Abstract
Purpose
First-line medical therapy for acromegaly management includes first-generation somatostatin receptor ligands (fgSRLs), but resistance limits their use. Despite international guidelines, the choice of second-line therapy is debated.
Methods
We aim to discuss resistance to fgSRLs, identify second-line therapy determinants and assess glycemia’s impact to provide valuable insights for acromegaly management in clinical practice. A group of Italian endocrinologists expert in the pituitary field participated in a two-round Delphi panel between July and September 2023. The Delphi questionnaire encompassed a total of 75 statements categorized into three sections: resistance to fgSRLs therapy and predictors of response; determinants for the selection of second-line therapy; the role of glycemia in the therapeutic management. The statements were rated on a 6-point Likert scale.
Results
Fifty-nine (79%) statements reached a consensus. IGF-1 levels resulted central for evaluating resistance to fgSRLs, that should be defined considering also symptomatic clinical response, degree of tumor shrinkage and complications, using clinician- and patient-reported outcome tools available. Factors to be evaluated for the choice of second-line medical therapy are hyperglycemia—that should be managed as in non-acromegalic patients—tumor remnant, resistant headache and compliance. Costs do not represent a main determinant in the choice of second-line medical treatment.
Conclusion
The experts agreed on a holistic management approach to acromegaly. It is therefore necessary to choose currently available highly effective second-line medical treatment (pegvisomant and pasireotide) based on the characteristics of the patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acromegaly is a chronic, disabling, rare endocrine disorder characterized by the excessive production of the growth hormone (GH) and insulin-like growth factor 1 (IGF-1) [1,2,3]. Systemic long-term complications of acromegaly include cardiovascular and metabolic complications, respiratory disorders, arthropathy, and depression, with an impact on quality of life (QoL); it is overall associated with potentially life-threatening effects [1, 4].
First-line multimodal management of acromegalic patients includes surgical interventions aiming to remove the pituitary adenoma responsible for excessive GH overproduction. Subsequent medical therapy aims to control tumor growth, inhibit GH hypersecretion, normalize IGF-1 levels, and reduce the burden of comorbidities. Finally, radiotherapy is used when surgery and medical treatments are not curative or have significant risks [5, 6].
The first-line medical treatment includes first-generation somatostatin receptor ligands (fgSRLs) such as octreotide and lanreotide [5, 6], although resistance to fgSRLs is common in clinical practice and is reported in up to 66% of patients [7, 8]. Several predictors of resistance have been described and include the patients’ gender, age, initial GH and IGF-1 levels, tumor volume, tumor hyperintensity on T2-weighted magnetic resonance imaging, and the expression of somatostatin receptor subtypes [7]. For patients who are not controlled while on fgSRLs, second-line therapies are available to achieve biochemical control of the disease. These include GH receptor antagonist pegvisomant (PEGV) and second-generation SRLs pasireotide (PASI) [5, 6, 9], while dopamine agonist cabergoline is only used in selected cases [10]. Treatment with PASI has been associated with a dysregulation of glucose metabolism and the onset of diabetes [11], while treatment with PEGV has been associated with an improvement in glycemic control [12].
Despite several international consensus and guidelines provide recommendations on the therapeutic algorithm for the management of acromegaly based on different patient’s characteristics [5, 11], a specific document providing indication on the current management of acromegalic patients in clinical practice in Italy is missing. To this end, Italian endocrinologists expert in acromegaly’s management were involved in a Delphi panel to obtain indications on the definition of resistance to fgSRLs, the biochemical and clinical determinants to drive the second-line therapy and the impact of glycemia in the therapeutic approach.
Methods
The Delphi method
The Delphi method involves multiple rounds of anonymous surveys that allow experts to provide feedback and revise their responses based on the collective insights of the group. The method facilitates the achievement of a consensus [13].
The fundamental prerequisites of a high-quality Delphi study are anonymity, iteration, controlled feedback, and statistical stability of consensus [14]. The details of Delphi methods and statements’ definition are reported in Supplementary material.
Participant selection
For Delphi studies it is crucial to assemble a panel of experts, essential for generating valuable insights and reliable answers [15, 16]. Participants were each individually selected by the Steering Committee (AG, Silvia G, and Pietro M) based on their qualifications and expertise in the management of acromegaly, to form a diverse and knowledgeable panel that ensures a comprehensive coverage of the endocrinology centers nationwide. Selected panelists belonged to referral centers for neuroendocrinology and had long-term experience in acromegaly management. In the invitation email, the panelists were provided with an explanation of the nature and the aim of the project. The invited endocrinologists were not involved in creating the survey, but only to express their opinion to the statements.
Questionnaires
The present questionnaire encompassed a total of 75 statements categorized into three sections:
-
Incidence, definition of resistance to fgSRLs therapy, and predictors of response (21 items)
-
Biochemical and clinical determinants for the selection of second-line therapy (29 items)
-
The role of glycemia in the therapeutic management of the patient (25 items).
Participants provided ratings on a 6-point Likert scale, ranging from strong disagreement to full agreement. The survey was available for completion through a dedicated online platform (SurveyMonkey®) over 10 days in July 2023. The results of the first round were discussed by the Steering Committee and, as per Delphi method, statements that did not reach consensus threshold in the first round were resubmitted for further consideration in a second round, on the same platform, for another 10 days in September 2023. A total of 28 statements were resubmitted to the panelists. Slight revisions to two statements were made to eliminate any potential ambiguities or misinterpretations (Supplementary material).
In 2017, a similar Delphi questionnaire was proposed to a panel of experts. That study was not published, but the results were made available to the authors for discussion and comparison with the current results, taking into consideration that many statements had to be changed to be in line with the most recent clinical development. The 2017 Delphi encompassed 61 statements, subdivided in the following topics: SRLs therapy, resistance to SRLs, second-line choice, evaluation of hyperglycemia and comorbidities in patients with acromegaly. The questionnaire was evaluated on 5-point Likert scale. The complete text of the 2017 Delphi questionnaire is reported in the Supplementary material.
