Abstract
Blunt cerebrovascular injuries (BCVI) have been shown to affect the pediatric population and have long term neurologic morbidity. The reported incidence is low which likely represents under diagnosis due to poor screening efforts. No large prospective studies have been conducted to specifically address practice guidelines for children with BCVI. Increased awareness and knowledge is needed to improve quality of care and patient outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Injuries to the carotid and vertebral vessels are poorly recognized sequelae of blunt trauma to the head, face, and neck, presenting either with subtle signs of cerebral ischemia or with catastrophic neurologic deficits from a hemispheric ischemic infarct. Initial studies, consisting of case series, serve to better characterize the natural history this disease [1–5]. From these studies, two aspects become evident: (1) recognition of a latent period between time of injury and development of ischemic symptoms, and (2) timely institution of anticoagulation prevents development of ischemic symptoms. This renders blunt cerebrovascular injuries (BCVI) as a modifiable, and thus relevant, injury despite its relatively low incidence among blunt trauma patients.
Furthermore, over the past decade combined efforts have led to the development of screening criteria, diagnostic modalities, and treatment options, all of which have contributed to a timely injury recognition, treatment implementation and overall decrease in BCVI-associated morbidity and mortality [6–9, 10•, 11, 12]. Despite growing knowledge in the adult population, there is scant literature evaluating BCVI in children; however, the incidence is thought to be considerably lower, likely due to underdiagnosis [13, 14••, 15]. We aim to present the current understanding of BCVI in children.
Epidemiology
The first case of blunt carotid injury was reported by Verneuil in 1872. By 1980 only 96 cases of BCVI had been reported in the literature. With widespread screening, the incidence of adult BCVI currently ranges between 1 and 2 % of blunt trauma admissions [8, 9, 16, 17]. By contrast, a literature review by Duke et al. [18] identified that by 1997 only 16 cases of pediatric BCVI had been reported in the literature. Initial studies on pediatric BCVI estimated its incidence to be 0.03 % [13]; however, recent retrospective cohort studies approximate an incidence of 0.4–0.9 % [14••, 15, 19, 20].
Across age groups, blunt cerebrovascular injuries are more common in males and most commonly occur after motorized vehicle collisions (automobile, motorcycles, all—terrain vehicles, etc.) [1, 6–13, 14••, 15].
Anatomy
Knowledge of relevant anatomy is paramount in understanding cerebrovascular injuries and their clinical presentation, a concept that was implored by Dr. Timothy Fabian in his 2012 Scudder Oration on Trauma while presenting the implications of anatomical variants in the Circle of Willis with the occurrence of ischemic infarcts and associated outcomes in patients sustaining BCVIs [21].
First described in 1664 by Richard Lower, the Circle of Willis represents the primary collateral system connecting the right and left, and the anterior and posterior cerebral circulatory systems (Fig. 1). Theoretically, in absence of distal clot embolization or atherosclerotic disease, only bilateral carotid or vertebral injuries should result in ischemic infarcts. However, approximately 80 % of the population represents anatomical “variants,” with hypoplastic or absent posterior or anterior communicating arteries [22]. This has serious implications in patients with acute flow-limiting injuries, in which no alternate vasculature are present to sustain regional blood flow and oxygen consumption. Thus, variations in underlying anatomy might, in part, explain why some lesions remain clinically silent while others produce devastating neurological deficits, including death.
Pathophysiology
Injury mechanisms leading to a BCVI are numerous (Table 1), but all involve stretch and shear stress mechanisms, leading to intimal disruption, vessel wall dissection, platelet activation and aggregation, and subsequent thrombus formation. Dissection and thrombus formation represent an injury spectrum which can lead to partial or complete vessel occlusion, pseudoaneurysms, or alternately promote clot embolization, and distal ischemia. Regardless of where an individual injury lies on this spectrum, both mechanical and chemical processes need to be addressed in an attempt to prevent or minimize clinically overt signs and symptoms of central nervous system infarcts. In 1999 Biffl et al. [7] developed the BCVI grading system, with prognostic and therapeutic implications, which has since served to guide practice guidelines (Table 2).
