Abstract
Background
Overall, up to one-third of epilepsy patients have drug-resistant epilepsy. However, there was previously no meta-analysis to support the guidelines for broad-spectrum antiseizure medication selection for the adjunctive treatment of refractory epilepsy. In the present meta-analysis, we assessed the efficacy and safety of three second-generation broad-spectrum antiseizure medications, lamotrigine (LTG), levetiracetam (LEV), and topiramate (TPM), and two third-generation broad-spectrum antiseizure medications, perampanel (PER) and lacosamide (LCM), for the adjunctive treatment of refractory epilepsy.
Methods
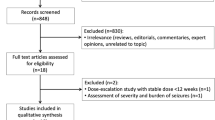
We systematically searched PubMed, Embase, and CENTRAL from inception to July 15, 2022. The studies included in the meta-analysis were required to meet the following criteria: (1) be randomized, double-blind clinical trials; (2) include patients aged >2 years with a clinical diagnosis of drug-resistant epilepsy; (3) have at least 8 weeks for the treatment period excluding the titration phase; and (4) report the outcomes of seizure response, seizure freedom and the withdrawal rate due to treatment-emergent adverse effects. Data were extracted, and the risk of bias for each study was assessed by two authors independently using RoB2 tools. We performed the network meta-analysis for each outcome through a group of programs in the mvmeta and network packages in Stata. Relative odds ratios with 95% confidence intervals were calculated as the result of the analyses. The surface under the cumulative ranking curve (SUCRA) and mean ranks were used to rank these treatments.
Results
Forty-two randomized controlled trials (RCTs) (LTG-placebo: n = 6, LEV-placebo: n = 13, TPM-placebo: n = 9, PER-placebo: n = 6, LCM-placebo: n = 7, LEV-TPM: n = 1) with 10257 participants (LTG = 569, LEV = 1626, TPM = 701, PER = 1734, LCM = 1908, placebo = 3719) were included. Levetiracetam had subequal efficacy in 50 % seizure frequency reduction to TPM [odds ratio (OR) 1.00, 95% confidence interval (CI) 0.73–1.38], and LEV had a higher rate of ≥ 50% seizure frequency reduction than LCM (OR 1.49, 95% CI 1.11–2.01) and PER (OR 1.68, 95% CI 1.24–2.29). Levetiracetam was also related to a higher proportion of seizure freedom participants than TPM (OR 1.87, 95% CI 1.20–2.89), PER (OR 2.23, 95% CI 1.12–4.43), and LCM (OR 2.97, 95% CI 1.46–6.05). In addition, LEV was associated with a lower risk of experiencing at least one treatment-emergent adverse event (TEAE) than PER (OR 0.63, 95% CI 0.46–0.85) and TPM (OR 0.51, 95 % CI 0.36–0.72) and a lower proportion of patients experiencing TEAEs leading to discontinuation than PER (OR 0.51, 95% CI 0.27–0.97) and TPM (OR 0.50, 95 % CI 0.27–0.93).
Conclusions
Third-generation drugs (PER and LCM) had no advantages in terms of efficacy and safety for adjunctive treatment of refractory epilepsy compared with several second-generation drugs (LEV and LTG). Levetiracetam was the priority choice for adjunctive treatment of refractory epilepsy. Perampanel and LCM had no advantages in terms of efficacy and safety among the five drugs.
Registration
PROSPERO registration number, CRD42022344153; last edited on December 23, 2022.










Similar content being viewed by others
References
Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–82.
Milligan TA. Epilepsy: a clinical overview. Am J Med. 2021;134(7):840–7.
Thijs RD, Surges R, O’Brien TJ, Sander JW. Epilepsy in adults. Lancet (London, England). 2019;393(10172):689–701.
Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993;34(3):453–68.
Saxena S, Li S. Defeating epilepsy: a global public health commitment. Epilepsia open. 2017;2(2):153–5.
