Abstract
Background and Objectives
High-dose melphalan is an integral part of conditioning chemotherapy prior to both autologous and allogeneic hematopoietic cell transplantation. While underexposure may lead to relapse, overexposure may lead to toxicities include mucositis, diarrhea, bone marrow suppression, and rarely sinusoidal obstruction syndrome. In this study, we describe the population pharmacokinetics of high-dose melphalan as a first step towards individualized dosing.
Methods
Melphalan samples were collected in patients receiving an allogeneic or autologous hematopoietic cell transplantation between August 2016 and August 2020 at the Memorial Sloan Kettering Cancer Center. A population-pharmacokinetic model was developed using NONMEM.
Results
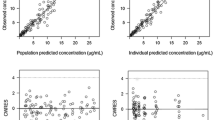
Based on a total of 3418 samples from 452 patients receiving a median cumulative dose of 140 mg/m2, a two-compartment population-pharmacokinetic model was developed. Fat-free mass was a covariate for clearance, central volume of distribution, and inter-compartmental clearance, while glomerular filtration rate predicted clearance. Simulation studies showed that based on fixed body surface area-based dosing, renal impairment has a higher impact in increasing melphalan exposure compared with obesity.
Conclusions
The proposed model adequately describes the population pharmacokinetics of melphalan in adult patients receiving a hematopoietic cell transplantation. This model can be used to define the therapeutic window of melphalan, and subsequently to develop individualized dosing regimens aiming for that therapeutic window in all patients.



Similar content being viewed by others
References
Papac R, Galton D, Till M, Wiltshaw E. Preliminary clinical trial of p-di-2-chloroethyl-amino-L-phenylalanine (CB 3025, melphalan) and of di-2-chloroethyl methanesulfonate (CB 1506). Ann N Y Acad Sci. 1958;68:1126–7.
Barlogie B, Shaughnessy J, Tricot G, et al. Treatment of multiple myeloma. Blood. 2016;103:20–33.
Verhoef C, Wilt JHW, Grünhagen DJ, Geel AN, Hagen TLM, Eggermont AMM. Isolated limb perfusion with melphalan and TNF-α in the treatment of extremity sarcoma. Curr Treat Options Oncol. 2007;8:417–27.
Samuels BL, Bitran JD. High-dose intravenous melphalan: a review. J Clin Oncol. 1995;13:1786–99.
Hsieh T, Liao A, Francis JH, et al. Comparison of efficacy and toxicity of intravitreal melphalan formulations for retinoblastoma. PLoS ONE. 2020;15:4–11.
Bayraktar UD, Bashir Q, Qazilbash M, Champlin RE, Ciurea SO. Fifty years of melphalan use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:344–56.
Jain T, Alahdab F, Firwana B, Sonbol MB, Almader-Douglas D, Palmer J. Choosing a reduced-intensity conditioning regimen for allogeneic stem cell transplantation, fludarabine/busulfan versus fludarabine melphalan: a systematic review and meta-analysis. Biol Blood Marrow Transplant. 2019;25:728–33.
Dahi PB, Lazarus HM, Sauter CS, Giralt SA. Strategies to improve outcomes of autologous hematopoietic cell transplant in lymphoma. Bone Marrow Transplant. 2019;54:943–60.
Aljitawi OS, Ganguly S, Abhyankar SH, et al. Phase IIa cross-over study of propylene glycol-free melphalan (LGD-353) and alkeran in multiple myeloma autologous transplantation. Bone Marrow Transplant. 2014;49:1042–5.
Miller KC, Gertz MA, Buadi FK, et al. Comparable outcomes using propylene glycol-free melphalan for autologous stem cell transplantation in multiple myeloma. Bone Marrow Transplant. 2019;54:587–94.
Shaw PJ, Nath CE, Lazarus HM. Not too little, not too much-just right (better ways to give high dose melphalan). Bone Marrow Transplant. 2014;49:1457–65.
Choi T. Is autologous stem cell transplantation still relevant for multiple myeloma? Curr Opin Hematol. 2019;26:386–91.
