Abstract
Background and Objectives
Endoxifen is the active metabolite of tamoxifen, and a minimal plasma concentration of 16 nM has been suggested as a threshold above which it is effective in reducing the risk of breast cancer recurrence. The aim of the current analysis was to investigate the cost-effectiveness of therapeutic drug monitoring (TDM)-guided tamoxifen dosing.
Methods
A cost-effectiveness analysis was performed from a Dutch healthcare perspective, using a partitioned survival model and a lifetime horizon. The reduction in subtherapeutic treatment following TDM is modelled as improved rates of recurrence-free survival (RFS) and overall survival (OS) in comparison to standard tamoxifen treatment. A probabilistic sensitivity analysis (PSA) and a series of scenario analyses were performed to assess the robustness of the results.
Results
Base-case results estimated a total increase in life years and quality-adjusted life years (QALYs) for TDM of 0.40 and 0.53, respectively. Total costs for TDM and standard tamoxifen treatment are €32,893 and €39,524, respectively. The TDM intervention results in both more QALYs and less healthcare costs, indicating a dominating effect for TDM. The PSA results indicate that the probability of TDM being cost-effective is 92% when using a willingness-to-pay threshold of €20,000.
Conclusions
TDM-guided dose optimization of tamoxifen is estimated to save costs and increase QALYs for early breast cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The probability of therapeutic drug monitoring (TDM)-guided tamoxifen dosing being cost-effective relative to standard tamoxifen is 92% at a willingness-to-pay (WTP) threshold of €20,000 per QALY gained. |
This analysis suggests that TDM dominates standard tamoxifen care in terms of cost-effectiveness, gaining QALYs, life years and saving costs. |
1 Introduction
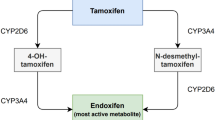
For more than 40 years, tamoxifen has been the standard adjuvant treatment for estrogen receptor-positive (ER+) breast cancer [1]. Treatment regimens often consist of 5 years of tamoxifen for premenopausal women and at least 2.5 years of tamoxifen followed by an aromatase inhibitor (i.e., letrozole, anastrazole or exemestane) in postmenopausal women [2,3,4]. Tamoxifen treatment reduces recurrence rates by approximately one-third compared with control (no tamoxifen treatment) [1]. The effectiveness of tamoxifen is exerted through its active metabolite endoxifen [2].
The rate at which tamoxifen is converted to endoxifen varies greatly between individuals. This means that the same tamoxifen dose does not necessarily translate to the same endoxifen plasma concentrations at the individual level [5]. The results from two retrospective studies investigating the effectiveness of tamoxifen at different plasma concentrations of endoxifen suggested that a minimal endoxifen concentration of 14–16 nM is needed for an optimal risk reduction for recurrence [6,7,8]. In the main retrospective analysis of these two studies, Madlensky et al. reported an endoxifen threshold of 16 nM. This study included 1,370 pre- and postmenopausal early breast cancer patients who were treated with tamoxifen in the adjuvant setting for 5 years. Patients with an endoxifen concentration below the lowest quintile (< 16 nM) showed a 26% lower disease-free survival compared to patients with endoxifen levels above 16 nM [6]. Findings from subsequent studies indicate that endoxifen concentrations remained below 16 nM in 20–24% of patients treated with tamoxifen [6, 8, 9]. These findings suggested that the effectiveness of tamoxifen treatment may be enhanced in patients with subtherapeutic endoxifen concentrations.
Therapeutic drug monitoring (TDM)-guided dose individualization of tamoxifen is a strategy to attain endoxifen levels above a predefined threshold, thereby enhancing its therapeutic effectiveness. TDM consists of regular monitoring of endoxifen plasma concentrations and increasing tamoxifen dosage when the endoxifen concentration is below 16 nM. The feasibility of TDM for breast cancer patients treated with adjuvant tamoxifen has recently been established in the TOTAM study, in which the number of patients with subtherapeutic endoxifen levels was reduced by 50% within 3 months from the first assessment of endoxifen levels [10]. As such, TDM is expected to increase the effectiveness of tamoxifen treatment at the expense of additional healthcare resource use due to TDM. This raises the question of whether TDM is a cost-effective intervention relative to standard tamoxifen treatment.
The cost-effectiveness of TDM of tamoxifen in the Netherlands was investigated previously, in the absence of data from a clinical trial on TDM, from a theoretical perspective [11]. This study relied on several assumptions, for example regarding the outcomes of TDM, additional healthcare resource use and patients’ quality of life, which were not in line with the current implementation and results of TDM in TOTAM. The results of that study indicated that TDM is cost-effective, but an important question remains regarding whether the same outcome is obtained from a cost-effectiveness analysis (CEA) based on the implementation and results of a prospective TDM clinical trial. Here we reported the results of a CEA of TDM-guided adjuvant tamoxifen therapy in hormone-sensitive breast cancer from a Dutch healthcare perspective [12, 13].