Tian N, Boring M, Kobau R, Zack MM, Croft JB. Active epilepsy and seizure control in adults—United States, 2013 and 2015. MMWR Morb Mortal Wkly Rep. 2018;67(15):437–42.
Yuen AWC, Keezer MR, Sander JW. Epilepsy is a neurological and a systemic disorder. Epilepsy Behav E&B. 2018;78:57–61.
Ryvlin P, Cross JH, Rheims S. Epilepsy surgery in children and adults. Lancet Neurol. 2014;13(11):1114–26.
Genton P, Gelisse P, Thomas P, Dravet C. Do carbamazepine and phenytoin aggravate juvenile myoclonic epilepsy? Neurology. 2000;55(8):1106–9.
Chaves J, Sander JW. Seizure aggravation in idiopathic generalized epilepsies. Epilepsia. 2005;46(Suppl 9):133–9.
Marson AG, Al-Kharusi AM, Alwaidh M, Appleton R, Baker GA, Chadwick DW, et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalised and unclassifiable epilepsy: an unblinded randomised controlled trial. Lancet (London, England). 2007;369(9566):1016–26.
Marson A, Burnside G, Appleton R, Smith D, Leach JP, Sills G, et al. The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalised and unclassifiable epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomised controlled trial. Lancet (London, England). 2021;397(10282):1375–86.
Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51(6):1069–77.
Kanner AM, Ashman E, Gloss D, Harden C, Bourgeois B, Bautista JF, et al. Practice guideline update summary: efficacy and tolerability of the new antiepileptic drugs II: treatment-resistant epilepsy: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2018;91(2):82–90.
Nanau RM, Neuman MG. Adverse drug reactions induced by valproic acid. Clin Biochem. 2013;46(15):1323–38.
Hu Q, Zhang F, Teng W, Hao F, Zhang J, Yin M, et al. Efficacy and safety of antiepileptic drugs for refractory partial-onset epilepsy: a network meta-analysis. J Neurol. 2018;265(1):1–11.
Lattanzi S, Trinka E, Zaccara G, Striano P, Russo E, Del Giovane C, et al. Third-generation antiseizure medications for adjunctive treatment of focal-onset seizures in adults: a systematic review and network meta-analysis. Drugs. 2022;82(2):199–218.
GlaxoSmithKline. LAMICTAL® (lamotrigine). US prescribing information 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020241s064,020764s057,022251s028lbl.pdf. Accessed 21 Jul 2023.
UCB, Inc. KEPPRA® (levetiracetam). US prescribing information 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/204417s004lbl.pdf. Accessed 21 Jul 2023.
Janssen Pharmaceuticals, Inc. TOPAMAX® (topiramate). US prescribing information 2012. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020844s041lbl.pdf. Accessed 21 Jul 2023.
Eisai Inc. FYCOMPA® (perampanel). US prescribing information 2012. thttps://www.accessdata.fda.gov/drugsatfda_docs/label/2019/202834s016,208277s004lbl.pdf. Accessed 21 Jul 2023.
Eisai Inc. VIMPAT® (lacosamide). US prescribing information 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/022253s050,022254s040,022255s032lbl.pdf. Accessed 21 Jul 2023.
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84.
Perucca E. Antiepileptic drugs: evolution of our knowledge and changes in drug trials. Epileptic Disord Int Epilepsy J Videotape. 2019;21(4):319–29.
Luchini C, Veronese N, Nottegar A, Shin JI, Gentile G, Granziol U, et al. Assessing the quality of studies in meta-research: review/guidelines on the most important quality assessment tools. Pharm Stat. 2021;20(1):185–95.
Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synthesis Methods. 2012;3(2):80–97.
Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29(7–8):932–44.
Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS ONE. 2013;8(10): e76654.
Matsuo F, Bergen D, Faught E, Messenheimer JA, Dren AT, Rudd GD, et al. Placebo-controlled study of the efficacy and safety of lamotrigine in patients with partial seizures. U.S. Lamotrigine Protocol 0.5 Clinical Trial Group. Neurology. 1993;43(11):2284–91.