Presentation
Patients with carotid artery injuries usually develop contralateral sensorimotor deficits in the distribution of the affected vessel. Conversely, patients with vertebral artery injuries often present with cerebellar symptoms, visual defects, and/or vomiting. Hassan et al. [23], in a literature review of 68 children with vertebral artery injuries, report that the most common presenting symptoms are: eye movement deficits (72 %), paresis/paralysis (54 %), ataxia (53 %), vomiting (37 %), and loss of consciousness (34 %).
While a subset of BCVI patients develop neurologic symptoms within an hour of injury, the majority exhibit a clinically silent period due to injury progression to a flow-limiting stenosis or development of emboli. The majority of patients develop neurologic symptoms within 10 to 72 h post-injury; however, there have been reports of patients presenting up to 14 years post-injury [1–4, 9, 11, 24]. This clinically silent period has also been appreciated in pediatric patients [25]. Nevertheless, several case series in children diagnosed with vertebral artery dissections, undergoing long term follow up, have reported additional delayed complications, including thrombosis, pseudoaneurysms and recurrent strokes [26, 27].These studies suggest the need for long term monitoring in children with BCVI.
The goal is to identify injuries while clinically silent, allowing for timely intervention and greater risk reduction in strokes, neurological deficits, and death. This is supported by the fact that stroke rates in adults with BCVI has been reduced from 21 % in the untreated patient, to 0.2–4 % in those receiving medical and/or endovascular treatment [9–11]. Despite these accomplishments, in-hospital BCVI-associated mortality has not been completely avoided and fluctuates between 6 and 30 % [9, 10•, 12, 21].
Screening
Early studies report that, of adult patients undergoing diagnostic arteriography for BCVI, 77 to 100 % underwent testing due to onset of neurological symptoms [1–6]. In 1996, Fabian et al. reported favorable results on the effects of heparin therapy in patients with BCVI, especially in patients undergoing therapy prior to the onset of symptoms [6]; this rendered BCVI as a modifiable injury and has led to a widespread interest. Despite the growing interest, existing literature at the time documented that 50 to 90 % of patients with a diagnosis of BCVI did not have physical exam findings suggestive of cervical trauma [2]. Additionally, up to half of patients diagnosed with BCVI had concomitant closed head injury, which resulted in a depressed Glasgow Coma Scale (GCS) that masked localizing neurological deficits [2, 28]. Due to lack of reliable clinical findings, along with technical difficulties and risks associated with the use of four-vessel digital subtraction arteriography (DSA) and head and neck computed tomographic angiography (CTA), it quickly became evident that screening criteria was needed to guide diagnostic efforts.
The ensuing years were dedicated to identifying injury mechanisms and independent factors that were predictive of BCVI. Specifically, the University of Colorado at Denver and the University of Tennessee Health Science Center in Memphis took the lead in formulating and refining adult BCVI screening criteria [29–31]. While both centers developed their own guidelines, both agreed on mechanisms of injury (MOI) for which patients should undergo screening (Table 1) [29–31].
The initial Memphis screening criteria specifically included patients with the designated MOI and neurologic examination findings inconsistent with head CT, neck hematoma, Horner’s Syndrome, skull base fractures extending through the foramen lacerum, cervical spine fractures through the transverse foramen, or severe complex facial fractures [31]. However, the current guidelines include any cervical spine fracture (see below).
Conversely, the initial Denver screening criteria included patients with the designated MOI and any of the following signs or symptoms suggestive of BCVI: arterial hemorrhage, cervical bruit, expanding hematoma, focal neurological deficit, neurological examination incongruous with head CT scan findings, or CT-proven stroke. Additionally, patients would be screened if found to have a high-energy transfer mechanism with soft tissue injury of the anterior neck, cervical spine fracture, displaced mid-face or mandibular fracture, or basilar skull fracture with carotid canal involvement [29]. Additional criteria were added in 1999, when Biffl, with the Denver group, showed that a GCS less than 6, diffuse axonal injury, petrous bone fractures, and Le Fort II or III fractures were found to be independently associated with the presence of carotid artery injury (CAI); however, only cervical spine fractures were associated with vertebral artery injuries (VAI) [30].
While the initial Denver and Memphis groups’ screening criteria resembled each other in principle, their differences have been debated over the past two decades. The aim is to capture the approximate 20 % of patients with BCVI who do not meet the conventional screening criteria [16, 30].
