Abstract
Direct oral anticoagulants (DOACs) are recommended for the prevention of thromboembolism in patients with atrial fibrillation (AF), and are now preferred over vitamin K antagonists due to their beneficial efficacy and safety profile. However, all oral anticoagulants carry a risk of gastrointestinal (GI) bleeding. Although the risk is well documented and acute bleeding well codified, there is limited high-quality evidence and no guidelines to guide physicians on the optimal management of anticoagulation after a GI bleeding event. The aim of this review is to provide a multidisciplinary critical discussion of the optimal management of GI bleeding in patients with AF receiving oral anticoagulants to help physicians provide individualized treatment for each patient and optimize outcomes. It is important to perform endoscopy when a patient presents with bleeding manifestations or hemodynamic instability to determine the bleed location and severity of bleeding and then perform initial resuscitation. Administration of all anticoagulants and antiplatelets should be stopped and bleeding allowed to resolve with time; however, anticoagulant reversal should be considered for patients who have life-threatening bleeding or when the bleeding is not controlled by the initial resuscitation. Anticoagulation needs to be timely resumed considering that bleeding risk outweighs thrombotic risk when anticoagulation is resumed early after the bleeding event. To prevent further bleeding, physicians should prescribe anticoagulant therapy with the lowest risk of GI bleeding, avoid medications with GI toxicity, and consider the effect of concomitant medications on potentiating the bleeding risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
While oral anticoagulants are recommended for the prevention of thromboembolism in patients with atrial fibrillation (AF), a major complication of their use is an increased risk of bleeding, including gastrointestinal (GI) bleeding. |
Severe GI bleeding is associated with poor prognosis; however, reintroduction of oral anticoagulants after GI bleeding management is associated with increased survival rates. |
The optimal management of GI bleeding in patients receiving anticoagulants relies on multidisciplinary care, including gastroenterologists and cardiologists, as well as intensivists and hemostasis specialists in particular cases, to provide an individualized optimal balance of benefit and risk. |
1 Introduction
Atrial fibrillation (AF) is the most common type of cardiac arrhythmia that increases the risk of cardiovascular events such as stroke, systemic embolism, and heart failure and promotes the worsening of cardiac and noncardiac conditions [1, 2]. According to the Global Burden of Disease Study, there were 37.57 million [95% uncertainty interval (UI) 32.55–42.59] prevalent cases of AF and 3.05 million (95% UI 2.61–3.51) incident cases of AF worldwide in 2017 [2]. The prevalence of AF has nearly doubled between 1990 and 2017, and rates are expected to keep increasing due to the aging population [2].
European guidelines recommend the use of direct oral anticoagulants (DOACs) such as dabigatran, rivaroxaban, apixaban, or edoxaban for the prevention of stroke and systemic embolism in AF [3, 4]. Their mechanism of action is based on the direct inhibition of activated coagulation factors; dabigatran inhibits thrombin (factor IIa), while rivaroxaban, apixaban, and edoxaban inhibit factor Xa [5,6,7,87].
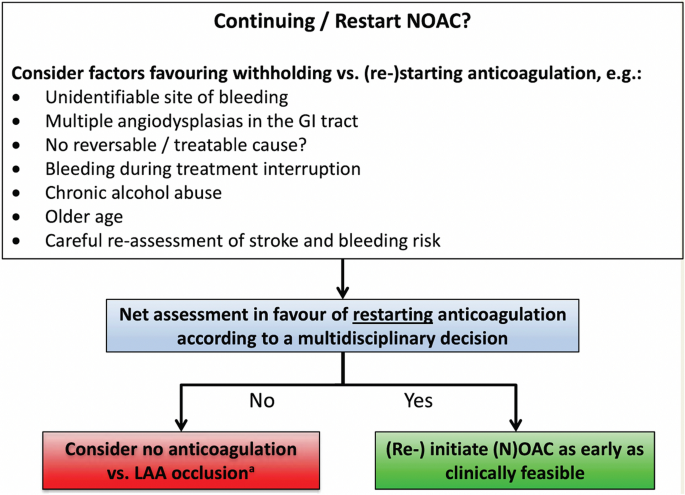
In the latest version of their guidelines, the EHRA changed their point of view, with the decision-making process suggesting a net assessment in favor of resuming anticoagulation and a recommendation to resume DOACs as early as clinically feasible (Fig. 2) [3].
