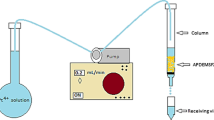
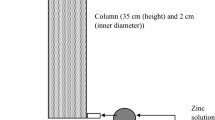
Abstract
Treatment of wastewater and reuse of purified water in an industrial process can provide an alternative source of fresh water as well as reduce pollution load by discharging a lower quantity of wastewater. When adsorption is used for treatment, the regeneration of the used adsorbent may also account for a large portion of the operational cost and cause secondary pollution. This problem may be solved by cyclic repetition of adsorption/regeneration cycles using a column method. In this paper, a total of nine successive cycles of zinc capture and zeolite bed regeneration using a column method have been investigated. The derived form of the breakthrough curve was used for analysing mass transfer in the column. For that purpose, the Dose–response, the Thomas, the Bohart-Adams, the Yoon-Nelson and the Wolborska models were used for modelling the breakthrough curve by nonlinear regression analysis. Simulation results and mathematical similarities between the models were discussed. This is the first study that used derived form of Dose–response model to analyse the inflection points of the breakthrough curve and mass transfer during nine consecutive sorption-regeneration cycles of zinc ions on natural zeolite. Obtained peak shape rate profiles were analysed for all cycles. Optimal operation conditions were evaluated with respect to the inflection point, the model parameters, and the residence time.













Similar content being viewed by others
Availability of data and materials
The dataset analyzed during the current study are available from the corresponding author.
References
Ahmadi E, Yousefzadeh S, Mokammel A, Miri M, Ansari M, Arfaeinia H, Badi MY, Ghaffari HR, Rezaei S, Mahvi AH. Kinetic study and performance evaluation of an integrated two-phase fixed-film baffled bioreactor for bioenergy recovery from wastewater and bio-wasted sludge. Renew Sust Energ Rev. 2020;121:109674. https://doi.org/10.1016/j.rser.2019.109674.
Ostad-Ali-Askari K, Eslamian S. Water reuse in industry: necessities and possibilities. Am J Eng Applied Sci. 2021;14(1):94–102. https://doi.org/10.3844/ajeassp.2021.94.102.
New Trends in Removal of Heavy Metals from Industrial Wastewater. In: Shah Maulin P, Rodriguez Couto S, Kumar V, editors. Elsevier; 1st edition. 2021. https://doi.org/10.1016/C2019-0-04585-2.
Joseph L, Jun BM, Flora JRV, Park CM, Yoon Y. Removal of heavy metals from water sources in the develo** world using low-cost materials: A review. Chemosphere. 2019;2019(229):142–59. https://doi.org/10.1016/j.chemosphere.2019.04.198.
Baskar AV, Bolan N, Hoang SA, Sooriyakumar P, Kumar M, Singh L, Jasemizad T, Padhye LP, Singh G, Vinu A, Sarkar B, Kirkham MB, Rinklebe J, Wang S, Wang H, Balasubramanian R, Siddique KHM. Recovery, regeneration and sustainable management of spent adsorbents from wastewater treatment streams: A review. Sci Total Environ. 2022;822:153555. https://doi.org/10.1016/j.scitotenv.2022.153555.
Phuengphai P, Singjanusong T, Kheangkhun N, Wattanakornsiri A. Removal of copper(II) from aqueous solution using chemically modified fruit peels as efficient low-cost biosorbents. Water Sci Eng. 2021;14(4):286–94. https://doi.org/10.1016/j.wse.2021.08.003.
Hidayat AE, Moersidik SS, Adityosulindro S. Adsorption and desorption of zinc and copper in acid mine drainage onto synthesized zeolite from coal fly ash. J Phys Conf Ser. 2021;1811:012045. https://doi.org/10.1088/1742-6596/1811/1/012045.
Chatterjee A, Abraham J. Desorption of heavy metals from metal loaded sorbents and e-wastes: A review. Biotechnol Lett. 2019;41(3):319–33. https://doi.org/10.1007/s10529-019-02650-0.
Patel H. Batch and continuous fixed bed adsorption of heavy metals removal using activated charcoal from neem (Azadirachta indica) leaf powder. Sci Rep. 2020;10:16895. https://doi.org/10.1038/s41598-020-72583-6.