Inclusion of “Limited Cervical Spine Criteria”
In 2000, the Denver group showed that 39 % of patients with cervical fractures had an associated VAI; however, they did not find any correlations between occurrence of VAIs and cervical fracture type or level [32]. A subsequent study in 2002, from Memphis, showed that 92 % of patients with VAIs would be identified by only screening patients with cervical spine transverse foramen fractures or subluxations; similar findings had been previously reported by Willis et al. in 1994 [8, 33]. Based on these findings, the Denver group conducted further studies which identified three fracture patterns most commonly associated with VAI: fractures extending to the transverse foramina, subluxations, and C1 to C3 fractures [34], and modified their screening criteria accordingly. This version of the Denver BCVI screening criteria was adopted in 2009 by the Western Trauma Association (WTA) and in 2010 by the Eastern Association for the Surgery of Trauma (EAST) as practice management guidelines for BCVI [35, 36].
In 2011, the Memphis group challenged this “limited cervical spine criteria” by presenting the largest number of cervical fractures analyzed, showing that C4 to C7 fractures, while less common than C1 to C3 fractures, were considerably associated with BCVI. Specifically, they state that 9 % of cerebrovascular injuries would have been missed if only using the limited cervical criteria; they continue to advocate screening of patients with any cervical fracture [37].
Additional Screening Criteria
Additionally, the 2011 Memphis study advocated for the use of CTA as a screening tool in patients with significant mechanism of injury who were undergoing CT scan of the head, cervical spine and/or face. They identified 44 patients who, based on irregular CTA findings, went on to have a DSA, finding a 43 % (19/44) incidence of BCVI. These 19 patients represented 16 % of all BCVIs detected in the study and would have been missed, had they not included “irregular CTA findings” as a screening criterion [37]. This new recommendation essentially advocates for the use of CTA in blunt trauma patients with any significant injury to head, face, and/or neck requiring non-contrast CT scan; this was implemented in their latest screening guidelines (Table 3), with the purpose of identifying the elusive 20 % patients with BCVI not identified based on initial conventional criteria.
In 2011, the Denver group focused on identifying the most common injury patterns in patients with BCVI not meeting standard criteria (20 % of the BCVIs in their study). Based on their findings they redefined their screening criteria to include patients with mandible fractures, complex frontal, basilar or occipital condyle fractures, high grade mechanisms with traumatic brain injury (TBI) with thoracic injuries, scalp degloving, and thoracic vascular injuries or blunt cardiac rupture (Table 4) [55••]. These criteria were in accordance with previous studies looking at additional risk factors for BCVI [11, 15].
Special Considerations in Children
Literature addressing screening criteria for children with BCVI is scarce. In 1999, Lew et al. [13] reviewed injury patterns in children with blunt carotid artery injuries (BCAI) and found a 4-fold increase in patients with chest trauma or basilar skull fractures, a 6-fold increase in patients with intracranial hemorrhage or combined head and chest trauma, and an 8-fold increase in patients with clavicle fractures. Some case series have speculated that the presence of congenital cervical spine anomalies place children at additional risk for sustaining vascular injuries, even in the presence of minimal trauma [27, 38]. Due to the absence of literature devoted to pediatric patients, the 2010 EAST practice guidelines suggested using adult screening criteria [36].
Subsequently, several studies have attempted to evaluate whether adult criteria translate to the pediatric population, with mixed results. The Denver group, in reviewing their cohort of 45 pediatric patients, found that only 30 % of symptomatic children with BCVI met adult screening criteria [20]. In contrast, other studies show that approximately 90 % of children meet adult screening criteria [14••, 15, 19].
More importantly, these latter studies show that only 16–42 % of patients meeting screening criteria are formally evaluated for the presence of BCVI and that those who were screened presented with significantly higher injury severity scores (ISS) than those who were not screened [14••, 15, 19, 39]. This finding suggests that work up for BCVI tends to be driven by the severity of patient’s condition and that widespread education on usage of a screening algorithm should result in improved injury recognition and improved patient care. Currently, a prospective multi-institutional study is underway to detect the true incidence of BCVI and missed injury rate encountered after systematic application of the adult screening criteria in the pediatric population.