Motte J, Trevathan E, Arvidsson JF, Barrera MN, Mullens EL, Manasco P. Lamotrigine for generalized seizures associated with the Lennox-Gastaut syndrome. Lamictal Lennox-Gastaut Study Group. N Engl J Med. 1997;337(25):1807–12.
Duchowny M, Pellock JM, Graf WD, Billard C, Gilman J, Casale E, et al. A placebo-controlled trial of lamotrigine add-on therapy for partial seizures in children. Lamictal Pediatric Partial Seizure Study Group. Neurology. 1999;53(8):1724–31.
Biton V, Sackellares JC, Vuong A, Hammer AE, Barrett PS, Messenheimer JA. Double-blind, placebo-controlled study of lamotrigine in primary generalized tonic-clonic seizures. Neurology. 2005;65(11):1737–43.
Naritoku DK, Warnock CR, Messenheimer JA, Borgohain R, Evers S, Guekht AB, et al. Lamotrigine extended-release as adjunctive therapy for partial seizures. Neurology. 2007;69(16):1610–8.
Biton V, Di Memmo J, Shukla R, Lee YY, Poverennova I, Demchenko V, et al. Adjunctive lamotrigine XR for primary generalized tonic-clonic seizures in a randomized, placebo-controlled study. Epilepsy Behav E&B. 2010;19(3):352–8.
Betts T, Waegemans T, Crawford P. A multicentre, double-blind, randomized, parallel group study to evaluate the tolerability and efficacy of two oral doses of levetiracetam, 2000 mg daily and 4000 mg daily, without titration in patients with refractory epilepsy. Seizure. 2000;9(2):80–7.
Cereghino JJ, Biton V, Abou-Khalil B, Dreifuss F, Gauer LJ, Leppik I. Levetiracetam for partial seizures: results of a double-blind, randomized clinical trial. Neurology. 2000;55(2):236–42.
Shorvon SD, Löwenthal A, Janz D, Bielen E, Loiseau P. Multicenter double-blind, randomized, placebo-controlled trial of levetiracetam as add-on therapy in patients with refractory partial seizures. European Levetiracetam Study Group. Epilepsia. 2000;41(9):1179–86.
Glauser TA, Ayala R, Elterman RD, Mitchell WG, Van Orman CB, Gauer LJ, et al. Double-blind placebo-controlled trial of adjunctive levetiracetam in pediatric partial seizures. Neurology. 2006;66(11):1654–60.
Tsai JJ, Yen DJ, Hsih MS, Chen SS, Hiersemenzel R, Edrich P, et al. Efficacy and safety of levetiracetam (up to 2000 mg/day) in Taiwanese patients with refractory partial seizures: a multicenter, randomized, double-blind, placebo-controlled study. Epilepsia. 2006;47(1):72–81.
Berkovic SF, Knowlton RC, Leroy RF, Schiemann J, Falter U. Placebo-controlled study of levetiracetam in idiopathic generalized epilepsy. Neurology. 2007;69(18):1751–60.
Noachtar S, Andermann E, Meyvisch P, Andermann F, Gough WB, Schiemann-Delgado J. Levetiracetam for the treatment of idiopathic generalized epilepsy with myoclonic seizures. Neurology. 2008;70(8):607–16.
Peltola J, Coetzee C, Jiménez F, Litovchenko T, Ramaratnam S, Zaslavaskiy L, et al. Once-daily extended-release levetiracetam as adjunctive treatment of partial-onset seizures in patients with epilepsy: a double-blind, randomized, placebo-controlled trial. Epilepsia. 2009;50(3):406–14.
Wu XY, Hong Z, Wu X, Wu LW, Wang XF, Zhou D, et al. Multicenter double-blind, randomized, placebo-controlled trial of levetiracetam as add-on therapy in Chinese patients with refractory partial-onset seizures. Epilepsia. 2009;50(3):398–405.