Algorithm for the resumption of direct oral anticoagulants after a gastrointestinal hemorrhage according to the European Heart Rhythm Association guidelines [3]. GI gastrointestinal, LAA left atrial appendage, NOAC novel oral anticoagulants. Reproduced from Steffel et al. [3] by permission of Oxford University Press
The American College of Cardiology guidelines recommend determining the optimal timing for oral anticoagulant resumption on the basis of whether there is a greater risk of thromboembolism or bleeding. In conditions with high thrombotic risk, the recommendation is for early resumption of anticoagulation once hemostasis is achieved and the patient is clinically stable; for patients with moderate or high re-bleeding risk, individualized strategies are more appropriate [14].
Further studies and randomized controlled trials are urgently needed to establish optimal timing of DOAC resumption in patients after a GI hemorrhage according to baseline patient characteristics (age, comorbidities, indication for anticoagulants, source and severity of bleeding, risk of re-bleeding or thrombosis).
8 Minimizing the Risk of Recurrences in Patients Resuming Anticoagulants after GI Bleeding
As previously described, resuming anticoagulant treatment after a GI bleeding event generally provides clinical benefit. To prevent recurrent bleeding after a GI bleeding episode, it is important to evaluate the main risk factors favoring the occurrence of GI bleeding [88], such as the presence of a digestive luminal disease, older age, renal or liver dysfunction, hypertension, anemia, history of hemorrhage or stroke, genetic factors, malignancy, and concomitant treatments and diseases [11, 28, 30, 74]. Overall, despite a potential 23% increase in GI bleeding, DOACs have a 14% trend toward a relative risk reduction in major bleeding relative to warfarin [89]. Since the risk of bleeding appears to be higher with VKAs than with DOACs, it may be advisable to resume with a DOAC after a significant GI bleed.
However, several retrospective cohort studies of real-world patients starting DOAC therapy for AF have shown that, after adjustment for potential confounders, apixaban was associated with a significantly lower risk of major bleeding and GI bleeding compared with rivaroxaban or dabigatran [90,91,92].
Guidelines stress the need to minimize bleeding risk for all patients on oral anticoagulants by addressing modifiable risk factors such as concomitant use of aspirin, which increases the hazard for major bleeding events by at least 50% [12].
In summary, to prevent further bleeding, physicians should ensure the following steps are taken in a patient resuming anticoagulant therapy:
-
Preferentially prescribe DOACs with the lowest risk of GI bleeding rather than VKAs. The choice of DOAC cannot be determined by evidence-based medicine but should be determined by the risk of GI bleeding.
-
Comply with all guidelines and prescribing information, especially avoiding DOAC accumulation related to kidney disease.
-
Consider the effect of concomitant medications on potentiating the bleeding risk (e.g., CYP3A4 or P-gp inhibitors, antiplatelet agents) [93].
-
Avoid medications with GI toxicity (nonsteroidal antiinflammatory drugs).
-
Initiate treatment with PPIs to reduce the risk of bleeding (although be cognizant of a possible interaction between PPIs and dabigatran).
-
Test patients with peptic ulcers for Helicobacter pylori and initiate eradication therapy as needed.
9 Conclusions
Guidelines now recommend DOACs over VKAs for the prevention of thromboembolism in patients with AF due to their safety and efficacy profiles. However, the major complication with DOAC treatment is the increased risk of bleeding, particularly GI bleeding. Management of GI bleeding and resumption of oral anticoagulants in patients with AF are associated with increased survival rates. The optimal approach for patients with GI bleeding who are taking anticoagulants involves multidisciplinary care to provide an individualized optimal balance of benefit and risk for each patient.
References
Bassand J-P, Apenteng PN, Atar D, Camm AJ, Cools F, Corbalan R, et al. GARFIELD-AF: a worldwide prospective registry of patients with atrial fibrillation at risk of stroke. Future Cardiol. 2021;17(1):19–38. https://doi.org/10.2217/fca-2020-0014.