Diagnosis
The reference standard for screening and diagnosis of BCVI has been DSA [35, 36]; however, this imaging modality is technically demanding, expensive, invasive, and with associated complications including puncture site hematomas and groin vessel injury [24, 37, 40–42]. More so, arteriography poses a minimal but real risk of stroke or dissection of the carotid and vertebral vasculature in 1 % of those undergoing DSA [24, 36, 37, 40–42], which emphasizes the need for alternative imaging tools. Duplex ultrasonography and magnetic resonance angiograms (MRA) were found to have unsatisfactory sensitivity, reliability and availability [8, 28, 36, 39, 42–45]. Therefore, the focus has been on CTA as an attractive alternative for diagnosis of BCVI. However, while less invasive and more readily available than DSA, CTA is associated with significant radiation exposure with a variable sensitivity. A meta-analysis by Roberts et al. in 2013, looking at studies which directly compared CTA versus DSA, reported CTA sensitivities ranging from 51 to 82 % (overall, per vessel) [46•].
The 2009 WTA and the 2010 EAST recommendations for the diagnosis of BCVI stated that CTA is the preferred screening tool for BCVI (level II and III evidence) [35, 36]. In absence of CTA, arteriography should be performed. Likewise, in the presence of irregular or indeterminate CTA findings in patients with high index of suspicion for BCVI, DSA must be performed to formally exclude the diagnosis. In the absence of both CTA and DSA, hospitals should consider transferring patients to specialized trauma centers in which these diagnostic modalities are available.
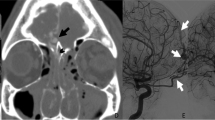
Since approximately 2009 the Denver group adopted CTA as a screening and diagnostic modality, using DSA only for therapeutic purposes (Fig. 2) [42]. Conversely, until 2013 the Memphis group only accepted CT angiograms as a screening criterion. Abnormal or inconclusive CTA findings still required DSA to confirm diagnosis. Negative CTA findings in patients meeting conventional BCVI screening criteria still required DSA to exclude the diagnosis (Fig. 3). This approach was based on results published by their group in 2011 which showed that 32-multidetector CTA’s sensitivity was merely 51 % [47]. A recent publication on 64-channel CTA found a screening sensitivity of 68 % [48••]. Based on an extrapolation of missed injuries’ estimated stroke rate, they concluded that only 0.4 % of the study population would have been harmed due to false negative CT angiograms. Memphis now accepts CTA as a screening tool and states that negative CTA will not require further testing with DSA. On the other hand, they do continue to use DSA as a diagnostic tool to confirm BCVI in patients with positive CTA.
Current screening criteria for blunt cerebrovascular injuries at Denver Health Medical Center [54••]. BCVI blunt cerebrovascular injuries
Memphis BCVI Screening and Management Guideline [10•]
The advent of multidetector CT scanners, improved image acquisition protocols, along with increasing expertise on radiologic interpretation of images, has allowed for acceptable sensitivity and widespread acceptance as the preferred screening and diagnostic tool for detection of BCVI. A 2011 survey among trauma surgeons, neurosurgeons, and radiologists, reported that 60 % of practitioners in North America used CTA for screening and diagnosis of BCVI; only 15 % continued to use the reference standard of DSA [49].
Special Considerations in Children
Studies on the accuracy of the different diagnostic tools do not include pediatric patients and definite recommendations for this population are not available. More so, there are two relevant concerns when considering use of CTA and DSA in children: radiation exposure and technical difficulties in performing DSA on small vessels.
In an attempt to avoid excessive radiation, Magnetic Resonance Arteriogram (MRA) has been documented as a helpful tool for the diagnosis and follow up of children with BCVI [26, 27]. Despite this practice, comparisons on the diagnostic accuracy between CTA and MRA yield mixed results [20, 36, 43, 45]. Given the current data, and the devastating potential of undiagnosed BCVI, our pediatric trauma center continues to advocate the use of CTA for screening, diagnosis and follow up of BCVI.
With highly specialized pediatric neuro-interventialists, as in adult trauma centers we utilize DSA for therapeutic purposes in children with high grade BCVI’s. However, since some studies have documented that most vascular injuries in children are iatrogenic, technical feasibility along with institutional capability should be weighed in decisions to use DSA therapeutically [50].