**ao Z, Li JM, Wang XF, **ao F, ** ZQ, Lv Y, et al. Efficacy and safety of levetiracetam (3, 000 mg/Day) as an adjunctive therapy in Chinese patients with refractory partial seizures. Eur Neurol. 2009;61(4):233–9.
Inoue Y, Yagi K, Ikeda A, Sasagawa M, Ishida S, Suzuki A, et al. Efficacy and tolerability of levetiracetam as adjunctive therapy in Japanese patients with uncontrolled partial-onset seizures. Psychiatry Clin Neurosci. 2015;69(10):640–8.
Wu L, Yagi K, Hong Z, Liao W, Wang X, Zhou D, et al. Adjunctive levetiracetam in the treatment of Chinese and Japanese adults with generalized tonic-clonic seizures: a double-blind, randomized, placebo-controlled trial. Epilepsia Open. 2018;3(4):474–84.
Manreza MLG, Pan TA, Carbone EQ, Vattimo ACA, Herrera R, Morais DC, et al. Efficacy and safety of levetiracetam as adjunctive therapy for refractory focal epilepsy. Arq Neuropsiquiatr. 2021;79(4):290–8.
Ben-Menachem E, Henriksen O, Dam M, Mikkelsen M, Schmidt D, Reid S, et al. Double-blind, placebo-controlled trial of topiramate as add-on therapy in patients with refractory partial seizures. Epilepsia. 1996;37(6):539–43.
Faught E, Wilder BJ, Ramsay RE, Reife RA, Kramer LD, Pledger GW, et al. Topiramate placebo-controlled dose-ranging trial in refractory partial epilepsy using 200-, 400-, and 600-mg daily dosages. Topiramate YD Study Group. Neurology. 1996;46(6):1684–90.
Sharief M, Viteri C, Ben-Menachem E, Weber M, Reife R, Pledger G, et al. Double-blind, placebo-controlled study of topiramate in patients with refractory partial epilepsy. Epilepsy Res. 1996;25(3):217–24.
Tassinari CA, Michelucci R, Chauvel P, Chodkiewicz J, Shorvon S, Henriksen O, et al. Double-blind, placebo-controlled trial of topiramate (600 mg daily) for the treatment of refractory partial epilepsy. Epilepsia. 1996;37(8):763–8.
Biton V, Montouris GD, Ritter F, Riviello JJ, Reife R, Lim P, et al. A randomized, placebo-controlled study of topiramate in primary generalized tonic-clonic seizures. Topiramate YTC Study Group. Neurology. 1999;52(7):1330–7.
Topiramate in medically intractable partial epilepsies: double-blind placebo-controlled randomized parallel group trial. Korean Topiramate Study Group. Epilepsia. 1999;40(12):1767–74.
Elterman RD, Glauser TA, Wyllie E, Reife R, Wu SC, Pledger G. A double-blind, randomized trial of topiramate as adjunctive therapy for partial-onset seizures in children. Topiramate YP Study Group. Neurology. 1999;52(7):1338–44.
Yen DJ, Yu HY, Guo YC, Chen C, Yiu CH, Su MS. A double-blind, placebo-controlled study of topiramate in adult patients with refractory partial epilepsy. Epilepsia. 2000;41(9):1162–6.
Chung SS, Fakhoury TA, Hogan RE, Nagaraddi VN, Blatt I, Lawson B, et al. Once-daily USL255 as adjunctive treatment of partial-onset seizures: randomized phase III study. Epilepsia. 2014;55(7):1077–87.
Lee SK, Lee SA, Kim DW, Loesch C, Pelgrims B, Osakabe T, et al. A randomized, open-label, multicenter comparative trial of levetiracetam and topiramate as adjunctive treatment for patients with focal epilepsy in Korea. Epilepsy Behav E&B. 2019;97:67–74.