Dai H, Zhang Q, Much AA, Maor E, Segev A, Beinart R, et al. Global, regional, and national prevalence, incidence, mortality, and risk factors for atrial fibrillation, 1990–2017: results from the Global Burden of Disease Study 2017. Eur Heart J Qual Care Clin Outcomes. 2020;7(6):574–82. https://doi.org/10.1093/ehjqcco/qcaa061.
Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG, et al. 2021 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace. 2021;23(10):1612–76. https://doi.org/10.1093/europace/euab065.
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. https://doi.org/10.1093/eurheartj/ehaa612.
Bathala MS, Masumoto H, Oguma T, He L, Lowrie C, Mendell J. Pharmacokinetics, biotransformation, and mass balance of edoxaban, a selective, direct factor Xa inhibitor, in humans. Drug Metab Dispos. 2012;40(12):2250–5. https://doi.org/10.1124/dmd.112.046888.
Chan NC, Hirsh J, Ginsberg JS, Eikelboom JW. Betrixaban (PRT054021): pharmacology, dose selection and clinical studies. Future Cardiol. 2014;10(1):43–52. https://doi.org/10.2217/fca.13.98.
Gulseth MP, Michaud J, Nutescu EA. Rivaroxaban: an oral direct inhibitor of factor Xa. Am J Health Syst Pharm. 2008;65(16):1520–9. https://doi.org/10.2146/ajhp070624.
He K, Luettgen JM, Zhang D, He B, Grace JE, **n B, et al. Preclinical pharmacokinetics and pharmacodynamics of apixaban, a potent and selective factor Xa inhibitor. Eur J Drug Metab Pharmacokinet. 2011;36(3):129–39. https://doi.org/10.1007/s13318-011-0037-x.
Shantsila E, Lip GY. Apixaban, an oral, direct inhibitor of activated factor Xa. Curr Opin Investig Drugs. 2008;9(9):1020–33.
Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost. 2009;15(Suppl 1):9s–16s. https://doi.org/10.1177/1076029609343004.
Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–962. https://doi.org/10.1093/eurheartj/ehw210.
January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104–32. https://doi.org/10.1016/j.jacc.2019.01.011.
Cheung K-S, Leung WK. Gastrointestinal bleeding in patients on novel oral anticoagulants: risk, prevention and management. World J Gastroenterol. 2017;23(11):1954. https://doi.org/10.3748/wjg.v23.i11.1954.
Tomaselli GF, Mahaffey KW, Cuker A, Dobesh PP, Doherty JU, Eikelboom JW, et al. 2020 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants. J Am Coll Cardiol. 2020;76(5):594–622. https://doi.org/10.1016/j.jacc.2020.04.053.
Bouget J, Viglino D, Yvetot Q, Oger E. Major gastrointestinal bleeding and antithrombotics: characteristics and management. World J Gastroenterol. 2020;26(36):5463–73. https://doi.org/10.3748/wjg.v26.i36.5463.
Raymond J, Imbert L, Cousin T, Duflot T, Varin R, Wils J, et al. Pharmacogenetics of direct oral anticoagulants: a systematic review. J Pers Med. 2021;11(1):37. https://doi.org/10.3390/jpm11010037.
Desai J, Granger CB, Weitz JI, Aisenberg J. Novel oral anticoagulants in gastroenterology practice. Gastrointest Endosc. 2013;78(2):227–39. https://doi.org/10.1016/j.gie.2013.04.179.
Vaduganathan M, Bhatt DL. Gastrointestinal bleeding with oral anticoagulation: understanding the scope of the problem. Clin Gastroenterol Hepatol. 2017;15(5):691–3. https://doi.org/10.1016/j.cgh.2016.12.033.
Hellenbart EL, Faulkenberg KD, Finks SW. Evaluation of bleeding in patients receiving direct oral anticoagulants. Vasc Health Risk Manag. 2017;13:325–42. https://doi.org/10.2147/VHRM.S121661.
Thapa N, Shatzel J, Deloughery TG, Olson SR. Direct oral anticoagulants in gastrointestinal malignancies: is the convenience worth the risk? J Gastrointest Oncol. 2019;10(4):807–9. https://doi.org/10.21037/jgo.2019.02.07.
Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation. 2011;123(21):2363–72. https://doi.org/10.1161/circulationaha.110.004747.
Ruff CT, Giugliano RP, Antman EM, Crugnale SE, Bocanegra T, Mercuri M, et al. Evaluation of the novel factor Xa inhibitor edoxaban compared with warfarin in patients with atrial fibrillation: design and rationale for the Effective aNticoaGulation with factor xA next GEneration in Atrial Fibrillation-Thrombolysis In Myocardial Infarction study 48 (ENGAGE AF-TIMI 48). AM Heart J. 2010;160(4):635-41.e2. https://doi.org/10.1016/j.ahj.2010.06.042.
Sherwood MW, Nessel CC, Hellkamp AS, Mahaffey KW, Piccini JP, Suh E-Y, et al. Gastrointestinal bleeding in patients with atrial fibrillation treated with rivaroxaban or warfarin: ROCKET AF trial. J Am Coll Cardiol. 2015;66(21):2271–81. https://doi.org/10.1016/j.jacc.2015.09.024.
Granger CB, Lopes RD, Hanna M, Ansell J, Hylek EM, Alexander JH, et al. Clinical events after transitioning from apixaban versus warfarin to warfarin at the end of the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Am Heart J. 2015;169(1):25–30. https://doi.org/10.1016/j.ahj.2014.09.006.
Cannon CP, Kohli P. Danger ahead: watch out for indirect comparisons! J Am Coll Cardiol. 2012;60(8):747–8. https://doi.org/10.1016/j.jacc.2012.05.012.
Desai JC, Chatterjee P, Friedman K, Aisenberg J. Incidence and clinical presentation of gastrointestinal bleeding in atrial fibrillation patients taking direct oral anticoagulants. Am J Gastroenterol Suppl. 2016;3(1):13. https://doi.org/10.1038/ajgsup.2016.3.
Sengupta N, Tapper EB, Patwardhan VR, Ketwaroo GA, Thaker AM, Leffler DA, et al. Risk factors for adverse outcomes in patients hospitalized with lower gastrointestinal bleeding. Mayo Clin Proc. 2015;90(8):1021–9. https://doi.org/10.1016/j.mayocp.2015.04.024.
Undas A, Drabik L, Potpara T. Bleeding in anticoagulated patients with atrial fibrillation: practical considerations. Kardiol Pol. 2020;78(2):105–16. https://doi.org/10.33963/KP.15205.
White EM, Coons JC. Direct oral anticoagulant use in special populations: elderly, obesity, and renal failure. Curr Cardiol Rep. 2021;23(4):27. https://doi.org/10.1007/s11886-021-01456-9.
Gunasekaran K, Rajasurya V, Devasahayam J, Singh Rahi M, Chandran A, Elango K, et al. A review of the incidence diagnosis and treatment of spontaneous hemorrhage in patients treated with direct oral anticoagulants. J Clin Med. 2020;9(9):2984. https://doi.org/10.3390/jcm9092984.
Soliman EZ, Prineas RJ, Go AS, **e D, Lash JP, Rahman M, et al. Chronic kidney disease and prevalent atrial fibrillation: the Chronic Renal Insufficiency Cohort (CRIC). Am Heart J. 2010;159(6):1102–7. https://doi.org/10.1016/j.ahj.2010.03.027.
Padrini R. Clinical pharmacokinetics and pharmacodynamics of direct oral anticoagulants in patients with renal failure. Eur J Drug Metab Pharmacokinet. 2019;44(1):1–12. https://doi.org/10.1007/s13318-018-0501-y.
Ha JT, Neuen BL, Cheng LP, Jun M, Toyama T, Gallagher MP, et al. Benefits and harms of oral anticoagulant therapy in chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med. 2019;171(3):181–9. https://doi.org/10.7326/m19-0087.
Weinberg EM, Palecki J, Reddy KR. Direct-acting oral anticoagulants (DOACs) in cirrhosis and cirrhosis-associated portal vein thrombosis. Semin Liver Dis. 2019;39(2):195–208. https://doi.org/10.1055/s-0039-1679934.