Management and Outcomes
Historically, anticoagulation with heparin was the initial treatment of choice; however, antiplatelet therapy and endovascular approaches have gained acceptance in the management of BCVIs. Antiplatelet therapy was initially proposed as an alternate therapy for BCVI patients with contraindications to anticoagulation (i.e., ongoing intracranial bleeding or complex pelvic fractures). Alternatively, patients with a history of intracranial bleeding or solid organ injuries and stable CT findings can be considered for initiation of anticoagulation with heparin [12]. Nevertheless, given the ease of long term administration, antiplatelet medications have gained popularity as a first line treatment. Multiple efficacy studies comparing antiplatelet therapy to heparin have reported equivalence in preventing injury progression on follow up imaging, and in reducing mortality, stroke rate and neurologic deficit [9, 10•, 12, 24, 31, 40, 41, 51]. Of note, an early report from the Denver group raised concerns about stent occlusion and increased stroke rates with endovascular repair [52]; however, recent reports have documented the safety and efficacy in patients with grade II, III and V injuries [10•, 12].
While debate exists regarding treatment guidelines, a study by the Memphis group confirmed that patients who suffered BCVI-related strokes had poorer long-term functional outcomes; this highlights the need for timely management [53]. Treatment decisions should be a collaborative agreement between the neurosurgeons, interventional radiologists, and trauma surgeons involved in the patient’s care.
Management and Outcomes for Grade I Injuries
A grade I injury, consisting of a raised intimal flap, with or without thrombus formation and vasospasm, and a resultant intimal stenosis less than 25 %, account for the majority of lesions identified on retrospective and prospective reviews and may be clinically innocuous [7, 9, 31, 40, 51]. It has been reported that up to 88 % will remain stable or heal, regardless of treatment of choice [7, 9, 31, 40]. Nonetheless, approximately 7 % of patients with grade I injuries have developed strokes, suggesting that these lesions should not be ignored and should receive anticoagulation or antiplatelet therapy [7, 40]. Both Denver and Memphis advocate for use of anticoagulation in the acute setting, and transitioning to antiplatelet therapy upon discharge; this is based on the ease of reversing the effects of heparin should bleeding complications occur [9, 47, 48••, 54••]. Interval re-imaging at 6 months is advocated in an attempt to document lesion regression and to determine discontinuation of antiplatelet therapy [48••, 54••].
Management and Outcomes for Grade II and Grade III Injuries
Grade II lesions consist of intimal flaps, intramural hematomas, or dissections occluding more than 25 % of the luminal diameter, with the potential of develo** flow-limiting stenosis, either by progression to occlusion or by distal embolization of clots. Grade II injuries account for approximately 20 % of lesions identified on retrospective and prospective reviews [7, 40, 51]. Only 30–50 % will remain stable or heal, and up to 50–70 % will progress to pseudoaneurysms or occlusions, regardless of the treatment of choice [7, 9, 40]. The documented stroke rate for grade II injuries is approximately 10 to 25 % [7, 31, 40, 48••].
Pseudoaneurysms, categorized as grade III injuries, persist or progress to occlusion in 90 % of lesions, despite anticoagulation [7, 9, 40, 48••]. Moreover, they are associated with up to a 17–33 % stroke rate [7, 9, 40]. Endovascular stenting has become an attractive means to tack down the intima, exclude the pseudoaneurysm and potential emboli from circulation, and potentially reduce the stroke rate. A recent study by DiCocco et al. showed a 7–9 % post-diagnosis stroke rate, including patients with strokes diagnosed at long term follow up. This treatment failure rate is lower than previous reports by their group, and is in part attributed to the addition of endovascular stents to their treatment algorithm [10•].
Given the above data, Memphis’ current approach for adults with grade II and III injuries consists of initial treatment with heparin (no loading dose, with a goal partial thromboplastin time of 40–50 s). Anticoagulation should be withheld from patients with hemodynamic instability, ongoing hemorrhage, or intracranial bleeding with mass effect. Interval reassessment should be used to determine the appropriate timing in which to initiate antithrombotic therapy. Patients undergo arteriogram 7–10 days post-injury, and, if warranted, definitive repair with an endovascular stent is attempted at that time. Patients should receive a loading dose of antiplatelet therapy (ASA and Clopidogrel) the day prior to arteriogram in preparation for possible stent placement. Patients must then continue a dual antiplatelet regimen for 6 months before undergoing reimaging to assess stent patency and determine the need for further medical therapy [10•, 48••].