2 Methods
2.1 Patients, Intervention and Comparator
The results from the prospective, open-label TOTAM study (Dutch Trial Registry; NL6918) that included 145 patients with breast cancer who were treated with TDM-guided adjuvant tamoxifen dosing were used [10]. Patient and disease characteristics are presented in Appendix I. This study was designed to investigate the feasibility of establishing a predefined endoxifen concentration (≥ 16 nM) in a period of 6 months after start with tamoxifen. All patients initially received a tamoxifen dose of 20 mg once daily. The tamoxifen dose was escalated over time to a maximum of 40 mg once daily for patients with an endoxifen concentration below 16 nM. Furthermore, a switch to an aromatase inhibitor (i.e., letrozole, anastrozole or exemestane) can be a valid option for the subgroup of patients with persistently low endoxifen levels after a tamoxifen dose adjustment to 40 mg. Endoxifen plasma concentrations were monitored at 3, 4.5 and 6 months after start of treatment, followed by dose escalations when applicable. The results from TOTAM showed that in 20.7% of the patients the endoxifen concentration was below 16 nM after 3 months of treatment. After 6 months, 11.0% of the patients remained below the threshold (p = 0.007). Standard tamoxifen treatment was assumed to consist of 20 mg once daily, as recommended in the treatment guideline for early breast cancer. Given the lack of (dose-related) adverse events that are associated with tamoxifen, these were not included in the analysis [9, 14, 15].
2.2 Model Structure
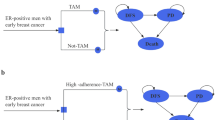
A partitioned survival model was constructed in Excel (Microsoft 2016, Redmond, WA, USA) that consisted of three health states: recurrence-free survival (RFS), recurrent disease (RD), and death (Fig. 1). All patients started the model in RFS, where they could either remain in the next model cycle, transition to RD upon diagnosis with a local recurrence or distant metastases, or die. Patients in RD can either remain in RD in the next model cycle or die. A model cycle length of 3 months is used, which corresponds with the duration of data collection to reach the primary endpoint in the TOTAM study.
2.3 Perspective, Time Horizon and Discounting
The analysis was performed from a Dutch healthcare perspective. A scenario analysis was performed that included costs due to lost productivity from paid and unpaid work to approximate a societal perspective. A lifetime time horizon was used to capture all relevant costs and effects, which were discounted—according to the Dutch guidelines for economic evaluations in healthcare—at an annual rate of 4% and 1.5%, respectively [16, 17].
2.4 Model Input Parameters
2.4.1 Survival Estimates
Data on RFS and overall survival (OS) were extracted from a meta-analysis by the early breast cancer trialists’ collaborative group (EBCTCG) on 10,238 women who received treatment with adjuvant tamoxifen for about 5 years or no tamoxifen, with a follow-up time period of 10–15 years [1]. Patient-level data were estimated using the methods described by Tierney et al. [18] and Hoyle and Henley [19]. Extrapolations of RFS and OS were performed using the package ‘survival’ in R-statistics, using exponential, Weibull, lognormal and log-logistic parametric functions [20]. For both RFS and OS, the Weibull curves were selected based on statistical fit as indicated by the Akaike Information Criterion (AIC), visual fit and clinical plausibility. OS extrapolations were adjusted for background mortality using life tables provided by Statistics Netherlands (CBS). To prevent logical inconsistencies, RFS was restricted using OS as its upper bound in th model.
The clinical benefit of TDM was modelled based on the assumption that the RFS and OS curves of patients treated with a standard tamoxifen dose (20 mg tamoxifen once daily; without the intervention TDM) included 20% of patients with subtherapeutic endoxifen levels below 16 nM, which was reduced to 10% with TDM in line with results from TOTAM and other studies [6, 9, 21]. It was assumed that RFS and OS of patients with subtherapeutic endoxifen levels are equal to RFS and OS of patients who were not treated with tamoxifen in the EBCTCG study. This assumption was supported by clinical and pharmacological expert opinion [21].
2.4.2 Health-Related Quality of Life
Health-related quality-of-life data were collected in TOTAM using the EQ-5D-5L [22]. Subsequently, the descriptive health profiles were valued using the Dutch tariff to generate utilities for patients in RFS [23]. The average utility value obtained after 3 months of treatment (i.e., before any dose adjustments) was used to represent patients receiving standard tamoxifen treatment, and the average utility value obtained after 6 months of treatment (i.e., after dose adjustments were performed if needed) was used to represent patients receiving TDM-guided adjuvant tamoxifen dosing. In absence of data from TOTAM on the utilities of patients in RD, a utility value for RD was sourced from the literature. The results from a study in Finland that assessed the utilities of patients in different stages of breast cancer provided a value of 0.74 (standard deviation (SD) ± 0.26) for metastatic disease [24]. The utilities that were used in the model are presented in Table 1 alongside other model input parameters. An age-dependent decline in utility was applied using the method by Ara and Brazier [25].