Lobraico-Fernandez J, Baksh S, Nemec E. Elderly bleeding risk of direct oral anticoagulants in nonvalvular atrial fibrillation: a systematic review and meta-analysis of cohort studies. Drugs R D. 2019;19(3):235–45. https://doi.org/10.1007/s40268-019-0275-y.
Abraham NS, Noseworthy PA, Yao X, Sangaralingham LR, Shah ND. Gastrointestinal safety of direct oral anticoagulants: a large population-based study. Gastroenterology. 2017;152(5):1014-22.e1. https://doi.org/10.1053/j.gastro.2016.12.018.
Lip GYH. Implications of the CHA2DS2-VASc and HAS-BLED scores for thromboprophylaxis in atrial fibrillation. Am J Med. 2011;124(2):111–4. https://doi.org/10.1016/j.amjmed.2010.05.007.
Borre ED, Goode A, Raitz G, Shah B, Lowenstern A, Chatterjee R, et al. Predicting thromboembolic and bleeding event risk in patients with non-valvular atrial fibrillation: a systematic review. Thromb Haemost. 2018;118(12):2171–87. https://doi.org/10.1055/s-0038-1675400.
Steinberg BA, Shrader P, Kim S, Thomas L, Fonarow GC, Ansell J, et al. How well does physician risk assessment predict stroke and bleeding in atrial fibrillation? Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J. 2016;181:145–52. https://doi.org/10.1016/j.ahj.2016.07.026.
Srygley FD, Gerardo CJ, Tran T, Fisher DA. Does this patient have a severe upper gastrointestinal bleed? JAMA. 2012;307(10):1072–9. https://doi.org/10.1001/jama.2012.253.
Barnert J, Messmann H. Diagnosis and management of lower gastrointestinal bleeding. Nat Rev Gastroenterol Hepatol. 2009;6(11):637–46. https://doi.org/10.1038/nrgastro.2009.167.
Gralnek IM, Dumonceau J-M, Kuipers EJ, Lanas A, Sanders DS, Kurien M, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2015;47(10):a1–46. https://doi.org/10.1055/s-0034-1393172.
Gaiani F, De’Angelis N, Kayali S, Manfredi M, Di Mario F, Leandro G, et al. Clinical approach to the patient with acute gastrointestinal bleeding. Acta Biomed Ateneo Parmense. 2018;89(8-S):12–9. https://doi.org/10.23750/abm.v89i8-S.7861.
Rodrigues A, Carrilho A, Almeida N, Baldaia C, Alves Â, Gomes M, et al. Interventional algorithm in gastrointestinal bleeding-an expert consensus multimodal approach based on a multidisciplinary team. Clin Appl Thromb Hemost. 2020;26:1076029620931943. https://doi.org/10.1177/1076029620931943.
McNulty PH, King N, Scott S, Hartman G, McCann J, Kozak M, et al. Effects of supplemental oxygen administration on coronary blood flow in patients undergoing cardiac catheterization. Am J Physiol Heart Circ Physiol. 2005;288(3):H1057–62. https://doi.org/10.1152/ajpheart.00625.2004.
Schulman S, Angeras U, Bergqvist D, Eriksson B, Lassen MR, Fisher W, et al. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost. 2010;8(1):202–4. https://doi.org/10.1111/j.1538-7836.2009.03678.x.
Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692–4. https://doi.org/10.1111/j.1538-7836.2005.01204.x
Pernod G, Godier A, Gozalo C, Tremey B, Sie P. French National Authority for Health French clinical practice guidelines on the management of patients on vitamin K antagonists in at-risk situations (overdose, risk of bleeding, and active bleeding). Thromb Res. 2010;126(3):e167–74. https://doi.org/10.1016/j.thromres.2010.06.017
Haute Autorité de Santé. Oral anticoagulants. Saint-Denis La Plaine. 2018. https://www.has-sante.fr/jcms/c_2851086/fr/les-anticoagulants-oraux. Accessed 22 July 2021.
Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet. 2000;356(9238):1318–21. https://doi.org/10.1016/S0140-6736(00)02816-6.