It is important to note that a 2014 report by the Denver group states that the use of endovascular stents should be limited to patients with neurological symptoms or markedly enlarging pseudoaneurysms [54••]. These recommendations are based on an increased stroke rate in patients with grade II and III injuries treated with stents prior to 2005, in comparison to those treated with antithrombotics after 2005. However, the patients treated with stents did not receive long term antiplatelet therapy, which has been documented to be associated with an increased stroke rate and could have falsely elevated the stroke rates in their study. While their results cannot be used to argue against the use of stents, they seem to point toward the efficacy of medical therapy in patients with grade II and III injuries, and poses as safe treatment alternative for institutions in which endovascular treatment is not available.
Management and Outcomes for Grade IV Injuries
Grade IV injuries consist of vessel occlusions. While known to be less prominent than grade III injuries, a study by Berne et al. [15] reported that 70 % of their patients with VAIs were found to have complete occlusions at time of diagnosis [15]. Over 80 % of grade IV lesions show no changes on repeat imaging and are associated with a stroke rate of up to 50 % [7, 9, 31, 40]. While heparin and antiplatelet therapy does not seem to result in recanalization, treatment has been associated with reduced strokes rates. Patients with grade IV injuries are generally started on aspirin, without the need for additional inpatient arteriogram [10•, 21]. Whether treatment should be discontinued at 6 months or continued throughout the patient’s lifetime has not been directly addressed; decisions should be based on individual clinical scenarios.
Management and Outcomes for Grade V Injuries
Grade V lesions consist of arterial transections with active contrast extravasation or carotid-cavernous fistula formation. While rare, these injuries are known for having high stroke and mortality rates and, upon identification, should be treated with immediate surgical or endovascular repair [7, 10•, 21, 24, 31, 40, 42].
Special Considerations in Children
There are no formal studies or guidelines regarding treatment of pediatric BCVI. Duke et al. (1997) reported on five patients with BCVI, documenting that children seemed to be more susceptible to increased intracranial pressures (ICP) associated to infarcted tissue. These patients were managed with aggressive ICP monitoring and surgical resection of infarcted tissue [18]. Additionally, a study by the Denver group published in 2008, recommended use of low molecular weight heparin (LMWH) for long term management of pediatric patients with BCVI, based on previous studies documenting the safety, favorable pharmokinetics, and ease of monitoring for patients using LMWH as DVT prophylaxis and treatment [55, 56].
In view of the paucity of data regarding treatment and outcomes on pediatric BCVI, and given the availability of pediatric neurointerventionalists, our institution has adopted Memphis’ adult treatment guideline. Prospective studies to validate this approach are warranted in order to improve patient care at specialized pediatric trauma centers.
Conclusions
The diagnostic modalities and treatment of BCVI have been debated over the past 20 years, yet while disagreement on some approaches to these patients remains, agreement on the serious clinical significance and devastating nature of undiagnosed and untreated injuries is clear. Unfortunately, there is no good prospective data on the diagnosis and treatment of BCVI in the pediatric population. Currently, we recommend screening high risk patients with CTA, and management with anticoagulation and antiplatelet therapy. DSA with endovascular repair should be used when clinically appropriate.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Batzdorf U, et al. Blunt trauma to the high cervical carotid artery. Neurosurgery. 1979;5(2):195–201.
Krajewski LP, Hertzer NR. Blunt carotid artery trauma: report of two cases and review of the literature. Ann Surg. 1980;191(3):341–6.
Perry MO, et al. Carotid artery injuries caused by blunt trauma. Ann Surg. 1980;192(1):74–7.
Mokri B, et al. Traumatic dissections of the extracranial internal carotid artery. J Neurosurg. 1988;68(2):189–97.
Watridge CB, et al. Traumatic carotid artery dissection: diagnosis and treatment. J Neurosurg. 1989;71(6):854–7.
Fabian TC, et al. Blunt carotid injury Importance of early diagnosis and anticoagulant therapy. Ann Surg. 1996;223(5):513–22 (discussion 522–515).
Biffl WL, et al. Blunt carotid arterial injuries: implications of a new grading scale. J Trauma. 1999;47(5):845–53.
Miller PR, et al. Prospective screening for blunt cerebrovascular injuries: analysis of diagnostic modalities and outcomes. Ann Surg. 2002;236(3):386–93 (discussion 393–385).