Data analysis
The data were analyzed by descriptive statistics. Clear a priori criteria were established to define the conditions under which the Delphi study’s conclusions would be considered reached [17]. Consensus was to be deemed achieved when a minimum agreement threshold of 67% was met. The same criteria were applied to the Delphi questionnaire performed in 2017, albeit with some minor differences in the scale used (Supplementary material).
Results
A total of 66 endocrinologists were involved in the Delphi panel; during the first round, 54 experts submitted a response to at least one statement, and 48 completed the full questionnaire. The second round involved the 53 experts who responded to at least one statement of the first round (one of the panelists agreed not to proceed further) and 36 full responses were collected. Overall, 59 out of 75 (79%) statements reached a consensus, either in agreement or disagreement. In the 16 cases where the agreement threshold was not met, the values from the first round, which had the highest number of respondents, were considered as the final results.
Incidence, definition of resistance to fgSRLs therapy, and predictors of response
The first 21 statements explored the resistance to fgSRLs in the clinical management of acromegaly, with 17 reaching a consensus (Table 1). The highest level of agreement was reached for the lack of somatostatin receptors on adenoma and genetic predisposition as characteristic predicting resistance to fgSRLs (96% and 92%, respectively), and for IGF-1 levels being central for evaluating resistance (89% agreement). Seventy-eight percent of experts stated that IGF-1 alone should be considered in case of discrepancy with GH values, and that multiple measurements over time are needed in case of moderately increased values (83% agreement). Beside IGF-1, GH levels should also be considered to monitor fgSRL resistance (75% agreement for considering both values) but it should not be used alone (71% disagreement). More frequent assessment of IGF-1/GH levels is pivotal for patients not achieving disease control (88% of agreement); slightly elevated IGF-1/GH levels are tolerated for patients who underwent radiosurgery (75% agreement), but not for those over 50 years that have been recently diagnosed (76% disagreement). No agreement was achieved in specific statements exploring results in discrepant patients.
A good level of agreement was reached for all statements exploring the integration of different parameters (symptomatic clinical response, degree of tumor shrinkage, complications, use of clinician-reported outcomes [CROs]/patient-reported outcomes [PROs] tools) in the definition of fgSRLs resistance (range 69–86%), while hyperglycemia is not seen as a parameter to be integrated for the biochemical definition of resistance (68% disagreement).
Biochemical and clinical determinants for the choice of second-line medical therapy
Statements from 22 to 50 analyzed the determinants influencing the choice of second-line medical therapy, with 23 reaching a consensus (Table 2). The highest level of agreement was for cost not influencing the choice of fgSRLs therapy (90%). PEGV monotherapy or a combination of fgSRLs + PEGV are a second-line choice for 88% of experts, while PASI is for 86% of them.
Overall, the panel reached a consensus on the indications for the shift to PEGV monotherapy and the related possible causes of concern. For instance, the presence of type 2 diabetes mellitus (T2DM) and IGF-1 levels > 1.5 × ULN motivate to PEGV shift after SRLs resistance (86% each), and the recurrence of previously controlled headaches is a concern for 83% of the panelists.
PASI can be used with no concern in the absence of T2DM and in case of severe headache regardless of the response to other therapies (73% and 69% agreement, respectively); previous therapy with PEGV (either as a monotherapy or combination with fgSRLs) is not a prerequisite for switching to this therapy (81% disagreement). Costs influence the therapy choice only in case of PASI + PEGV, which did not reach an agreement. Considering patient’s compliance and adherence, 85% of experts would choose PASI, 83% PEGV as monotherapy and 79% a combination of fgSRLs + PEGV.
Statements not reaching an agreement concerned fgSRLs and cabergoline combination therapy, PASI and PEGV combination therapy, and the management of T2DM in a patient starting PASI therapy.
Role of blood glucose levels in the therapeutic management of the patient
Twenty-five statements investigated the influence of blood glucose levels on the choice of treatment, with 19 reaching a consensus (Table 3). The panel disagreed on impaired fasting glucose (IFG) being a contraindication for the use of PASI, or a strict indication for switching to PEGV monotherapy in young acromegalic patients (83% and 75%, respectively). A good level of consent was achieved for the statements exploring the management of hyperglycemia, with the highest consensus on target HbA1c being age-dependent (94%). Ninety-four percent of panelists also agreed that a controlled patient who develops T2DM should be maintained on fgSRLs; 90% disagreed in switching to PEGV monotherapy in this condition, which should be initiated only if hyperglycemia control is not achieved (69% agreement). In case of resistance to fgSRLs, experts did not agree on PASI therapy to be started only in association with PEGV (92% disagreement), while for 67% HbA1c levels guide the choice of the second-line treatment.
When presented with a clinical case scenario of a woman with previous gestational diabetes mellitus (GDM) and a GH-secreting macroadenoma resistant to fgSRLs, experts agreed on neurosurgery as the next therapeutic step (90%), followed by association of fgSRLs + PEGV (75%) or a switch to PASI monotherapy (75%), while a switch to PEGV monotherapy or fgSRL + cabergoline association therapy were not deemed adequate options (81% and 67% disagreement respectively).
Six statements did not reach consensus: IFG guiding the choice of therapy or being an indication for the addition of PEGV, the need to refer acromegalic diabetic patients to a Diabetology center, the consideration on the use of DPP-4 inhibitors or GLP-1 receptor agonists in a controlled patient, and the influence of T2DM in the therapy choice in case of fgSRL resistance or the necessity to add PEGV in such patients.