Piazza VM, Popenko NA, Winograd S. An evidence-based review of gastrointestinal bleeding evaluation and management in the emergency department. Emerg Med Rep Relias Media. 2019. https://www.reliasmedia.com/articles/144557-an-evidence-based-review-of-gastrointestinal-bleeding-evaluation-and-management-in-the-emergency-department. Accessed 27 Apr 27.
Jung K, Moon W. Role of endoscopy in acute gastrointestinal bleeding in real clinical practice: an evidence-based review. World J Gastrointest Endosc. 2019;11(2):68–83. https://doi.org/10.4253/wjge.v11.i2.68.
U.S. Food and Drug Administration. SAVAYSA™ (edoxaban) prescribing information. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/206316s015lbl.pdf. Accessed 2 Aug 2021.
U.S. Food and Drug Administration. PRADAXA® (dabigatran etexilate) prescribing information. 2021. https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. Accessed 4 Aug 2021.
U.S. Food and Drug Administration. XARELTO (rivaroxaban) prescribing information. 2021. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/XARELTO-pi.pdf. Accessed 4 Aug 2021.
U.S. Food and Drug Administration. ELIQUIS® (apixaban) prescribing information. 2021. https://packageinserts.bms.com/pi/pi_eliquis.pdf. Accessed 4 Aug 2021.
Chen A, Stecker E, Bruce AW. Direct oral anticoagulant use: a practical guide to common clinical challenges. J Am Heart Assoc. 2020;9(13):e017559. https://doi.org/10.1161/jaha.120.017559.
Ur-Rahman A, Guan J, Khalid S, Munaf A, Sharbatji M, Idrisov E, et al. Both full Glasgow–Blatchford score and modified Glasgow-Blatchford score predict the need for intervention and mortality in patients with acute lower gastrointestinal bleeding. Dig Dis Sci. 2018;63(11):3020–5. https://doi.org/10.1007/s10620-018-5203-4.
Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastrointestinal bleeding. Lancet. 1974;2(7877):394–7. https://doi.org/10.1016/s0140-6736(74)91770-x.
Lee S, Ahn JY, Jung HY, Jung KW, Lee JH, Kim DH, et al. Effective endoscopic treatment of Mallory-Weiss syndrome using Glasgow-Blatchford score and Forrest classification. J Dig Dis. 2016;17(10):676–84. https://doi.org/10.1111/1751-2980.12409.
Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38(3):316–21. https://doi.org/10.1136/gut.38.3.316.
Barkun AN, Bardou M, Kuipers EJ, Sung J, Hunt RH, Martel M, et al. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152(2):101–13. https://doi.org/10.7326/0003-4819-152-2-201001190-00009.
Strate LL, Gralnek IM. ACG clinical guideline: management of patients with acute lower gastrointestinal bleeding. Am J Gastroenterol. 2016;111(4):459–74. https://doi.org/10.1038/ajg.2016.41.
Pasha SF, Shergill A, Acosta RD, Chandrasekhara V, Chathadi KV, Early D, et al. The role of endoscopy in the patient with lower GI bleeding. Gastrointest Endosc. 2014;79(6):875–85. https://doi.org/10.1016/j.gie.2013.10.039.
Conway JD, Adler DG, Diehl DL, Farraye FA, Kantsevoy SV, Kaul V, et al. Endoscopic hemostatic devices. Gastrointest Endosc. 2009;69(6):987–96. https://doi.org/10.1016/j.gie.2008.12.251.
Cuker A, Burnett A, Triller D, Crowther M, Ansell J, Van Cott EM, et al. Reversal of direct oral anticoagulants: guidance from the anticoagulation forum. Am J Hematol. 2019;94(6):697–709. https://doi.org/10.1002/ajh.25475.
Levy JH, Ageno W, Chan NC, Crowther M, Verhamme P, Weitz JI, et al. When and how to use antidotes for the reversal of direct oral anticoagulants: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(3):623–7. https://doi.org/10.1111/jth.13227.
U.S. Food and Drug Administration. PRAXBIND® (idarucizumab) prescribing information. 2015. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/761025lbl.pdf. Accessed 2 Aug 2021.
