Abstract
Background and Objective
SUVN-502, a selective 5-HT6 receptor antagonist, was found to be active in preclinical models of cognitive deterioration suggesting a potential role in the treatment of dementia related to Alzheimer’s disease. The objective of this study was to characterize the safety, tolerability and pharmacokinetics of SUVN-502 in healthy young adults and elderly subjects following single and multiple oral doses.
Methods
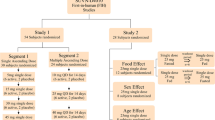
Single doses (5, 15, 50, 100 and 200 mg SUVN-502) and multiple doses (50, 100 and 130 mg SUVN-502 once daily for 7 days) were evaluated in healthy young adults and multiple doses (50 and 100 mg SUVN-502 once daily for 14 days) were evaluated in elderly subjects using randomized, double-blind, placebo-controlled, dose-escalating study designs. The effect of food, gender and age on SUVN-502 pharmacokinetics (100 mg single dose) was evaluated using an open-label, two-period, randomized, fed and fasted in a crossover design. SUVN-502 and M1 (major metabolite of SUVN-502) were monitored using validated analytical methods.
Results
SUVN-502 is safe and well tolerated up to the highest tested single dose of 200 mg in healthy young adults and multiple doses up to 130 mg for 7 days and 100 mg for 14 days in healthy young adults and elderly subjects, respectively. Exposures of SUVN-502 and M1 were more than dose-proportional over the evaluated dose range. Food and gender did not have a clinically meaningful effect on SUVN-502 exposure. The mean SUVN-502 total (AUC0–∞, and AUC0–last) and peak exposures (Cmax) were 2.9- and 2.2-fold higher, respectively, in elderly subjects compared to young subjects. Steady-state was achieved for SUVN-502 and M1 within 7 days after once-daily dosing of SUVN-502.
Conclusions
SUVN-502 exhibited an acceptable safety, tolerability and pharmacokinetic profile in healthy young adults and elderly subjects. Based on the above results, 50 and 100 mg once-daily doses of SUVN-502 were advanced to Phase 2 evaluation in patients with moderate AD.






Similar content being viewed by others
References
Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J Neurol Neurosurg Psychiatry. 1999;66(2):137–47.
Thompson S, Lanctot KL, Herrmann N. The benefits and risks associated with cholinesterase inhibitor therapy in Alzheimer’s disease. Expert Opin Drug Saf. 2004;3:425–40.
Schneider LS, Dagerman KS, Higgins JP, McShane R. Lack of evidence for the efficacy of memantine in mild Alzheimer disease. Arch Neurol. 2011;68:991–8.
Nirogi R, Shinde A, Kambhampati RS, Mohammed AR, Saraf SK, Badange RK, et al. Discovery and development of 1-[(2-bromophenyl)sulfonyl]-5-methoxy-3-[(4-methyl-1-piperazinyl)methyl]-1H-indole dimesylate monohydrate (SUVN-502): a novel, potent, selective and orally active serotonin 6 (5-HT6) receptor antagonist for potential treatment of Alzheimer’s disease. J Med Chem. 2017;60(5):1843–59.
Nirogi R, Kandikere V, Mudigonda K, Bhyrapuneni G, Shinde A, Kambhampati R, Jayarajan P, Abraham R, Mohamad SM, Muddana N, Benade V, Saralaya R. SUVN-502: a potent and selective 5-HT6 antagonist, potential drug for the treatment of Alzheimers disease. Alzheimer’s Dement. 2011;7(4):S659.
Vishwakarma SL, Abraham R, Jayarajan P, Shirsath VS, Nirogi RV. Active metabolite M1 of SUVN-502: efficacy profiling in rodent models of cognition. Neuroscience. 26th annual meeting of Society for Neuroscience (plus poster). Abstract 666; 2006.
ICH Harmonized Tripartite Guideline, guideline for good clinical practice, E6 (R1), Current Step 4 version, dated 10 June 1996.
Nirogi R, Ajjala DR, Aleti R, Rayapati L, Pantangi HR, Boggavarapu RK. Padala NSP development and validation of sensitive LC–MS/MS method for the quantification of SUVN-502 and its metabolite and its application for first in human pharmacokinetic study. J Pharm Biomed Anal. 2017;145:423–30.
Kinirons MT, O’Mahony MS. Drug metabolism and ageing. Br J Clin Pharmacol. 2004;57(5):540–4.
Association Alzheimer’s. 2017 Alzheimer’s disease facts and figures. Alzheimers Dement. 2017;13(4):325–73.
Acknowledgements
The authors wish to acknowledge the principal investigators, Seiberling M, Swiss Pharma contract, Lettenweg, Switzerland and Thomas Murtaugh, Quintiles Phase I services LLC, Overland Park, for their support in the conduct of the studies reported in this manuscript. The authors acknowledge Venkateswarlu Jasti, CEO, Suven Life Sciences Ltd, for his moral and financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
All authors are employees of Suven Life Sciences Ltd, and are responsible for the content and writing of this manuscript.
Funding
The Phase I study was funded by Suven Life Sciences Ltd, the owner of the compound.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nirogi, R., Mudigonda, K., Bhyrapuneni, G. et al. Safety, Tolerability and Pharmacokinetics of the Serotonin 5-HT6 Receptor Antagonist, SUVN-502, in Healthy Young Adults and Elderly Subjects. Clin Drug Investig 38, 401–415 (2018). https://doi.org/10.1007/s40261-018-0618-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-018-0618-4