French JA, Krauss GL, Biton V, Squillacote D, Yang H, Laurenza A, et al. Adjunctive perampanel for refractory partial-onset seizures: randomized phase III study 304. Neurology. 2012;79(6):589–96.
Krauss GL, Edwards HB, Lin B. Lacosamide for the treatment of epilepsy. Ann Med. 2012;44(7):674–9.
French JA, Krauss GL, Steinhoff BJ, Squillacote D, Yang H, Kumar D, et al. Evaluation of adjunctive perampanel in patients with refractory partial-onset seizures: results of randomized global phase III study 305. Epilepsia. 2013;54(1):117–25.
French JA, Krauss GL, Wechsler RT, Wang XF, DiVentura B, Brandt C, et al. Perampanel for tonic-clonic seizures in idiopathic generalized epilepsy A randomized trial. Neurology. 2015;85(11):950–7.
Lagae L, Villanueva V, Meador KJ, Bagul M, Laurenza A, Kumar D, et al. Adjunctive perampanel in adolescents with inadequately controlled partial-onset seizures: a randomized study evaluating behaviOR efficacy, and safety. Epilepsia. 2016;57(7):1120–9.
Nishida T, Lee SK, Inoue Y, Saeki K, Ishikawa K, Kaneko S. Adjunctive perampanel in partial-onset seizures: Asia-Pacific, randomized phase III study. Acta Neurol Scand. 2018;137(4):392–9.
Ben-Menachem E, Biton V, Jatuzis D, Abou-Khalil B, Doty P, Rudd GD. Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial-onset seizures. Epilepsia. 2007;48(7):1308–17.
Halász P, Kälviäinen R, Mazurkiewicz-Beldzińska M, Rosenow F, Doty P, Hebert D, et al. Adjunctive lacosamide for partial-onset seizures: efficacy and safety results from a randomized controlled trial. Epilepsia. 2009;50(3):443–53.
Chung S, Sperling MR, Biton V, Krauss G, Hebert D, Rudd GD, et al. Lacosamide as adjunctive therapy for partial-onset seizures: a randomized controlled trial. Epilepsia. 2010;51(6):958–67.
Hong Z, Inoue Y, Liao W, Meng H, Wang X, Wang W, et al. Efficacy and safety of adjunctive lacosamide for the treatment of partial-onset seizures in Chinese and Japanese adults: a randomized, double-blind, placebo-controlled study. Epilepsy Res. 2016;127:267–75.
Farkas V, Steinborn B, Flamini JR, Zhang Y, Yuen N, Borghs S, et al. Efficacy and tolerability of adjunctive lacosamide in pediatric patients with focal seizures. Neurology. 2019;93(12):e1212–26.
Vossler DG, Knake S, O’Brien TJ, Watanabe M, Brock M, Steiniger-Brach B, et al. Efficacy and safety of adjunctive lacosamide in the treatment of primary generalised tonic-clonic seizures: a double-blind, randomised, placebo-controlled trial. J Neurol Neurosurg Psychiatry. 2020;91(10):1067–75.
Inoue Y, Liao W, Wang X, Du X, Tennigkeit F, Sasamoto H, et al. Safety and efficacy of adjunctive lacosamide in Chinese and Japanese adults with epilepsy and focal seizures: a long-term, open-label extension of a randomized, controlled trial. Epilepsy Res. 2021;176: 106705.
Poochikian-Sarkissian S, Sidani S, Wennberg R, Devins GM. Seizure freedom reduces illness intrusiveness and improves quality of life in epilepsy. The Canadian journal of neurological sciences Le journal canadien des sciences neurologiques. 2008;35(3):280–6.
Bodalia PN, Grosso AM, Sofat R, Macallister RJ, Smeeth L, Dhillon S, et al. Comparative efficacy and tolerability of anti-epileptic drugs for refractory focal epilepsy: systematic review and network meta-analysis reveals the need for long term comparator trials. Br J Clin Pharmacol. 2013;76(5):649–67.