Marano G, Vaglio S, Pupella S, Liumbruno GM, Franchini M. How we treat bleeding associated with direct oral anticoagulants. Blood Transfus. 2016;14(5):465–73. https://doi.org/10.2450/2016.0180-15.
Sheikh-Taha M. Idarucizumab for reversal of dabigatran: single-center real-world experience. Am J Cardiovasc Drugs. 2019;19(1):59–64. https://doi.org/10.1007/s40256-018-0300-5.
Chaudhary R, Sharma T, Garg J, Sukhi A, Bliden K, Tantry U, et al. Direct oral anticoagulants: a review on the current role and scope of reversal agents. J Thromb Thrombolysis. 2020;49(2):271–86. https://doi.org/10.1007/s11239-019-01954-2.
U.S. Food and Drug Administration. ANDEXXA® (coagulation factor Xa) prescribing information. 2018. https://www.fda.gov/media/113279/download. Accessed 02 Aug 2021.
Sheikh-Taha M, Crawley RM. Reversal of apixaban and rivaroxaban using activated prothrombin complex concentrates in patients with major bleeding. Am J Cardiovasc Drugs. 2020;20(3):295–9. https://doi.org/10.1007/s40256-019-00383-z.
Sengupta N, Feuerstein JD, Patwardhan VR, Tapper EB, Ketwaroo GA, Thaker AM, et al. The risks of thromboembolism vs recurrent gastrointestinal bleeding after interruption of systemic anticoagulation in hospitalized inpatients with gastrointestinal bleeding: a prospective study. Am J Gastroenterol. 2015. https://doi.org/10.1038/ajg.2014.398.
Proietti M, Romiti GF, Romanazzi I, Farcomeni A, Staerk L, Nielsen PB, et al. Restarting oral anticoagulant therapy after major bleeding in atrial fibrillation: a systematic review and meta-analysis. Int J Cardiol. 2018;261:84–91. https://doi.org/10.1016/j.ijcard.2018.03.053.
Little D, Chai-Adisaksopha C, Hillis C, Witt DM, Monreal M, Crowther MA, et al. Resumption of anticoagulant therapy after anticoagulant-related gastrointestinal bleeding: a systematic review and meta-analysis. Thromb Res. 2019;175:102–9. https://doi.org/10.1016/j.thromres.2019.01.020.
Tapaskar N, Pang A, Werner DA, Sengupta N. Resuming anticoagulation following hospitalization for gastrointestinal bleeding is associated with reduced thromboembolic events and improved mortality: results from a systematic review and meta-analysis. Dig Dis Sci. 2021;66(2):554–66. https://doi.org/10.1007/s10620-020-06248-9.
Kido K, Scalese MJ. Management of oral anticoagulation therapy after gastrointestinal bleeding: whether to, when to, and how to restart an anticoagulation therapy. Ann Pharmacother. 2017;51(11):1000–7. https://doi.org/10.1177/1060028017717019.
Radaelli F, Fuccio L, Paggi S, Bono CD, Dumonceau JM, Dentali F. What gastroenterologists should know about direct oral anticoagulants. Dig Liver Dis. 2020;52(10):1115–25. https://doi.org/10.1016/j.dld.2020.04.032.
Witt DM. What to do after the bleed: resuming anticoagulation after major bleeding. Hematology. 2016;2016(1):620–4. https://doi.org/10.1182/asheducation-2016.1.620.
Albaladejo P, Pernod G, Godier A, de Maistre E, Rosencher N, Mas JL, et al. Management of bleeding and emergency invasive procedures in patients on dabigatran: updated guidelines from the French Working Group on perioperative haemostasis (GIHP)—September 2016. Anaesth Crit Care Pain Med. 2018;37(4):391–9. https://doi.org/10.1016/j.accpm.2018.04.009.
Majeed A, Wallvik N, Eriksson J, Höijer J, Bottai M, Holmström M, et al. Optimal timing of vitamin k antagonist resumption after upper gastrointestinal bleeding a risk modelling analysis. Thromb Haemost. 2017;117(3):491–9. https://doi.org/10.1160/TH16-07-0498.