Gyurkocza B, Sandmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood. 2014;124:344–53.
Dimopoulos MA, Terpos E, Chanan-Khan A, et al. Renal impairment in patients with multiple myeloma: a consensus statement on behalf of the International Myeloma Working Group. J Clin Oncol. 2010;28:4976–84.
Shultes KC, Arp C, Stockerl-goldstein K, Trinkaus K, Defrates S. Transplantation impact of dose-adjusted melphalan in obese patients undergoing autologous stem cell transplantation. Biol Blood Marrow Transplant. 2018;24:687–93.
Nath CE, Shaw PJ, Montgomery K, Earl JW. Population pharmacokinetics of melphalan in paediatric blood or marrow transplant recipients. Br J Clin Pharmacol. 2007;64:151–64.
Sezer O, Heider U, Meineke I, et al. Population pharmacokinetics of melphalan and glutathione S-transferase polymorphisms in relation to side effects. Clin Pharmacol Ther. 2008;83:749–57.
Cho YK, Sborov DW, Lamprecht M, et al. Associations of high-dose melphalan pharmacokinetics and outcomes in the setting of a randomized cryotherapy trial. Clin Pharmacol Ther. 2017;102:511–9.
Mougenot P, **uet F, Fabbro M, et al. Population pharmacokinetics of melphalan, infused over a 24-hour period, in patients with advanced malignancies. Cancer Chemother Pharmacol. 2004;53:503–12.
Mizuno K, Dong M, Fukuda T, Chandra S. Population pharmacokinetics and optimal sampling strategy for model-based precision dosing of melphalan in patients undergoing hematopoietic stem cell transplantation. Clin Pharmacokinet. 2018;57:625–36.
Nath CE, Shaw PJ, Trotman J, et al. Population pharmacokinetics of melphalan in patients with multiple myeloma undergoing high dose therapy. Br J Clin Pharmacol. 2010;69:484–97.
Shah GL, Lin A, Kamrowski A, et al. Successful personalization of propylene glycol free melphalan (PGF-MEL) for multiple myeloma (MM) and AL amyloidosis (AL) patients undergoing autologous hematopoietic stem cell transplant (AHCT) using pharmacokinetic (PK)-directed dosing. Biol Blood Marrow Transplant. 2020;26:S154–5.
Nath CE, Trotman J, Tiley C, et al. High melphalan exposure is associated with improved overall survival in myeloma patients receiving high dose melphalan and autologous transplantation. Br J Clin Pharmacol. 2016;82:149–59.
Schofield RC, Landau HJ, Giralt SA, et al. Measurement of the DNA alkylating agents busulfan and melphalan in human plasma by mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2019;1125:121711.
Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28:481–504.
Keizer RJ, van Benten M, Beijnen JH, Schellens JHM, Huitema ADR. Piraña and PCluster: a modeling environment and cluster infrastructure for NONMEM. Comput Methods Programs Biomed. 2011;101:72–9.
Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44:1051–65.
Dosne AG, Bergstrand M, Harling K, Karlsson MO. Improving the estimation of parameter uncertainty distributions in nonlinear mixed effects models using sampling importance resampling. J Pharmacokinet Pharmacodyn. 2016;43:583–96.
Wählby U, Thomson AH, Milligan PA, Karlsson MO. Models for time-varying covariates in population pharmacokinetic-pharmacodynamic analysis. Br J Clin Pharmacol. 2004;58:367–77.
Evomela Summary of Product Characteristics. Spectrum Pharmaceuticals. 2012.
Alberts DS, Chang SY, Chen HS, et al. Kinetics of intravenous melphalan. Clin Pharmacol Ther. 1979;26:73–80.
Alberts DS, Chang SY, George Chen HS, Evans TL, Moon TE. Oral melphalan kinetics. Clin Pharmacol Ther. 1979;26:737–45.
Levey A, Stevens L, Schmid C, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
Green B, Duffull SB. What is the best size descriptor to use for pharmacokinetic studies in the obese? Br J Clin Pharmacol. 2004;58:119–33.