Dynamic comparison between 2017 and 2023 Delphi panels
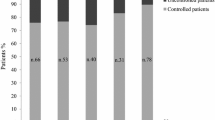
A dynamic comparison with the findings of the prior 2017 Delphi study was performed, although many statements had to be changed to be in line with the most recent clinical development. The previous study involved 78 endocrinologists. As shown in Table 4, for most of the comparable statements, a significant overlap of the responses was noticed, indicating a certain level of consistency in the viewpoints of clinicians regarding the various issues raised. However, discrepancies on some points were highlighted as shown in Figs. 1 and 2.
Dynamic comparison–Sect. 2, statement 6-% of consensus
Dynamic comparison–Sect. 2, statement 7-% of consensus
Discussion
In this work, by means of the Delphi methodology, a panel of Italian endocrinologists assessed factors determining fgSRL resistance, second-line medical treatment [18,19,20], and T2DM’s role in patient management. IGF-1 is unequivocally established as the primary parameter for monitoring treatment efficacy in clinical practice. The panel revealed low confidence and interest in the use of cabergoline in second-line, while all the other choices are considered according to patient’s condition. Hyperglycemia is an important issue, and it could influence the choice of therapy, especially in certain categories of patients.
Incidence, definition of resistance to therapy with fgSRLs, and predictors of response
Although both GH and IGF-1 levels are central in defining resistance to treatment (statement 1.4), the panel acknowledges that IGF-1 is the “gold standard” (statement 1.1). The role of IGF-1 is consolidated [21, 22], as it was similarly recognized as determinant also in 2017. On the other hand, the agreement on the role of GH results weaker compared to 2017 (75% vs 83% in 2023 vs 2017, respectively), in line with recent consensus [3].
In case of discrepant IGF-1/GH values, resistance is primarily determined by the levels of IGF-1(statement 2.4). Indeed, in one third of acromegalic patients, GH levels may give discrepant information compared to IGF-1, likely due to assay or cut-off issues, GHR polymorphism, timing of post-surgical assessment, or different biological significance of the two parameters [11, 23,24,25]. However, no consensus was reached on some statements on the definition of acromegaly under control when IGF-1 and GH levels show different responses to therapies (statements 2.5 and 2.6). In fact, for some of the experts, normalization of GH should be a clinically relevant goal irrespective of IGF-1 normalization, possibly because of the fear of a prospective increase of IGF-1 levels in the absence of GH control; however, this concept has not been included in new Consensus recommendations [3]. Nevertheless, the responses suggest potentially significant implications for clinical practice, since it could involve treatment intensification or increased follow-up even in cases of IGF-1 normalization. This might also indicate a preference for a different second-line drug that lowers GH rather than IGF-1 in cases of discrepancies.
Due to IGF-1 assay variability, particularly in mildly elevated cases, experts stress the importance of repeated evaluations over time before confirming resistance (statement 1.2). This aligns with the 2017 Delphi, albeit with lower agreement (91% in 2017 and 83% in 2023). No consensus was reached regarding whether slightly elevated IGF-1 levels should be considered an indication of effective treatment (statement 1.3). Indeed, not all the guidelines suggest as acceptable target levels up to 1.3 × ULN [4, 11]. A less ambitious target in patients who underwent radiotherapy is acceptable since progressive improvement of disease activity’s control is expected (statement 2.2). However, IGF-1 levels slightly above the normal range are not considered an appropriate target in elderly patients with a recent diagnosis, where the disease could be less aggressive (statement 2.1). Despite acceptable range in these two conditions changed between the two surveys (up to 1.5 x ULN in 2017 and up to 1.3 x ULN in 2023), this did not affect the consensus, corroborating the idea of a possible stricter IGF-1 target nowadays.
Overall, biochemical and clinical parameters should be more frequently checked in uncontrolled patients (statement 2.3) and should be integrated into the definition of resistance to fgSRLs (statement 3.1 and 3.3). In particular, a comprehensive evaluation of acromegaly, possibly through AcroDAT ® [26] and SAGIT® [27, 28], is considered valuable (statement 3.4). The absent or modest (< 20%) adenoma shrinkage is less relevant than biochemical measures in defining resistance (statement 3.2; agreement reached only in the second round); this may be due to the lack of evidence on a specific diameter/volume threshold to define clinically relevant shrinkage, particularly in small post-surgical remnants where MRI evaluation may be impacted by different artifacts [24]. Side-effects, such as hyperglycemia, should not be included in the definition of resistance to fgSRLs (statement 3.5), likely due to the reported marginal impact of fgSRLs on glucose homeostasis [29].
The possibility to predict resistance to fgSRLs would allow personalized interventions in patients identified at risk, avoiding long and ineffective attempts to control the disease [7, 11, 30]. Familiar history of pituitary adenomas and positive genetics are recognized as a predictive parameter for development of resistance (statement 4.1), particularly in young patients [31]. Absence of SSTRs and expression of molecular markers of aggressiveness in surgical pituitary tissue are strongly identified as markers of expected poor response to fgSRLs (statement 4.4–4.5). This is of great interest, since it implies that experts rely substantially on novel pathological techniques, recently found not to be routinely available also in Pituitary Tumor Centers of Excellence [32,33,34].
Finally, size of adenoma is not believed to be a predictor of resistance to fgSRLs (statement 4.2), while no consensus was reached on the role of elevated post-surgical GH/IGF-1 (statement 4.3); this is an interesting finding since virtually all guidelines report on the low likelihood of biochemical control in these two conditions. In the experience of part of the group, therefore, control could still be obtained in this context with fgSRLs. This opinion may be based on the long diagnostic delay in acromegaly, which is one of the main determinants of adenoma overgrowth and excessive GH and IGF-1 levels, with no implications on potential response to treatment [35, 36].