2.4.3 Resource Use and Costs
Drug acquisition costs for TDM-guided adjuvant tamoxifen were calculated using the proportions of patients receiving each dose (i.e., 20, 30 or 40 mg once daily) in TOTAM or 20 mg once daily for standard tamoxifen. The costs of tamoxifen and anastrozole were sourced from the Dutch national healthcare institute (Zorginstituut Nederland, Diemen, The Netherlands) [26, 27]. It was assumed that tamoxifen was provided per 3-monthly prescription, for which pharmacy drug dispensing costs were included. Healthcare resource use in RFS was based on the Dutch (adopted from the ESMO guidelines) treatment guideline for breast cancer [28,29,30,31], and included outpatient hospital visits and visits to the general practitioner (GP). Health state costs for RFS were subdivided to account for differences in resource use during the first year, use experienced between years 1 and 5, and after 5 years. Intervention costs occurred only during the first year and consisted of three additional outpatient oncology visits, a phone consultation and two endoxifen tests (€95 each). It was assumed that after 1 year, resource use in RFS was the same for both treatments. Unit costs were derived from the Dutch manual for cost research: methodology of cost research and reference prices for economic evaluations in health care, and published hospital declaration prices. The health-state costs in RD were assumed to be the same for both interventions, and were informed by published healthcare cost estimates of Dutch patients with local and distant recurrence during the first year and thereafter [32]. A weighted, per-cycle cost estimate is calculated assuming an average time spent in RD of 3 years and equal proportions of local and distant metastases [33]. The RD health-state costs included drug costs, surgical procedures, radiotherapy, diagnostic resources, and in- and outpatient visits. All costs included in the analysis are expressed in 2019 euros, and costs sourced from a prior year were updated using the consumer price indexes provided by Statistics Netherlands (CBS). An overview of the health-state costs for RFS and RD is provided in Table 1.
2.4.4 Productivity Costs
Productivity loss data were collected in TOTAM using the iMTA Productivity Costs Questionnaire and included short-term absence from paid work, presenteeism at paid work, and productivity losses at unpaid work as reported in the 28-day recall period [34]. Reported productivity losses as a result of long-term absenteeism starting before tamoxifen treatment, during primary cancer treatment, were excluded from the final estimate. Costs due to productivity losses at paid work were valued using the average hourly wage for women aged 55–60 years (€27.18/h) as provided by Statistics Netherlands (CBS). For unpaid work this was valued using the reference cost of informal care (€14.00/h) provided by the Dutch guidelines for health economic evaluations [29]. A maximum loss of productivity was assumed (i.e., based on the average of reported productivity at baseline) for both RD and death. Productivity losses were included for all subjects up to the current Dutch retirement age of 67 years.
2.5 Sensitivity Analyses
2.5.1 Probabilistic Sensitivity Analysis
A probabilistic sensitivity analysis is performed to assess the sensitivity of the results to the uncertainty surrounding all input parameters. Parameter uncertainty is expressed using as a distribution around the mean values with a corresponding standard error (SE). If SEs are not available, an arbitrary percentage of the mean value is applied using 10% of the mean for fixed unit costs and 20% for healthcare resource use parameters, reflecting the expectation that resource use is more variable than unit costs. The proportions of patients receiving a tamoxifen dose escalation (i.e., in TDM-guided adjuvant tamoxifen dosing) are varied using a Dirichlet distribution. The uncertainty surrounding the extrapolated survival curves is estimated using a Cholesky correlation matrix [35]. In each model simulation a random value is drawn for each parameter from its corresponding distribution. Simulations are repeated 1,000 times and cost-effectiveness outcomes are presented in a cost-effectiveness (CE)-plane. The probability of cost-effectiveness is represented by the percentage of simulations below the applied willingness-to-pay (WTP) threshold. The probability of cost-effectiveness is assessed at a WTP of €20,000, in line with the Dutch standard [36, 37].
2.6 Scenario Analyses
In addition to the base-case analysis, several scenario analyses were performed to assess the sensitivity of the results to alternative values for model input parameters. In scenario 1 (S1), three different sets of survival curves were used: an absolute increase of 20% (S1a; 100% on target), 15% (S1b; 95% on target) or 5% (S1c; 85% on target) of the patients in the whole population with an endoxifen level ≥ 16 nM, in contrast to the absolute increase of 10% (90% on target) who benefits as in the base case. S1a therefore describes the maximal attainable effectiveness of TDM for another distribution of patients below the predefined endoxifen threshold of 16 nM. S1b represents of a scenario where the TDM threshold is set at a lower value. S1c describes a scenario in which TDM is less effective at improving endoxifen serum concentrations. Analogous to the base case, it is assumed that that the effect of tamoxifen below this threshold is equal to control. A fourth set of survival curves (S1d) was constructed using the survival curve of S1a where all patients are above the threshold in combination with the assumption that an improved recurrence rate can be found in patients with endoxifen levels above 16 nM [hazard ratio (HR) 0.74] [6]. Due to uncertainty in duration of tamoxifen treatment in the Madlensky paper, the HR assumption of 0.74 was not included in the base-case scenario. After applying the HR, a curve is obtained representing the RFS below the 16 nM threshold [6]. This survival curve is again combined with the curve of S1a to construct a final curve where 90% is above the 16 nM threshold and 10% is below based on data obtained in the TOTAM trial.