National Library of Medicine (US): National Center for Biotechnology. PubChem compound summary for CID 460612, melphalan. 2004. https://pubchem.ncbi.nlm.nih.gov/compound/460612. Accessed 22 Nov 2021.
Parmar SR, Bookout R, Shapiro JF, et al. Comparison of 1-day vs 2-day dosing of high-dose melphalan followed by autologous hematopoietic cell transplantation in patients with multiple myeloma. Bone Marrow Transplant. 2014;49:761–6.
Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. N Engl J Med. 1996;335:91–7.
Admiraal R, van Kesteren C, Jol-van Der Zijde CM, et al. Association between anti-thymocyte globulin exposure and CD4+ immune reconstitution in paediatric haematopoietic cell transplantation: a multicentre , retrospective pharmacodynamic cohort analysis. Lancet Haematol. 2015;2:194–203.
Admiraal R, Nierkens S, de Witte MA, et al. Association between anti-thymocyte globulin exposure and survival outcomes in adult unrelated haemopoietic cell transplantation: a multicentre, retrospective, pharmacodynamic cohort analysis. Lancet Haematol. 2017;4:e183–91.
Bartelink IH, Lalmohamed A, van Reij EML, et al. Association of busulfan exposure with survival and toxicity after haemopoietic cell transplantation in children and young adults: a multicentre, retrospective cohort analysis. Lancet Haematol. 2016;3:e526–36.
Langenhorst JB, van Kesteren C, van Maarseveen EM, et al. Fludarabine exposure in the conditioning prior to allogeneic hematopoietic cell transplantation predicts outcomes. Blood Adv. 2019;3(14):2179–87.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was supported by funding from the Sawiris Family Research Fund, Melvin Berlin Family Fund for Myeloma Research, A.C. Israel Foundation, and the Donna and Patrick Martin Foundation. This research was supported in part by National Institutes of Health award number P01 CA23766 and National Institutes of Health/National Cancer Institute Cancer Center Support grant P30 CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The work was performed independently of all funders.
Conflicts of interest/Competing interests
GS reports research funding from Janssen and Amgen unrelated to the current project. MS has served as a consultant for McKinsey & Company, Angiocrine Bioscience, Inc., and Omeros Corporation; has received research funding from Angiocrine Bioscience, Inc. and Omeros Corporation; has served on an advisory board for Kite, a Gilead Company; and has served as a CME speaker for i3Health, all unrelated to the current project. JB has received honoraria for consulting for Race Oncology, AvroBio, Omeros, Sanofi, Advanced Clinical, and BlueRock, all unrelated to the current project. SG has consulted for and received research funding from Amgen, Actinium, Celgene, Johnson & Johnson, and Takeda; has consulted for Jazz Pharmaceuticals, Novartis, Kite, and Spectrum Pharmaceuticals; and has received research funding from Miltenyi unrelated to the current project. MS has received research support from Angiocrine Bioscience, Inc. and Omeros Corporation; has consulted for Angiocrine Bioscience, Inc., Omeros Corporation and McKinsey & Company; has advised for Kite , a Gilead Company; and received a speaking engagement from i3Health. RA has received research grants from Sanofi, unrelated to the current project.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The data and materials for this study are not available.
Code availability
The model codes are available upon request.
Author contributions
RA and GS designed and conducted the research, analyzed the data, and wrote the paper; DC and RS analyzed the samples and wrote the paper; JJB, SG, and MS initiated the research and wrote the paper; AL, NCS, AA, JR, AP, PD, and RT wrote the paper.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shah, G.L., Boelens, J.J., Carlow, D. et al. Population Pharmacokinetics of Melphalan in a Large Cohort of Autologous and Allogeneic Hematopoietic Cell Transplantation Recipients: Towards Individualized Dosing Regimens. Clin Pharmacokinet 61, 553–563 (2022). https://doi.org/10.1007/s40262-021-01093-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-021-01093-z