Biochemical and clinical determinants for the choice of second-line therapy
In case of ineffective control of acromegaly with fgSRLs, combination therapy with cabergoline [11] did not meet the consensus as further therapeutic step for the panelists (statement 5.1), even if, recently, this combination showed a good IGF-1 normalization rate (30–58% of cases) [37]. Different factors may have contributed to the absence of consensus, including limited controlled studies, off-label use of cabergoline, availability of alternative therapeutic options, weak GH suppression by this drug and potential long-term detrimental effects (of heart valves especially in the context of acromegalic cardiopathy). Cabergoline is not perceived as valuable option also when looking at compliance (statement 10.1), a finding supported by a recent real-world analysis of US administrative claims data, which reported lowest adherence and persistence for this drug [30].
In line with guidelines, experts agree that PEGV monotherapy (statement 5.2), in combination with fgSRLs (statement 5.3), or PASI (statement 5.4) could be used in patients resistant to fgSRLs [5, 11]. Although PEGV in initial studies was only used as monotherapy [38, 39], ACROSTUDY real-world cohort showed that PEGV is used in up to 50% of cases in combination with fgSRLs [40], albeit with differences among countries [41].
Guidelines suggest that PEGV monotherapy is useful in patients with glucose metabolism disorders and/or without problems related to the pituitary mass [11]. The Delphi panel agreed that the presence of T2DM motivates the choice of PEGV as monotherapy (statement 6.3), with increased consensus compared to 2017 (86% vs 72%), indicating a better knowledge of this positive impact of PEGV [12, 42]. In resistant patients, the switch to PEGV monotherapy is motivated by slightly elevated or frankly elevated IGF-1 levels (statement 6.1 and 6.2), with a stronger agreement achieved in this second cohort of patients compared to patients with a lower level of elevation of IGF-1 (69% vs 86%).
Despite reported only in a minority of cases in clinical trials [43], the growth of pituitary mass upon fgSRLs discontinuation is a concern for switching to PEGV monotherapy (statement 7.1), albeit with a decreasing consensus compared to 2017 (69% vs 74%, respectively). Previous radiotherapy is a determinant for switching to PEGV monotherapy (statement 6.5), indicating that in this cohort of patients the concern for pituitary mass growth is reduced. This statement did not achieve a consensus in 2017, possibly indicating a growing reliability of new radiotherapy techniques in controlling tumor mass [44].
PEGV daily injections schedule (statement 7.3), and the possible reappearance of headache after SRLs discontinuation (statement 7.4) are also reasons for concern; however, concern on compliance achieved a borderline consensus, lower than in 2017 (67% vs 83% in 2023 vs 2017, respectively), and in a subsequent statement (10.3) panelists agreed on PEGV monotherapy (even if not daily) being a possible therapeutic strategy considering the patient's compliance and adherence (83%).
Despite being a drawback in 2017, therapy costs do not seem to be a detrimental factor for PEGV therapy anymore (statement 7.2, 9.3), likely in light of the significant efficacy demonstrated [45,46,47] on various targets including glycemia, presumably associated with a reduction of indirect costs. In fact, according to an Incremental Cost Effectiveness Ratio analysis in the Spanish NHS [48], PEGV is the most cost-effective alternative in the treatment of acromegaly in fgSRLs-resistant patients.
According to the panel, the transition to PASI should not necessarily take place after initial treatment with PEGV (either in monotherapy or combination, statement 8.1). This is in line with the 2018 consensus [11] but not with the most recent 2020 consensus, which positioned PEGV monotherapy as a second-line option, while fgSRLs + PEGV or PASI were reported as a third-line option [5]. Still, this is consistent with the European and Italian label for the drugs, which position them both as options after ineffectiveness of fgSRLs, and it is possibly related to some recent real-life data reporting an increased efficacy of PASI in fgSRLs-resistant patients [49] compared to initial studies [50].
The experts agree" on the use of PASI without particular concern in case of no medical history of T2DM (statement 8.3). Indeed, PASI could induce a worsening of the metabolic picture by inhibiting insulin and incretin secretion, especially in patients starting therapy with increased basal glycemia. No consensus was reached on the optimal treatment approach for the management of PASI-associated hyperglycemia (statement 8.4 and 8.5). Still, a multicenter, randomized, open-label, Phase IV study reported that, in some acromegalic patients treated with PASI, hyperglycemia could be effectively controlled by metformin, eventually followed by incretin-based therapy [51].
Real-life studies have also identified a discrete action of PASI on headache [52, 53], as acknowledged by the experts (statement 8.2). As per PEGV, costs are not a determinant for switching to PASI monotherapy (statement 9.4), while the panel show some cost-related concern for PASI + PEGV combination (statements 9.5). This is possibly related to the significant higher costs of this combination, associated with the panel’s lower expertise on this approach.
To summarize, the panelists show a clear preference within the Italian NHS for an independent decision-making process, encompassing nearly all individual or combined therapy schedules (statements 9.1–9.4). PASI monotherapy achieves the highest consensus for compliance and adherence compared to the other second-line medical treatment options (statement 10.2), while a consensus was not achieved on PASI + PEGV combination therapy (statement 10.5).
Role of blood glucose levels in therapeutic management
Impaired glucose tolerance and T2DM are common in acromegaly [54] and guidelines suggest that T2DM should influence the choice of medical therapy. PASI is not recommended in uncontrolled T2DM patients because of the high risk of further glycemic control deterioration [4]; on the other hand, many data showed that PEGV treatment improves glucose metabolism [12, 43] and should be considered in patients with partial or no response to fgSRLs for whom glycemic control is challenging.