In scenario 2 (S2), two different per-cycle cost estimates for RD are used, being either almost twice as high (S2a) or almost half the original estimate (S2b) per cycle for the base-case analysis [32]. Parameter estimates are informed by the literatur,e which describes costs estimates of costs experienced after the first year of metastases (S2a) and costs experienced during the first year of recurrence (S2b). Using these alternative cost estimates, the influence of RD costs on cost-effectiveness is assessed. The cost estimates are also informed by the costs for local and distant recurrence. In this situation an unweighted average was applied [32]. For the low estimate, costs are based on local recurrence during the first year of disease. For the high estimate, experienced costs are based on the metastatic disease after the first year [32].
In scenario 3 (S3), costs due to productivity losses were included to approximate a societal perspective. Lastly, scenario analyses were performed based on alternative parametric functions for the extrapolations of RFS and OS, using loglogistic (S4a), lognormal (S4b) and exponential (S4c) parametric functions for both RFS and OS.
3 Results
3.1 Base-Case and Probabilistic Sensitivity Analysis
The base-case results are presented in Table 2 and show a total increase in QALYs and LYs for TDM of 0.53 and 0.40, respectively. Total lifetime costs were €32,893 for TDM-guided adjuvant tamoxifen therapy and €39,524 for standard tamoxifen (20 mg once daily, without TDM). In terms of cost-effectiveness, TDM dominates standard tamoxifen due to both positive incremental QALYs and negative incremental costs (i.e., TDM is cost saving in comparison to standard tamoxifen). An explanation for this result is the lower risk of recurrent disease—and hence, lower costs associated with this stage of disease—with an adequate endoxifen level. Probabilistic sensitivity analysis results for the base-case model are presented in the CE-plane in Fig. 2. The probability that TDM-guided adjuvant tamoxifen therapy is cost-effective relative to standard tamoxifen is 92% at a WTP threshold of €20,000 per QALY gained [37].
Cost-effectiveness plane of the probabilistic sensitivity analysis for base-case model in the cost-effectiveness analysis of therapeutic drug monitoring of tamoxifen adjuvant therapy versus standard of care (without TDM intervention). Straight line indicates the Dutch conservative willingness-to-pay threshold of €20,000. All model simulations below this threshold are considered cost-effective from a healthcare perspective
3.2 Scenario Analyses of Endoxifen Threshold (S1a, S1b and S1c)
S1a assessed the effect of assuming that all patients achieve endoxifen concentrations ≥ 16 nM. These survival curves aimed to estimate the maximum obtainable effect of TDM for tamoxifen treatment following our modelling approach. This scenario results in a total of 1.03 incremental QALYs, with a dominant incremental cost-effectiveness ratio (ICER) per QALY gained for TDM-guided adjuvant tamoxifen therapy. Deterministic results are presented in Table 2 and a graphic representation is shown in Fig. 3. In both the base-case scenario and most of the scenario analyses the intervention TDM dominates. Scenario 1b aims to provide an estimate of the cost-effectiveness of TDM when using a lower threshold of 14 nM. According to our results TDM-guided adjuvant tamoxifen therapy is dominant given this scenario as a result of an increase in LYs gained. Scenario 1c provides an opposing scenario where the effectiveness of TDM improving the endoxifen concentrations is lower than found by the TOTAM study. This scenario illustrates only 85% of the population achieving endoxifen concentrations ≥ 16 nM after TDM, rather than 90% of the population as in the base case. This results in an incremental increase in QALYs of 0.71 and an ICER of €2,177 per QALY gained, which means TDM-guided adjuvant tamoxifen therapy would induce higher costs though remaining well under the WTP threshold.
Tornado diagram illustrating the effect of alternative parameter values in a deterministic sensitivity analysis on the incremental cost-effectiveness ratio for therapeutic drug monitoring of tamoxifen adjuvant therapy versus standard of care (without TDM intervention). RFS recurrence-free survival, RD recurrent disease
3.3 Scenario Analyses Costs in Progressed Disease (S1d)
A third set of survival curves (S1d) is constructed using the survival curve of S1a and the hazard ratio of 0.74 for recurrence rate in patients with endoxifen levels above 16 nM based on the Madlensky data. This results in an increase in incremental QALYs of 0.76 and a dominant ICER for TDM-guided adjuvant tamoxifen therapy (Table 2).