Qureshi W, Mittal C, Patsias I, Garikapati K, Kuchipudi A, Cheema G, et al. Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. Am J Cardiol. 2014;113(4):662–8. https://doi.org/10.1016/j.amjcard.2013.10.044.
Witt DM, Delate T, Garcia DA, Clark NP, Hylek EM, Ageno W, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med. 2012;172(19):1484–91. https://doi.org/10.1001/archinternmed.2012.4261.
Held C, Hylek EM, Alexander JH, Hanna M, Lopes RD, Wojdyla DM, et al. Clinical outcomes and management associated with major bleeding in patients with atrial fibrillation treated with apixaban or warfarin: insights from the ARISTOTLE trial. Eur Heart J. 2015;36(20):1264–72. https://doi.org/10.1093/eurheartj/ehu463.
Van der Wall SJ, Lopes RD, Aisenberg J, Reilly P, van Ryn J, Glund S, et al. Idarucizumab for dabigatran reversal in the management of patients with gastrointestinal bleeding. Circulation. 2019;139(6):748–56. https://doi.org/10.1161/CIRCULATIONAHA.118.036710.
Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, et al. The 2018 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: executive summary. Europace. 2018;20(8):1231–42. https://doi.org/10.1093/europace/euy054.
Pipilis A, Makrygiannis S, Chrisanthopoulou E, Sourlas N, Kaliambakos S, Ntailianas P. Gastrointestinal bleeding in patients receiving antiplatelet and anticoagulant therapy: practical guidance for restarting therapy and avoiding recurrences. Hellenic J Cardiol. 2014;55(6):499–509.
Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955–62. https://doi.org/10.1016/S0140-6736(13)62343-0.
Lip GYH, Keshishian AV, Zhang Y, Kang A, Dhamane AD, Luo X, et al. Oral anticoagulants for nonvalvular atrial fibrillation in patients with high risk of gastrointestinal bleeding. JAMA Netw Open. 2021;4(8):e2120064. https://doi.org/10.1001/jamanetworkopen.2021.20064.
Ray WA, Chung CP, Stein CM, Smalley W, Zimmerman E, Dupont WD, et al. Association of rivaroxaban vs apixaban with major ischemic or hemorrhagic events in patients with atrial fibrillation. JAMA. 2021;326(23):2395–404. https://doi.org/10.1001/jama.2021.21222.
Souverein PC, van den Ham HA, Huerta C, Merino EM, Montero D, León-Muñoz LM, et al. Comparing risk of major bleeding between users of different oral anticoagulants in patients with nonvalvular atrial fibrillation. Br J Clin Pharmacol. 2021;87(3):988–1000. https://doi.org/10.1111/bcp.14450.
Sheikh-Taha M, Deeb ME. Assessment of non-vitamin K oral anticoagulants use in a tertiary care center in the USA: a chart review of 909 patients. Am J Cardiovasc Drugs. 2019;19(2):195–201. https://doi.org/10.1007/s40256-018-0310-3.
Acknowledgements
We would like to thank Catherine Rees and Alma Orts-Sebastien of Springer Healthcare Communications who wrote the outline and first draft, respectively. This medical writing assistance was funded by Pfizer and Bristol Myers Squibb.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Anne-Céline Martin: BMS/Pfizer, Bayer, Boehringer Ingelheim, Astra Zeneca, Novartis. Robert Benamouzig: no relevant conflicts of interest to declare. Isabelle Gouin-Thibault: Alliance BMS/Pfizer, Bayer, Leo Pharma, Abbvie. Jeannot Schmidt: no relevant conflicts of interest to declare.
Funding
Medical writing assistance was funded by Pfizer and Bristol Myers Squibb.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Code availability
Not applicable.
Author contributions
The content of this manuscript was originally based on discussions during expert meetings including all the authors. All authors critically reviewed the outline and first draft of the manuscript and have read and approved the final manuscript for submission.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Martin, AC., Benamouzig, R., Gouin-Thibault, I. et al. Management of Gastrointestinal Bleeding and Resumption of Oral Anticoagulant Therapy in Patients with Atrial Fibrillation: A Multidisciplinary Discussion. Am J Cardiovasc Drugs 23, 407–418 (2023). https://doi.org/10.1007/s40256-023-00582-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-023-00582-9