In the experts’ opinion, T2DM/metabolic alterations should be approached and treated as in the general population (statement 13.4, 13.5). Hyperglycemia onset should not modify acromegaly therapy, even if induced by it (statement 12.1). An HbA1c target below 7.0%, or further tailored to the patient’s age (statement 12.2, 12.4) and the management of cardiovascular risk factors (statement 12.6) are goals for acromegaly and hyperglycemia treatments. In the second round, the panel reached a consensus on a lower HbA1c target (below 6.5%, statement 12.3), a parameter that sparked disagreement in 2017. This finding, in line with the most recent T2DM guidelines [55], is possibly due to more efficacious and safer treatment options for diabetic patients, while a more stringent diabetes control is probably also seen as a proactive measure to mitigate cardiovascular mortality.
No consensus was reached on the referral of patients to a Diabetology Centre (statement 12.5), likely reflecting the different organization of Italian Endocrinology practices, where only some centers have Diabetology Units. It could be assumed that some neuroendocrinologists personally manage T2DM to avoid delocalization of patients, while others, considering acromegaly diabetes as non-specific form of diabetes, prefer referring these patients to diabetologists.
In fgSRL resistant patients, the choice of second-line depends on metabolic compensation (statement 14.1), but a consensus could not be reached on whether diabetes mellitus per se should influence the second-line medical therapy (statement 14.2) and on the addition of PEGV in these patients (statement 14.3). On the other hand, panelists do not perceive that in these cases the only possibility to start PASI is in association with PEGV (statement 14.4), probably also considering that PASI’s detrimental effect on glucose metabolism is only partially counteracted by PEGV [56]. Experts did not agree on IFG influencing the choice of second-line medical therapy, contraindicating, at least in young patients, the use of PASI (statement 11.1), or indicating the necessity to switch to PEGV monotherapy (statement 11.4). This is an interesting finding, since data from literature show that patients with IFG are at higher risk of develo** hyperglycemia when switching to PASI [57], while age seems to be protective against development of T2DM while on this therapy.
FgSRLs are not considered responsible for metabolic worsening, or at least not such as to disregard their effectiveness on biochemical or tumor mass control. The onset of T2DM in well-controlled patients is not a valid reason for fgSRLs therapy discontinuation (statement 13.1), which should be accompanied by the start of an antidiabetic drug (statement 13.2). This despite evidence demonstrating significant positive impact on glucose metabolism of PEGV, regardless of IGF-1 levels [43]. Therefore, in the experts’ opinion the attainment of effective control over acromegaly is the priority. The positive effects on GH/IGF-1 secretion and tumor mass are deemed more crucial, with any potential deterioration in glycemic control being considered a separate concern. It is noteworthy that these assertions achieved a more robust consensus than in 2017. However, when T2DM treatment is ineffective, the experts agree on a switch to PEGV monotherapy also in well-controlled patients (statement 13.3); this statement did not achieve a consensus in 2017, indicating that, at least in this subpopulation, the panelists agreed that the positive effect of PEGV on glucose metabolism may be exploited to manage both conditions.
Experts agree that in a patient with history of GDM and a GH-secreting macroadenoma resistant to fgSRLs, surgical intervention should be prioritized (statement 15.4). Another option is the combination of fgSRLs and PEGV (considering the diabetogenic and tumor growth risks if fgSRLs are discontinued and PEGV is used as monotherapy) (statement 15.2). An alternative that gains consensus in the second round is shifting to PASI (prioritizing the tumor mass issue over the diabetes concern, statement 15.1).
Limitations
This work presents the limitations of the Delphi studies: a decline in response rate between the rounds of 17% was observed, but this is within the limits described for Delphi studies, especially when dealing with a large number of statements [58, 59]. The consistency of answers in subsequent interactions indicated that the statements were correctly defined. Although some of the invited experts did not participate, those taking part well represented the real-world management of acromegaly in Italy. The lack of consensus for some statements underlines the open questions that need further research to be addressed.
Conclusions
A summary of the possible management of acromegalic patient resistant to first-line medical treatment based on what emerged from the answers provided by the Delphi panel is reported in Fig. 3.
The experts agreed on a holistic management approach to acromegaly, considering IGF-1 levels (with more stringent targets than in the past, at least in some categories of patients), tumor mass, complications, symptoms, and the patient’s QoL. This aligns with current guidelines, which emphasize the importance of incorporating CROs/PROs.
Hyperglycemia is determinant for second-line medical therapy choice. PEGV positive effect on glucose metabolism is not always valued, especially in well controlled patients with fgSRLs who develop glucose derangement. On the other hand, concern about the diabetogenic effect of PASI seems to be somehow attenuated, especially in young patients, and no consensus on its management was achieved. T2DM should be managed as in non-acromegalic patients, but with more strict targets than in the past, possibly due to the known cardiovascular risk of this population, further amplified by diabetes [4]. The direct cost of therapy is of minor importance likely because of a greater attention to the indirect costs associated with the complications, which increase with uncontrolled disease. It is therefore necessary to choose currently available highly effective second-line medical treatment (PEGV and PASI) based on the characteristics of the patients.
Data availability
Data available within the article or its supplementary materials.