3.4 Scenario Analyses Costs in Progressed Disease (S2a and S2b)
The second scenario included two alternative estimates, a high and a low estimate, for the per-cycle healthcare costs in RD. The results of these scenarios indicated that for both the high and the low estimate cost-effectiveness remains TDM-guided adjuvant tamoxifen therapy dominated, despite the large difference in total costs between the alternatives. These results illustrated that the costs associated with recurrence of breast cancer are an important driver of the cost-effectiveness of TDM-guided adjuvant tamoxifen therapy. Given that TDM aims to improve treatment effectiveness and therefore is expected to reduce recurrence, assuming higher costs for RD improves the cost-effectiveness of TDM relative to standard tamoxifen (Table 2).
3.5 Scenario Productivity Loss (S3)
In this scenario total incremental costs are increased to − €9549, resulting in an increasingly dominant ICER per QALY gained for TDM-guided adjuvant tamoxifen therapy. In this scenario, 30% of the difference in incremental costs can be attributed to the reduction in lost productivity resulting from fewer transitions to RD over time with TDM-guided dose individualization (Table 2).
3.6 Alternative Curve Fit (S4a–c)
In these scenarios the costs and effects of TDM-guided adjuvant tamoxifen therapy are presented when using alternative curve fits for survival extrapolation. Importantly, in all scenarios TDM-guided adjuvant tamoxifen therapy resulted in higher costs for resource use, and lower costs for treatment costs in RD and RFS. The difference in QALYs gained was very small and the increment in QALYs is mainly caused by the difference in life expectancy, because more people stayed longer in RFS (Table 2).
4 Discussion
Our study is the first CEA of TDM-guided dose individualization of tamoxifen in patients with early breast cancer that is based on data obtained from a clinical trial. The results indicated that TDM strategy is cost-effective for patients with early ER+ breast cancer compared to standard tamoxifen. Base-case results showed that TDM-guided adjuvant tamoxifen therapy is dominant over standard tamoxifen due to incremental QALYs of 0.53 and cost savings of €6631.
In a previous CEA, TDM of tamoxifen resulted in a similar outcome regarding the cost-saving potential of TDM when also assuming an endoxifen threshold of 16 nM [11]. In contrast to the current findings, the analysis by van Nuland et al. indicated an increment in QALYs of 0.0115, whereas our results showed a much higher estimate, including age-adjustment [11]. However, considerable differences between both studies can be identified in terms of methodology and data used to inform input parameters. In addition to using trial data from TOTAM and different literature sources to inform the current study, the main differences involve the assumptions for OS, and a different approach to the modelling of the clinical benefit of TDM. Regarding assumptions for OS, an important difference is that the current study assumed a difference in OS between standard care and TDM-guided tamoxifen dosing, whereas van Nuland et al. assumed equal OS between treatments. Regarding the modelling of the clinical benefit of TDM-guided adjuvant tamoxifen therapy, for the current study survival curves were constructed from patients who received about 5 years of tamoxifen or no tamoxifen based on the assumption that patients with subtherapeutic endoxifen levels have the same OS and RFS as patients who were not treated with tamoxifen, and represent 20% of the patients who were treated with standard tamoxifen. In contrast, van Nuland et al. applied an HR from Madlensky et al. to obtain RFS for high and low endoxifen levels. However, this HR did not represent patients who were treated with tamoxifen for a period approximating 5 years (Madlensky, personal communication), and therefore its applicability to the EBCTCG data is questionable.
The strengths of this economic evaluation included the availability of prospectively collected data by the TOTAM study, and therefore its representative description of the Dutch breast cancer population. Further, to date there have been no published QoL scores or productivity losses for Dutch breast cancer patients treated with tamoxifen in the adjuvant setting.
This analysis had some limitations. Foremost, these results are based on some important assumptions regarding the exposure-response relationship of endoxifen affecting the potential effectiveness of TDM and should, therefore, be interpreted carefully. Firstly, the improvement seen in the proportion of patients considered above the threshold is based on a threshold value for endoxifen of 16 nM. Also, the expected effect below this threshold is assumed to be equal to control in our model. However, based on the Madlensky data, it was not possible to construct overall survival rates, because of uncertainty in the duration of treatment with tamoxifen. Scenario 1d, which assumed that women with an endoxifen level ≥ 16 nM had a 26% lower recurrence rate, showed an enhanced dominant effect for TDM compared with the base-case scenario. Despite the initial evidence for an exposure-response relationship, two later prospective clinical studies reported no associations between endoxifen plasma concentrations and clinical outcome [38, 39]. However, based on the considerable amount of criticism published following these reported results, no affirmative prospective evidence exists on the complete absence of this relationship [40,41,42]. In addition to this, if a threshold exists, currently no agreement exists on the exact value of this threshold as two other thresholds have been suggested at a lower value of 14 nM and 9 nM [43,44,45]. Currently, the proportion of breast cancer patients with subtherapeutic endoxifen levels are determined and incorporated in the model based on the threshold first reported by Madlensky and colleagues of 16 nM [6]. Importantly, all issues of uncertainty in assumptions were included in the sensitivity analysis. If the true value of this threshold is lower (S1a–c), this could imply that a lower proportion of patients’ treatment is categorized as subtherapeutic before TDM. Because the method of survival extrapolation was based on these proportions, this could have implications for the effect of TDM, as well as lower its cost-effectiveness. The uncertainty regarding the effectiveness of tamoxifen below the current threshold of 16 nM is an important source of uncertainty. Yet, the cost-effectiveness model can be adjusted to re-evaluate the cost-effectiveness of TDM-guided adjuvant tamoxifen therapy in the light of new developments.