Patel H. Fixed-bed column adsorption study: a comprehensive review. Appl Water Sci. 2019;9(45):1–17. https://doi.org/10.1007/s13201-019-0927-7.
Osifo PO, Neomagus HWJP, Everson RC, Webster A, vd Gun MA. The adsorption of copper in a packed-bed of chitosan beads: Modeling, multiple adsorption and regeneration. J Hazard Mater. 2009;167(1–3):1242–5. https://doi.org/10.1016/j.jhazmat.2009.01.109.
Biswas S, Mishra U. Continuous Fixed-Bed Column Study and Adsorption Modeling: Removal of Lead Ion from Aqueous Solution by Charcoal Originated from Chemical Carbonization of Rubber Wood Sawdust. J Chem. 2015;907379:1–9. https://doi.org/10.1155/2015/907379.
Negrea A, Mihailescu M, Mosoarca G, Ciopec M, Duteanu N, Negrea P, Minzatu V. Estimation on Fixed-Bed Column Parameters of Breakthrough Behaviors for Gold Recovery by Adsorption onto Modified/Functionalized Amberlite XAD7. Int J Environ Res Public Health. 2020;17(18):6868. https://doi.org/10.3390/ijerph17186868.
Satya A, Harimawan A, Haryani GS, Johir MAH, Nguyen LN, Nghiem LD, Vigneswaran S, Ngo HH, Setiadi T. Fixed-bed adsorption performance and empirical modelingof cadmium removal using adsorbent prepared from the cyanobacterium Aphanothece sp cultivar. Environ Technol Innov. 2021;21:101194. https://doi.org/10.1016/j.eti.2020.101194.
Vukojević Medvidović N, Perić J, Trgo M. Column performance of lead removal from aqueous solutions by fixed bed of natural zeolite-clinoptilolite. Sep Purif Technol. 2006;49:237–44. https://doi.org/10.1016/j.seppur.2005.10.005.
Tan KL, Hameed BH. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J Taiwan Inst Chem E. 2017;74:25–48. https://doi.org/10.1016/j.jtice.2017.01.024.
Yoon YH, Nelson JH. Application of gas adsorption kinetics. I. A theoretical model for respirator cartridge service life. Am Ind Hyg Assoc J. 1984;45(8):509–16. https://doi.org/10.1080/15298668491400197.
Lin SH, Huang CY. Modeling of aqueous BTEX adsorption in column and multistage adsorbers. J Environ Eng. 2000;126:802–6. https://doi.org/10.1061/(ASCE)0733-9372(2000)126:9(802).
Vukojević Medvidović N, Perić J, Trgo M. Testing of breakthrough curves for removal of lead ions from aqueous solutions by natural zeolite-clinoptilolite according to the Clark kinetic equation. Sep Sci Technol. 2008;43:944–59. https://doi.org/10.1080/01496390701870622.
Hamdaoui O. Removal of copper (II) from aqueous phase by Purolite C100-MB cation exchange resin in a fixed bed columns: Modeling. J Hazard Matter. 2009;161:737–46. https://doi.org/10.1016/j.jhazmat.2008.04.016.
Aksu Z, Gonen F. Biosorption of phenol by immobilized activated sludge in a continuous packed bed: prediction of breakthrough curve. Process Biochem. 2004;39:599–613. https://doi.org/10.1016/S0032-9592(03)00132-8.
Hu Q, **e Y, Feng C, Zhang Z. Fractal-like kinetics of adsorption on heterogeneous surfaces in the fixed-bed column. Chem Eng J. 2019;358:1471–8. https://doi.org/10.1016/j.cej.2018.10.165.
Hu Q, **e Y, Zhang Z. Modification of breakthrough models in a continuous-flow fixed-bed column: mathematical characteristic of breakthrough curves and rate profile. Sep Purif Technol. 2020;238:116399. https://doi.org/10.1016/j.seppur.2019.116399.
Lee CG, Kim JH, Kang JK, Kim SB, Park SJ, Lee SH, Choi JW. Comparative analysis of fixed-bed sorption models using phosphate breakthrough curves in slag filter media. Desalin Water Treat. 2015;55:1795–805. https://doi.org/10.1080/19443994.2014.930698.