Cothren CC, et al. Treatment for blunt cerebrovascular injuries: equivalence of anticoagulation and antiplatelet agents. Arch Surg. 2009;144(7):685–90.
• DiCocco JM, et al. Optimal outcomes for patients with blunt cerebrovascular injury (BCVI): tailoring treatment to the lesion. J Am Coll Surg. 2011;212(4):549–57 (discussion 557–549). Important study which used long term data to assess the efficacy and safety of treatment for patients with BCVI.
Burlew CC, et al. Blunt cerebrovascular injuries: redefining screening criteria in the era of noninvasive diagnosis. J Trauma Acute Care Surg.2012;72(2):330-5 (discussion 336–337), quiz 539.
Edwards NM, et al. Antithrombotic therapy and endovascular stents are effective treatment for blunt carotid injuries: results from longterm followup. J Am Coll Surg. 2007;204(5):1007–13 (discussion 1014–1005).
Lew SM, et al. Pediatric blunt carotid injury: a review of the National Pediatric Trauma Registry. Pediatr Neurosurg. 1999;30(5):239–44.
•• Azarakhsh N, et al. Blunt cerebrovascular injury in children: underreported or underrecognized?: A multicenter ATOMAC study. J Trauma Acute Care Surg.2013;75(6):1006–11 (discussion 1011–1002). This is the first multicenter study assessing the current incidence of pediatric BCVI. Data showed that only 16% of patients meeting criteria were screened for the presence of BCVI and suggest that the low incidence is due to underdiagnosis.
Berne JD, et al. A multivariate logistic regression analysis of risk factors for blunt cerebrovascular injury. J Vasc Surg. 2010;51(1):57–64.
Bruns BR, et al. Blunt cerebrovascular injury screening guidelines: what are we willing to miss? J Trauma Acute Care Surg. 2014;76(3):691–5.
Franz RW, et al. A systematic review and meta-analysis of diagnostic screening criteria for blunt cerebrovascular injuries. J Am Coll Surg. 2012;214(3):313–27.
Duke BJ, Partington MD. Blunt carotid injury in children. Pediatr Neurosurg. 1996;25(4):188–93.
Kopelman TR, et al. Risk factors for blunt cerebrovascular injury in children: do they mimic those seen in adults?”. J Trauma. 2011;71(3):559–64 (discussion 564).
Jones TS, et al. Blunt cerebrovascular injuries in the child. Am J Surg. 2012;204(1):7–10.
Fabian TC. Blunt cerebrovascular injuries: anatomic and pathologic heterogeneity create management enigmas. J Am Coll Surg. 2013;216(5):873–85.
Riggs HE, Rupp C. Variation in form of circle of Willis. The relation of the variations to collateral circulation: anatomic analysis. Arch Neurol. 1963;8:8–14.
Hasan I, et al. Vertebral artery dissection in children: a comprehensive review. Pediatr Neurosurg. 2002;37(4):168–77.
Cothren CC, et al. Anticoagulation is the gold standard therapy for blunt carotid injuries to reduce stroke rate. Arch Surg. 2004;139(5):540–5 (discussion 545–546).
Fullerton HJ, et al. Arterial dissection and stroke in children. Neurology. 2001;57(7):1155–60.
Khurana DS, et al. Vertebral artery dissection: issues in diagnosis and management. Pediatr Neurol. 1996;14(3):255–8.
Tan MA, et al. Late complications of vertebral artery dissection in children: pseudoaneurysm, thrombosis, and recurrent stroke. J Child Neurol. 2009;24(3):354–60.
Cogbill TH, et al. The spectrum of blunt injury to the carotid artery: a multicenter perspective. J Trauma. 1994;37(3):473–9.
Biffl WL, et al. The unrecognized epidemic of blunt carotid arterial injuries: early diagnosis improves neurologic outcome. Ann Surg. 1998;228(4):462–70.
Biffl WL, et al. Optimizing screening for blunt cerebrovascular injuries. Am J Surg. 1999;178(6):517–22.
Miller PR, et al. Blunt cerebrovascular injuries: diagnosis and treatment. J Trauma. 2001;51(2):279–85 (discussion 285–276).