A prospective randomized controlled TDM study could provide clarity. However, such a trial is probably not feasible since it would require many thousands of patients to participate and a follow-up period of more than a decade [40]. To break out of this potential dead end, physicians are encouraged to implement TDM—a feasible intervention—in the meantime in clinical practice, pending further prospective data [21].
Further limitations pertained to data availability and uncertainty in underlying assumptions. First, the lack of prognostics on the influence of TDM-guided adjuvant tamoxifen therapy on RFS and OS based on prospective clinical trials required additional assumptions and computation of the expected effect, introducing additional uncertainty [40].
A final important consideration was based on the incidence at which women with ER+ early breast cancer are treated with tamoxifen for a total of 5 years. As described in the introduction, post-menopausal women often start treatment with tamoxifen and switch to an aromatase inhibitor (i.e., letrozole, anastrozole or exemestane) after 2–3 years of treatment [46]. Considering a significant proportion of women with breast cancer are post-menopausal, this implicates a smaller role for TDM in this subgroup of patients, as compared to breast cancer patients who are treated with tamoxifen for 5 years.
5 Conclusion
In conclusion, the current economic evaluation aimed to determine the cost-effectiveness of TDM for adjuvant tamoxifen therapy—resulting in dose optimization or a switch in pharmacotherapy to an aromatase inhibitor—compared to the current standard of care in the Netherlands. Our results indicated that TDM-guided adjuvant tamoxifen therapy dominated standard tamoxifen in terms of cost-effectiveness, gaining QALYs (0.53) and life years (0.40) and saving costs (€6631). The results of this economic evaluation indicate that TDM provides good value for money, which may support policy makers at the hospital, insurer and Dutch national level in decisions on the routine implementation of TDM for tamoxifen adjuvant therapy in the clinical setting.
References
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. https://doi.org/10.1016/S0140-6736(05)66544-0.
Sanchez-Spitman AB, Swen JJ, Dezentje VO, Moes DJAR, Gelderblom H, Guchelaar HJ. Clinical pharmacokinetics and pharmacogenetics of tamoxifen and endoxifen. Expert Rev Clin Pharmacol. 2019;12(6):523–36. https://doi.org/10.1080/17512433.2019.1610390.
Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO Clinical Practice Guideline Focused Update. J Clin Oncol. 2019;37(5):423–38. https://doi.org/10.1200/JCO.18.01160.
Visvanathan K, Fabian CJ, Bantug E, et al. Use of endocrine therapy for breast cancer risk reduction: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2019;37(33):3152–65. https://doi.org/10.1200/JCO.19.01472.
Antunes MV, Linden R, Santos TV, et al. Endoxifen levels and its association with CYP2D6 genotype and phenotype: evaluation of a Southern Brazilian population under tamoxifen pharmacotherapy. Ther Drug Monit. 2012;34(4):422–31. https://doi.org/10.1097/FTD.0b013e318260b46e.
Madlensky L, Natarajan L, Tchu S, et al. Tamoxifen metabolite concentrations, CYP2D6 genotype and breast cancer outcomes. Clin Pharmacol Ther. 2011;89(5):718–25. https://doi.org/10.1038/clpt.2011.32.
Koolen SLW, Bins S, Mathijssen RHJ. Individualized tamoxifen dose escalation—letter. Clin Cancer Res. 2016;22(24):6300. https://doi.org/10.1158/1078-0432.CCR-16-1967.
Jager NGL, Rosing H, Schellens JHM, Linn SC, Beijnen JH. Tamoxifen dose and serum concentrations of tamoxifen and six of its metabolites in routine clinical outpatient care. Breast Cancer Res Treat. 2013. https://doi.org/10.1007/s10549-013-2826-1.
Fox P, Balleine RL, Lee C, et al. Dose escalation of tamoxifen in patients with low endoxifen level: evidence for therapeutic drug monitoring —the TADE study. Clin Cancer Res. 2016;22(13):3164–71. https://doi.org/10.1158/1078-0432.CCR-15-1470.
Braal L, Jager A, Lommen KM, et al. 191P Therapeutic drug monitoring of tamoxifen to improve adjuvant treatment of hormone sensitive breast cancer: the TOTAM study. Ann Oncol. 2020;31:S319. https://doi.org/10.1016/j.annonc.2020.08.313.
van Nuland M, Vreman RA, ten Ham RMT, et al. Cost-effectiveness of monitoring endoxifen levels in breast cancer patients adjuvantly treated with tamoxifen. Breast Cancer Res Treat. 2018;172(1):143–50. https://doi.org/10.1007/s10549-018-4886-8.
