calcinosis: pathogenesis, clinical presentation, and challenges in management. Front Endocrinol (Lausanne). 2020;11:293. https://doi.org/10.3389/fendo.2020.00293.
Cheung WL, Hon KL, Fung CM, Leung AK. Tumor lysis syndrome in childhood malignancies. Drugs Context. 2020;9:1–14. https://doi.org/10.7573/dic.2019-8-2.
Hanudel MR, Moe SM, Salusky IB. Pathophysiology and treatment of chronic kidney disease–mineral and bone disorder. In: Bilezikian JP, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. 9th ed. Hoboken, NJ: American Society for Bone and Mineral Research; 2019. p. 695–704.
Nakanishi T, Hasuike Y, Nanami M, Yahiro M, Kuragano T. Novel iron-containing phosphate binders and anemia treatment in CKD: oral iron intake revisited. Nephrol Dial Transplant. 2016;31(10):1588–94. https://doi.org/10.1093/ndt/gfv268.
Hanudel MR, Laster M, Ramos G, Gales B, Salusky IB. Clinical experience with the use of ferric citrate as a phosphate binder in pediatric dialysis patients. Pediatr Nephrol. 2018;33(11):2137–42. https://doi.org/10.1007/s00467-018-3999-y.
Fouque D, Vervloet M, Ketteler M. Targeting gastrointestinal transport proteins to control hyperphosphatemia in chronic kidney disease. Drugs. 2018;78(12):1171–86. https://doi.org/10.1007/s40265-018-0950-2.
Thomas L, Xue J, Murali SK, Fenton RA, Dominguez Rieg JA, Rieg T. Pharmacological Npt2a inhibition causes phosphaturia and reduces plasma phosphate in mice with normal and reduced kidney function. J Am Soc Nephrol. 2019;30(11):2128–39. https://doi.org/10.1681/asn.2018121250.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pediatric Nephrology
Rights and permissions
About this article
Cite this article
Hanudel, M.R. Evaluation and Treatment of Disorders of Phosphate Balance. Curr Treat Options Peds 6, 227–240 (2020). https://doi.org/10.1007/s40746-020-00208-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40746-020-00208-1