References
Melmed S, Kaiser UB, Lopes MB, Bertherat J, Syro LV, Raverot G et al (2022) Clinical biology of the pituitary adenoma. Endocr Rev 43(6):1003–1037. https://doi.org/10.1210/endrev/bnac010
Fleseriu M, Langlois F, Lim DST, Varlamov EV, Melmed S (2022) Acromegaly: pathogenesis, diagnosis, and management. Lancet Diabetes Endocrinol 10(11):804–826. https://doi.org/10.1016/S2213-8587(22)00244-3
Giustina A, Biermasz N, Casanueva FF, Fleseriu M, Mortini P, Strasburger C et al (2023) Consensus on criteria for acromegaly diagnosis and remission. Pituitary. https://doi.org/10.1007/s11102-023-01360-1
Giustina A, Barkan A, Beckers A, Biermasz N, Biller BMK, Boguszewski C et al (2020) A consensus on the diagnosis and treatment of acromegaly comorbidities: an update. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgz096
Giustina A, Barkhoudarian G, Beckers A, Ben-Shlomo A, Biermasz N, Biller B et al (2020) Multidisciplinary management of acromegaly: a consensus. Rev Endocr Metab Disord 21(4):667–678. https://doi.org/10.1007/s11154-020-09588-z
Colao A, Grasso LFS, Giustina A, Melmed S, Chanson P, Pereira AM et al (2019) Acromegaly. Nat Rev Dis Primers 5(1):20. https://doi.org/10.1038/s41572-019-0071-6
Berton AM, Prencipe N, Bertero L, Baldi M, Bima C, Corsico M et al (2022) Resistance to somatostatin analogs in italian acromegaly patients: the MISS study. J Clin Med. https://doi.org/10.3390/jcm12010025
Gola M, Bonadonna S, Mazziotti G, Amato G, Giustina A (2006) Resistance to somatostatin analogs in acromegaly: an evolving concept? J Endocrinol Invest 29(1):86–93. https://doi.org/10.1007/BF03349183
Giustina A, di Filippo L, Uygur MM, Frara S (2023) Modern approach to resistant acromegaly. Endocrine 80(2):303–307. https://doi.org/10.1007/s12020-023-03317-7
Suda K, Inoshita N, Iguchi G, Fukuoka H, Takahashi M, Nishizawa H et al (2013) Efficacy of combined octreotide and cabergoline treatment in patients with acromegaly: a retrospective clinical study and review of the literature. Endocr J 60(4):507–515
Melmed S, Bronstein MD, Chanson P, Klibanski A, Casanueva FF, Wass JAH et al (2018) A consensus statement on acromegaly therapeutic outcomes. Nat Rev Endocrinol 14(9):552–561. https://doi.org/10.1038/s41574-018-0058-5
Feola T, Cozzolino A, Simonelli I, Sbardella E, Pozza C, Giannetta E et al (2019) Pegvisomant improves glucose metabolism in acromegaly: a meta-analysis of prospective interventional studies. J Clin Endocrinol Metab 104(7):2892–2902. https://doi.org/10.1210/jc.2018-02281
Scarpa M, Barbato A, Bisconti A, Burlina A, Concolino D, Deodato F et al (2023) Acid sphingomyelinase deficiency (ASMD): addressing knowledge gaps in unmet needs and patient journey in Italy-a Delphi consensus. Intern Emerg Med 18(3):831–842. https://doi.org/10.1007/s11739-023-03238-3
Nasa P, Jain R, Juneja D (2021) Delphi methodology in healthcare research: how to decide its appropriateness. World J Methodol 11(4):116–129. https://doi.org/10.5662/wjm.v11.i4.116
Boulkedid R, Abdoul H, Loustau M, Sibony O, Alberti C (2011) Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS ONE 6(6):e20476. https://doi.org/10.1371/journal.pone.0020476
Powell C (2003) The Delphi technique: myths and realities. J Adv Nurs 41(4):376–382. https://doi.org/10.1046/j.1365-2648.2003.02537.x
Diamond IR, Grant RC, Feldman BM, Pencharz PB, Ling SC, Moore AM et al (2014) Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol 67(4):401–409. https://doi.org/10.1016/j.jclinepi.2013.12.002
Puig-Domingo M, Bernabeu I, Pico A, Biagetti B, Gil J, Alvarez-Escola C et al (2021) Pasireotide in the personalized treatment of acromegaly. Front Endocrinol (Lausanne) 12:648411. https://doi.org/10.3389/fendo.2021.648411
Giustina A, Arnaldi G, Bogazzi F, Cannavo S, Colao A, De Marinis L et al (2017) Pegvisomant in acromegaly: an update. J Endocrinol Invest 40(6):577–589. https://doi.org/10.1007/s40618-017-0614-1
Fleseriu M, Biller BMK, Freda PU, Gadelha MR, Giustina A, Katznelson L et al (2021) A pituitary society update to acromegaly management guidelines. Pituitary 24(1):1–13. https://doi.org/10.1007/s11102-020-01091-7
Frara S, Maffezzoni F, Mazziotti G, Giustina A (2016) The modern criteria for medical management of acromegaly. Prog Mol Biol Transl Sci 138:63–83. https://doi.org/10.1016/bs.pmbts.2015.10.015
Paragliola RM, Corsello SM, Salvatori R (2017) Somatostatin receptor ligands in acromegaly: clinical response and factors predicting resistance. Pituitary 20(1):109–115. https://doi.org/10.1007/s11102-016-0768-4
Bianchi A, Giustina A, Cimino V, Pola R, Angelini F, Pontecorvi A et al (2009) Influence of growth hormone receptor d3 and full-length isoforms on biochemical treatment outcomes in acromegaly. J Clin Endocrinol Metab 94(6):2015–2022. https://doi.org/10.1210/jc.2008-1337
Amato G, Mazziotti G, Rotondi M, Iorio S, Doga M, Sorvillo F et al (2002) Long-term effects of lanreotide SR and octreotide LAR on tumour shrinkage and GH hypersecretion in patients with previously untreated acromegaly. Clin Endocrinol (Oxf) 56(1):65–71. https://doi.org/10.1046/j.0300-0664.2001.01438.x