Chu KH. Breakthrough curve analysis by simplistic models of fixed bed adsorption: in defence of the century-old Bohart-Adams model. Chem Eng J. 2020;380:122513. https://doi.org/10.1016/j.cej.2019.122513.
Hendricks D. Water Treatment Unit Processes. Boca Raton: Taylor and Francis Group; 2006.
Nuić I, Trgo M, Vukojević MN. The application of packed bed reactor theory to Pb and Zn uptake from the binary solution onto the fixed bed of natural zeolite. Chem Eng J. 2016;295:347–57. https://doi.org/10.1016/j.cej.2016.03.037.
Yan G, Viraraghavan T, Chen M. A new model for heavy metals removal in a biosorption column. Adsorp Sci Technol. 2001;19:25–43. https://doi.org/10.1260/0263617011493953.
Obiri-Nyarko F, Kwiatowska-Malina J, Malina G, Wolowiec K. Assessment of zeolite and composting-zeolite mixture as a permeable reactive materials for the removal of lead from a model acidic groundwater. J Cont Hydrol. 2020;229:103597. https://doi.org/10.1016/j.jconhyd.2019.103597.
Vera LM, Bermejo D, Uguna MF, Garcia N, Flores M, Gonzales E. Fixed bed column modelling of lead (II) and cadmium (II) ions biosorption on sugarcane bagasse. Environ Eng Res. 2019;24(1):31–7. https://doi.org/10.4491/eer.2018.042.
Han R, Ding D, Xu Y, Zou W, Wang Y, Li Y, Zou L. Use of rice husk for the adsorption of congo red from aqueous solution in column mode. Biores Technol. 2008;99:2938–46. https://doi.org/10.1016/j.biortech.2007.06.027.
Bohart GS, Adams EQ. Some aspects of the behaviour of charcoal with respect to chlorine. J Am Chem Soc. 1920;42:523–44. https://doi.org/10.1021/ja01448a018.
Yan J, Xue Y, Long L, Hu X. Adsorptive removal of As(V) by crawfish shell biochar: batch and column test. Environ Sci Pollut Res. 2018;25:34674–83. https://doi.org/10.1007/s11356-018-3384-1.
Wolborska A, Pustelnik P. A simplified method for determination of the break-through time of an adsorbent layer. Water Res. 1996;30:2643–50. https://doi.org/10.1016/S0043-1354(96)00166-2.
Sag Y, Aktay Y. Application of equilibrium and mass transfer models to dynamic removal of Cr(VI) ions by Chitin in packed column reactor. Process Biochem. 2001;36:1187–97. https://doi.org/10.1016/S0032-9592(01)00150-9.
Nuić I, Trgo M, Perić J, Vukojević MN. Analysis of breakthrough curves of Pb and Zn sorption from binary solutions on natural clinoptilolite. Micropor Mesopor Mat. 2013;167:55–61. https://doi.org/10.1016/j.micromeso.2012.04.037.
Ugrina M, Vukojević Medvidović N, Daković A. Characterization and environmental application of iron-modified zeolite from the Zlatokop deposit. Desalin Water Treat. 2015;53:3557–69. https://doi.org/10.1080/19443994.2013.873743.
Rodrigues AE. Chemical engineering and environmental challenges: Cyclic adsorption/reaction technologies: Materials and process together! J Enviro Chem Eng. 2020;8:103926. https://doi.org/10.1016/j.jece.2020.103926.
Funding
This work was supported by the institutional funds of the Faculty of Chemistry and Technology University of Split.
Author information
Authors and Affiliations
Contributions
Nediljka Vukojević Medvidović: Conceptualization, Formal analysis, Writing-original draft, Methodology; Supervision, Writing -review & editing.
Sandra Svilović: Conceptualization, Methodology, Validation, Supervision, Writing -review & editing. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors agreed to publish the data in this journal.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vukojević Medvidović, N., Svilović, S. Cyclic zinc capture and zeolite regeneration using a column method, mass transfer analysis of multi regenerated bed. J Environ Health Sci Engineer 21, 333–353 (2023). https://doi.org/10.1007/s40201-023-00861-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40201-023-00861-2