Dutch Trial Register [Internet]. NTR6918, the TOTAM study: Therapeutic Drug Monitoring guided tamoxifen dosing: a feasibility study in patients with hormone positive breast cancer. https://www.trialregister.nl/trial/6918
Food and Drug Administration [Internet]. Summary of Product Characteristics tamoxifen. https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/17970s053lbl.pdf. Accessed 9 June 2020.
Hertz DL, Deal A, Ibrahim JG, et al. Tamoxifen dose escalation in patients with diminished CYP2D6 activity normalizes endoxifen concentrations without increasing toxicity. Oncologist. 2016;21(7):795–803. https://doi.org/10.1634/theoncologist.2015-0480.
Dezentjé VO, Opdam FL, Gelderblom H, et al. CYP2D6 genotype- and endoxifen-guided tamoxifen dose escalation increases endoxifen serum concentrations without increasing side effects. Breast Cancer Res Treat. 2015;153(3):583–90. https://doi.org/10.1007/s10549-015-3562-5.
Dutch Institute National Health Care (Zorginstituut Nederland). Richtlijn voor het uitvoeren van economische evaluaties in de gezondheidzorg (Protocol for the execution of economic evaluation in healthcare). 2016;(november):120. https://www.ispor.org/PEguidelines/source/NL-Economic_Evaluation_Guidelines.pdf.
Hakkaart-van Roijen L, van der Linden N, Bouwmans C, Kanters T, Swan Tan S. Kostenhandleiding: Methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg. Zorginstituut Ned. 2016:1–73. [Internet] https://www.zorginstituutnederland.nl/publicaties/publicatie/2016/02/29/richtlijn-voor-het-uitvoeren-van-economische-evaluaties-in-de-gezondheidszorg.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8(1):16. https://doi.org/10.1186/1745-6215-8-16.
Hoyle MW, Henley W. Improved curve fits to summary survival data: Application to economic evaluation of health technologies. BMC Med Res Methodol. 2011;11(1):139. https://doi.org/10.1186/1471-2288-11-139.
Terneau T. A Package for Survival Analysis in R. R package version 3.2-10. 2021 [Internet]. https://cran.r-project.org/package=survival. Accessed 9 June 2020.
Braal CL, Jager A, Oomen-De Hoop E, et al. Therapeutic drug monitoring of endoxifen for tamoxifen precision dosing: feasible in patients with hormone-sensitive breast cancer. Clin Pharmacokinetic. 2021. https://doi.org/10.1007/S40262-021-01077-Z.
Janssen MF, Pickard AS, Golicki D, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res. 2013;22(7):1717–27. https://doi.org/10.1007/s11136-012-0322-4.
Versteegh M, Vermeulen K, Evers S, de Wit GA, Prenger R, Stolk E. Dutch tariff for the five-level version of EQ-5D. Value Health. 2016;19(4):343–52. https://doi.org/10.1016/j.jval.2016.01.003.
Rautalin M, Färkkilä N, Sintonen H, et al. Health-related quality of life in different states of breast cancer—comparing different instruments. Acta Oncol (Madr). 2018;57(5):622–8. https://doi.org/10.1080/0284186X.2017.1400683.
Ara R, Brazier JE. Populating an economic model with health state utility values: Moving toward better practice. Value Health. 2010;13(5):509–18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
Dutch Institute National Health Care (Zorginstituut Nederland). Drugprices [Internet] https://www.medicijnkosten.nl/. Accessed 9 June 2020.
Costs of Pharmaceutical care | Farmacotherapeutisch Kompas. [Internet]. https://www.farmacotherapeutischkompas.nl/algemeen/kosten. Accessed 9 June 2020.
Dutch Institute National Health Care (Zorginstituut Nederland). [Internet]. Verbetersignalement Zinnige nacontrole bij vrouwen behandeld voor borstkanker. 2016. https://www.zorginstituutnederland.nl/publicaties/rapport/2016/10/31/zinnige-zorg-verbetersignalement-zinnige-nacontrole-bij-vrouwen-behandeld-voor-borstkanker. Accessed 9 June 2020.
Hakkaart-van Roijen L, van der Linden N, Bouwmans C, Kanters T, Swan Tan S. Kostenhandleiding: methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg. Zorginstituut Ned. 2016:1–73 [internet] https://www.zorginstituutnederland.nl/richtlijnen.
Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194–220. https://doi.org/10.1093/annonc/mdz173.
Cardoso F, Senkus E, Costa A, et al. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4). Ann Oncol. 2018;29(8):1634–57. https://doi.org/10.1093/annonc/mdy192.
Seferina SC, Ramaekers BLT, de Boer M, et al. Cost and cost-effectiveness of adjuvant trastuzumab in the real world setting: a study of the Southeast Netherlands Breast Cancer Consortium. Oncotarget. 2017;8(45):79223–33. https://doi.org/10.18632/oncotarget.16985.