Giustina A, Bronstein MD, Casanueva FF, Chanson P, Ghigo E, Ho KK et al (2011) Current management practices for acromegaly: an international survey. Pituitary 14(2):125–133. https://doi.org/10.1007/s11102-010-0269-9
van der Lely AJ, Gomez R, Pleil A, Badia X, Brue T, Buchfelder M et al (2017) Development of ACRODAT((R)), a new software medical device to assess disease activity in patients with acromegaly. Pituitary 20(6):692–701. https://doi.org/10.1007/s11102-017-0835-5
Giustina A, Bevan JS, Bronstein MD, Casanueva FF, Chanson P, Petersenn S et al (2016) SAGIT(R): clinician-reported outcome instrument for managing acromegaly in clinical practice–development and results from a pilot study. Pituitary 19(1):39–49. https://doi.org/10.1007/s11102-015-0681-2
Giustina A, Bronstein MD, Chanson P, Petersenn S, Casanueva FF, Sert C et al (2021) International multicenter validation study of the SAGIT(R) instrument in acromegaly. J Clin Endocrinol Metab 106(12):3555–3568. https://doi.org/10.1210/clinem/dgab536
Cozzolino A, Feola T, Simonelli I, Puliani G, Pozza C, Giannetta E et al (2018) Somatostatin analogs and glucose metabolism in acromegaly: a meta-analysis of prospective interventional studies. J Clin Endocrinol Metab. https://doi.org/10.1210/jc.2017-02566
Fleseriu M, Barkan A, Brue T, Duquesne E, Houchard A, Del SchneiderPilar M et al (2023) Treatment patterns, adherence, persistence, and health care resource utilization in acromegaly: a real-world analysis. J Endocr Soc. https://doi.org/10.1210/jendso/bvad104
Denes J, Korbonits M (2021) The clinical aspects of pituitary tumour genetics. Endocrine 71(3):663–674. https://doi.org/10.1007/s12020-021-02633-0
MacFarlane J, Huynh KA, Powlson AS, Kolias AG, Mannion RJ, Scoffings DJ et al (2023) Novel imaging techniques in refractory pituitary adenomas. Pituitary 26(3):288–292. https://doi.org/10.1007/s11102-023-01304-9
Frara S, Rodriguez-Carnero G, Formenti AM, Martinez-Olmos MA, Giustina A, Casanueva FF (2020) Pituitary tumors centers of excellence. Endocrinol Metab Clin North Am 49(3):553–564. https://doi.org/10.1016/j.ecl.2020.05.010
Giustina A, Uygur MM, Frara S, Barkan A, Biermasz NR, Chanson P et al (2023) Pilot study to define criteria for pituitary tumors centers of excellence (PTCOE): results of an audit of leading international centers. Pituitary 26(5):583–596. https://doi.org/10.1007/s11102-023-01345-0
Giustina A (2016) Acromegaly: reducing diagnostic delay. Recenti Prog Med 107(8):450–451. https://doi.org/10.1701/2332.25074
Chiloiro S, Giampietro A, Gagliardi I, Bondanelli M, Veleno M, Ambrosio MR et al (2022) Impact of the diagnostic delay of acromegaly on bone health: data from a real life and long term follow-up experience. Pituitary 25(6):831–841. https://doi.org/10.1007/s11102-022-01266-4
Giraldi EA, Ioachimescu AG (2020) The role of dopamine agonists in pituitary adenomas. Endocrinol Metab Clin North Am 49(3):453–474. https://doi.org/10.1016/j.ecl.2020.05.006
van der Lely AJ, Hutson RK, Trainer PJ, Besser GM, Barkan AL, Katznelson L et al (2001) Long-term treatment of acromegaly with pegvisomant, a growth hormone receptor antagonist. Lancet 358(9295):1754–1759. https://doi.org/10.1016/s0140-6736(01)06844-1
Trainer PJ, Drake WM, Katznelson L, Freda PU, Herman-Bonert V, van der Lely AJ et al (2000) Treatment of acromegaly with the growth hormone-receptor antagonist pegvisomant. N Engl J Med 342(16):1171–1177. https://doi.org/10.1056/NEJM200004203421604
Ragonese M, Grottoli S, Maffei P, Alibrandi A, Ambrosio MR, Arnaldi G et al (2018) How to improve effectiveness of pegvisomant treatment in acromegalic patients. J Endocrinol Invest 41(5):575–581. https://doi.org/10.1007/s40618-017-0773-0
Grottoli S, Bianchi A, Bogazzi F, Bona C, Carlsson MO, Colao A et al (2022) Are there country-specific differences in the use of pegvisomant for acromegaly in clinical practice? an analysis from ACROSTUDY. J Endocrinol Invest 45(8):1535–1545. https://doi.org/10.1007/s40618-022-01789-4
Giustina A, Ambrosio MR, Beck Peccoz P, Bogazzi F, Cannavo S, De Marinis L et al (2014) Use of Pegvisomant in acromegaly an Italian Society of endocrinology guideline. J Endocrinol Invest 37(10):1017–30. https://doi.org/10.1007/s40618-014-0146-x
Barkan AL, Burman P, Clemmons DR, Drake WM, Gagel RF, Harris PE et al (2005) Glucose homeostasis and safety in patients with acromegaly converted from long-acting octreotide to pegvisomant. J Clin Endocrinol Metab 90(10):5684–5691. https://doi.org/10.1210/jc.2005-0331
Meral R, Selcukbiricik OS, Uzum AK, Sahin S, Okutan M, Barburoglu M et al (2023) Promising outcomes in acromegaly patients receiving cyberknife stereotactic hypofractionated radiotherapy. Cureus 15(10):e47936. https://doi.org/10.7759/cureus.47936
Pirchio R, Auriemma RS, Montini ME, Vergura A, Pivonello R, Colao A (2023) Control of acromegaly in more than 90% of patients after 10 years of pegvisomant therapy: an European referral centre real-life experience. J Endocrinol Invest 46(5):1027–1038. https://doi.org/10.1007/s40618-022-01980-7