Karaźniewicz-Łada M, Główka AK, Mikulska AA, Główka FK. Pharmacokinetic drug-drug interactions among antiepileptic drugs, including CBD, drugs used to treat COVID-19 and nutrients. Int J Mol Sci. 2021;22(17):9582.
Kwok CS, Johnson EL, Krauss GL. Comparing safety and efficacy of “third-generation” antiepileptic drugs: long-term extension and post-marketing treatment. CNS Drugs. 2017;31(11):959–74.
Rambeck B, Jürgens UH, May TW, Pannek HW, Behne F, Ebner A, et al. Comparison of brain extracellular fluid, brain tissue, cerebrospinal fluid, and serum concentrations of antiepileptic drugs measured intraoperatively in patients with intractable epilepsy. Epilepsia. 2006;47(4):681–94.
May TW, Brandt C, Helmer R, Bien CG, Cawello W. Comparison of lacosamide concentrations in cerebrospinal fluid and serum in patients with epilepsy. Epilepsia. 2015;56(7):1134–40.
Kinoshita Y, Ueno H, Kurata H, Ikeda C, Hori E, Okada T, et al. Infection-induced elevated plasma perampanel in a patient with hemimegalencephaly. Case Rep Pediatrics. 2022;2022:9844820.
Steinhoff BJ, Hübers E, Kurth C, Jürges K-K. Plasma concentration and clinical effects of perampanel-The Kork experience. Seizure. 2019;67:18–22.
Bialer M, Doose DR, Murthy B, Curtin C, Wang SS, Twyman RE, et al. Pharmacokinetic interactions of topiramate. Clin Pharmacokinet. 2004;43(12):763–80.
Tsai JJ, Wu T, Leung H, Desudchit T, Tiamkao S, Lim KS, et al. Perampanel, an AMPA receptor antagonist: from clinical research to practice in clinical settings. Acta Neurol Scand. 2018;137(4):378–91.
Sake JK, Hebert D, Isojärvi J, Doty P, De Backer M, Davies K, et al. A pooled analysis of lacosamide clinical trial data grouped by mechanism of action of concomitant antiepileptic drugs. CNS Drugs. 2010;24(12):1055–68.
Verrotti A, Lattanzi S, Brigo F, Zaccara G. Pharmacodynamic interactions of antiepileptic drugs: from bench to clinical practice. Epilepsy Behav E&B. 2020;104(Pt A): 106939.
Steinhoff BJ, Staack AM. Levetiracetam and brivaracetam: a review of evidence from clinical trials and clinical experience. Ther Adv Neurol Disord. 2019;12:1756286419873518.
Stefan H, Feuerstein TJ. Novel anticonvulsant drugs. Pharmacol Ther. 2007;113(1):165–83.
Acknowledgements
The authors thank Professor Yong Liu for his help in the network meta-analysis.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This work was financially supported by the Natural Science Foundation of Henan (222300420515), the Natural Science Foundation of Liaoning Province (2023-MS-096), and Fundamental Research Funds for the Central Universities (nos. DUT21RC(4)003 and DUT22YG107).
Conflicts of interest/competing interest
None of the authors has any conflict of interest to disclose.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
H-CW, H-RW, WZ and Y-SH contributed to the concept of the study and the study design. X-WN, X-MM, LZ, JZ contributed to the data search. H-CW, H-RW, YL, contributed to the data selection and data extraction. YZ, Y-LH and H-RW contributed to the quality assessment. H-RW and H-CW performed the statistical analysis. All authors contributed to the interpretation of the data. WZ and Y-SH supervised the work. H-CW, H-RW and Y-S H drafted the manuscript. All authors reviewed and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, H., Wang, H., Liu, Y. et al. Efficacy and Safety of Five Broad-Spectrum Antiseizure Medications for Adjunctive Treatment of Refractory Epilepsy: A Systematic Review and Network Meta-analysis. CNS Drugs 37, 883–913 (2023). https://doi.org/10.1007/s40263-023-01029-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-023-01029-0