Koleva-Kolarova RG, Oktora MP, Robijn AL, et al. Increased life expectancy as a result of non-hormonal targeted therapies for HER2 or hormone receptor positive metastatic breast cancer: a systematic review and meta-analysis. Cancer Treat Rev. 2017;55:16–25. https://doi.org/10.1016/j.ctrv.2017.01.001.
Bouwmans C, Krol M, Severens H, Koopmanschap M, Brouwer W, Van RLH. The iMTA productivity cost questionnaire: a standardized instrument for measuring and valuing health-related productivity losses. Value Health. 2015;18(6):753–8. https://doi.org/10.1016/j.jval.2015.05.009.
Hoyle MW, Henley W. Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med Res Methodol. 2011. https://doi.org/10.1186/1471-2288-11-139.
Dutch Institute National Health Care (Zorginstituut Nederland). Richtlijn voor het uitvoeren van economische evaluaties in de gezondheidzorg (Protocol for the execution of economic evaluation in healthcare). 2016;(november):120 [Internet] https://www.zorginstituutnederland.nl/publicaties.
Versteegh MM, Ramos IC, Buyukkaramikli NC, Ansaripour A, Reckers-Droog VT, Brouwer WBF. Severity-adjusted probability of being cost effective. Pharmacoeconomics. 2019;37(9):1155–63. https://doi.org/10.1007/s40273-019-00810-8.
Sanchez-Spitman A, Dezentjé V, Swen J, et al. Tamoxifen pharmacogenetics and metabolism: results from the prospective CypTAM study. J Clin Oncol. 2019;37(8):636–46. https://doi.org/10.1200/JCO.18.00307.
Neven P, Jongen L, Lintermans A, et al. Tamoxifen metabolism and efficacy in breast cancer: a prospective multicenter trial. Clin Cancer Res. 2018;24(10):2312–8. https://doi.org/10.1158/1078-0432.CCR-17-3028.
Braal CL, Beijnen JH, Koolen SLW, et al. Relevance of endoxifen concentrations: absence of evidence is not evidence of absence. J Clin Oncol. 2019;37(22):1980–1. https://doi.org/10.1200/JCO.19.00418.
Goetz MP, Suman VJ, Nakamura Y, Kiyotani K, Jordan VC, Ingle JN. Tamoxifen metabolism and breast cancer recurrence: a question unanswered by CYPTAM. J Clin Oncol. 2019;37(22):1982–3. https://doi.org/10.1200/JCO.19.00504.
Brauch H, Schroth W, Mürdter T, Schwab M. Tamoxifen pharmacogenetics and metabolism: the same is not the same. J Clin Oncol. 2019;37(22):1981–2. https://doi.org/10.1200/JCO.19.00507.
Sanchez-Spitman AB, Moes DJAR, Swen JJ, et al. Exposure–response analysis of endoxifen serum concentrations in early-breast cancer. Cancer Chemother Pharmacol. 2020. https://doi.org/10.1007/s00280-020-04089-x.
P S, T M, D E, et al. Tamoxifen metabolism predicts drug concentrations and outcome in premenopausal patients with early breast cancer. Pharmacogenom J. 2015;15(1):84–94. https://doi.org/10.1038/TPJ.2014.34.
Helland T, Henne N, Bifulco E, et al. Serum concentrations of active tamoxifen metabolites predict long-term survival in adjuvantly treated breast cancer patients. Breast Cancer Res. 2017;19(1):125. https://doi.org/10.1186/s13058-017-0916-4.
Morales L, Neven P, Paridaens R. Choosing between an aromatase inhibitor and tamoxifen in the adjuvant setting. Curr Opin Oncol. 2005;17(6):559–65. https://doi.org/10.1097/01.cco.0000180434.31991.bf.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by an MRACE grant of the Erasmus Medical Center, the Netherlands (grant number 2017-17108).
Conflict of interest
None declared (CLB, AK, AJ, SK, RM, ICR, PW, CUdG).
Ethical approval
The study was approved by the Local Ethics Committee (Erasmus MC, Rotterdam; MEC 17-548) and was registered at the Dutch Trial Registry (NL6918).
Consent to participate
Written informed consent was obtained from all patients participating in the TOTAM trial.
Consent for publication
Not applicable.
Availability of data and material
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Author contributions
LB collected and assembled the data and led the writing of the manuscript. LB and AK led the development of the cost effectiveness model. All authors participated in data analysis and interpretation, creation of figures and tables, and writing and editing of the manuscript.
Appendix
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Braal, C.L., Kleijburg, A., Jager, A. et al. Therapeutic Drug Monitoring-Guided Adjuvant Tamoxifen Dosing in Patients with Early Breast Cancer: A Cost-Effectiveness Analysis from the Prospective TOTAM Trial. Clin Drug Investig 42, 163–175 (2022). https://doi.org/10.1007/s40261-021-01114-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-021-01114-6