Fleseriu M, Fuhrer-Sakel D, van der Lely AJ, De Marinis L, Brue T, van der Lans-Bussemaker J et al (2021) More than a decade of real-world experience of pegvisomant for acromegaly: ACROSTUDY. Eur J Endocrinol 185(4):525–538. https://doi.org/10.1530/EJE-21-0239
Chiloiro S, Giampietro A, Mirra F, Donfrancesco F, Tartaglione T, Mattogno PP et al (2021) Pegvisomant and Pasireotide LAR as second line therapy in acromegaly: clinical effectiveness and predictors of response. Eur J Endocrinol 184(2):217–229. https://doi.org/10.1530/EJE-20-0767
Peral C, Cordido F, Gimeno-Ballester V, Mir N, Sanchez-Cenizo L, Rubio-Rodriguez D et al (2020) Cost-effectiveness analysis of second-line pharmacological treatment of acromegaly in Spain. Expert Rev Pharmacoecon Outcomes Res 20(1):105–114. https://doi.org/10.1080/14737167.2019.1610396
Gadelha M, Marques NV, Fialho C, Scaf C, Lamback E, Antunes X et al (2023) Long-term efficacy and safety of pasireotide in patients with acromegaly: 14 years’ single-center real-world experience. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgad378
Gadelha MR, Bronstein MD, Brue T, Coculescu M, Fleseriu M, Guitelman M et al (2014) Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, phase 3 trial. Lancet Diabetes Endocrinol 2(11):875–884. https://doi.org/10.1016/S2213-8587(14)70169-X
Samson SL, Gu F, Feldt-Rasmussen U, Zhang S, Yu Y, Witek P et al (2021) Managing pasireotide-associated hyperglycemia: a randomized, open-label. Phase IV study Pituit 24(6):887–903. https://doi.org/10.1007/s11102-021-01161-4
Lovato CM, Kapsner PL (2018) Analgesic effect of long-acting somatostatin receptor agonist pasireotide in a patient with acromegaly and intractable headaches. BMJ Case Rep. https://doi.org/10.1136/bcr-2017-219686
Shimon I, Adnan Z, Gorshtein A, Baraf L, Saba Khazen N, Gershinsky M et al (2018) Efficacy and safety of long-acting pasireotide in patients with somatostatin-resistant acromegaly: a multicenter study. Endocrine 62(2):448–455. https://doi.org/10.1007/s12020-018-1690-5
Frara S, Maffezzoni F, Mazziotti G, Giustina A (2016) Current and emerging aspects of diabetes mellitus in acromegaly. Trends Endocrinol Metab 27(7):470–483. https://doi.org/10.1016/j.tem.2016.04.014
Mannucci E, Candido R, Monache LD, Gallo M, Giaccari A, Masini ML et al (2023) 2023 update on Italian guidelines for the treatment of type 2 diabetes. Acta Diabetol 60(8):1119–1151. https://doi.org/10.1007/s00592-023-02107-x
Chiloiro S, Giampietro A, Visconti F, Rossi L, Donfrancesco F, Fleseriu CM et al (2021) Glucose metabolism outcomes in acromegaly patients on treatment with pasireotide-LAR or pasireotide-LAR plus Pegvisomant. Endocrine 73(3):658–666. https://doi.org/10.1007/s12020-021-02711-3
Gadelha MR, Gu F, Bronstein MD, Brue TC, Fleseriu M, Shimon I et al (2020) Risk factors and management of pasireotide-associated hyperglycemia in acromegaly. Endocr Connect 9(12):1178–1190. https://doi.org/10.1530/EC-20-0361
Gargon E, Crew R, Burnside G, Williamson PR (2019) Higher number of items associated with significantly lower response rates in COS Delphi surveys. J Clin Epidemiol 108:110–120. https://doi.org/10.1016/j.jclinepi.2018.12.010
Larreche J, Montgomery D (1977) A framework for the comparison of marketing models: a Delphi study. J Mark Res 14(4):487–498
Acknowledgements
We would like to thank the Italian Endocrine Delphi panel Group that completed the questionnaire. We thank the expert panelists who helped designing the questionnaire in 2017: Giorgio Arnaldi, Fausto Bogazzi, Salvatore Cannavò, Annamaria Colao, Laura De Marinis, Ernesto De Menis, Ezio Ghigo, Francesco Giorgino, Andrea Lania, Rosario Pivonello, Maria Chiara Zatelli. We thank Valeria Loiacono, who was the Rare Disease Medical Advisor in Pfizer overviewing the Delphi project in 2017. Paolo Mariani and Laura Benedan collaborated on the project on behalf of Health Publishing & Services Srl.
Funding
Open access funding provided by Università degli Studi di Torino within the CRUI-CARE Agreement. Silvia G., Pietro M., A.G. were paid consultants to Pfizer in connection with the development of this manuscript. Editorial support was provided by Barbara Bartolini, PhD at Health Publishing & Services Srl, and was funded by Pfizer.
Author information
Authors and Affiliations
Contributions
Silvia G., Pietro M., A.S.T., Simona G., A.G.: study conception. Paolo M. and L.B: methodology. All authors drafted and edited the manuscript, and approved the final version submitted.
Corresponding author
Ethics declarations
Conflict of interest
A.S.T. and Simona G. are Pfizer employees. Pietro M. is a PI involved in the ACROSTUDY.
Ethical approval
This research did not involve human and/or animal participants.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grottoli, S., Maffei, P., Tresoldi, A.S. et al. Insights from an Italian Delphi panel: exploring resistance to first-generation somatostatin receptor ligands and guiding second-line medical therapies in acromegaly management. J Endocrinol Invest (2024). https://doi.org/10.1007/s40618-024-02386-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40618-024-02386-3