Biffl WL, et al. The devastating potential of blunt vertebral arterial injuries. Ann Surg. 2000;231(5):672–81.
Willis BK, et al. The incidence of vertebral artery injury after midcervical spine fracture or subluxation. Neurosurgery. 1994;34(3):435–41 (discussion 441–432).
Cothren CC, et al. Cervical spine fracture patterns predictive of blunt vertebral artery injury. J Trauma. 2003;55(5):811–3.
Biffl WL, et al. Western Trauma Association critical decisions in trauma: screening for and treatment of blunt cerebrovascular injuries. J Trauma. 2009;67(6):1150–3.
Bromberg WJ, et al. Blunt cerebrovascular injury practice management guidelines: the Eastern Association for the Surgery of Trauma. J Trauma. 2010;68(2):471–7.
Emmett KP, et al. Improving the screening criteria for blunt cerebrovascular injury: the appropriate role for computed tomography angiography. J Trauma. 2011;70(5):1058–63 (discussion 1063–1055).
Capone C, Burjonrappa S. Congenital spine deformities: a new screening indication for blunt cerebrovascular injuries after cervical trauma? J Pediatr Surg. 2010;45(12):2444–6.
Tolhurst SR, et al. Cervical arterial injury after blunt trauma in children: characterization and advanced imaging. J Pediatr Orthop. 2013;33(1):37–42.
Biffl WL, et al. Treatment-related outcomes from blunt cerebrovascular injuries: importance of routine follow-up arteriography. Ann Surg. 2002;235(5):699–706 (discussion 706–697).
Cothren CC, Moore EE. Blunt cerebrovascular injuries. Clinics (Sao Paulo). 2005;60(6):489–96.
Burlew CC, Biffl WL. Blunt cerebrovascular trauma. Curr Opin Crit Care. 2010;16(6):587–95.
Biffl WL. Diagnosis of blunt cerebrovascular injuries. Curr Opin Crit Care. 2003;9(6):530–4.
Mutze S, et al. Blunt cerebrovascular injury in patients with blunt multiple trauma: diagnostic accuracy of duplex Doppler US and early CT angiography. Radiology. 2005;237(3):884–92.
Vertinsky AT, et al. Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. AJNR Am J Neuroradiol. 2008;29(9):1753–60.
• Roberts DJ, et al. Diagnostic accuracy of computed tomographic angiography for blunt cerebrovascular injury detection in trauma patients: a systematic review and meta-analysis. Ann Surg. 2013;257(4):621–32. Important systematic review of studies which assessed CTA’s accuracy in detecting BCVI in patients who underwent CTA and DSA imaging.
DiCocco JM, et al. Blunt cerebrovascular injury screening with 32-channel multidetector computed tomography: more slices still don’t cut it. Ann Surg. 2011;253(3):444–50.
•• Paulus EM, et al. Blunt cerebrovascular injury screening with 64-channel multidetector computed tomography: more slices finally cut it. J Trauma Acute Care Surg. 2014;76(2):279–83 (discussion 284–275). Important study reassessing the sensitivity of CTA as a screening tool for BCVI. Results led to change in practice at the study’s institution.
Harrigan MR, et al. Management of blunt extracranial traumatic cerebrovascular injury: a multidisciplinary survey of current practice. World J Emerg Surg. 2011;6:11.
Corneille MG, et al. Pediatric vascular injuries: acute management and early outcomes. J Trauma. 2011;70(4):823–8.
Wei CW, et al. Blunt cerebrovascular injuries: diagnosis and management outcomes. Can J Neurol Sci. 2010;37(5):574–9.
Cothren CC, et al. Carotid artery stents for blunt cerebrovascular injury: risks exceed benefits. Arch Surg. 2005;140(5):480–5 (discussion 485–486).
DiCocco JM, et al. Functional outcomes following blunt cerebrovascular injury. J Trauma Acute Care Surg. 2013;74(4):955–60.
•• Burlew CC, et al. Endovascular stenting is rarely necessary for the management of blunt cerebrovascular injuries. J Am Coll Surg. 2014;218(5):1012–17. Important study showing outcomes of Grade II and III BCVIs managed medically (without endovascular repairs).
Brunworth LS, et al. Pediatric blunt vertebral artery injury: case report and treatment plan. J Pediatr Surg. 2009;44(3):e5–9.
Dix D, et al. The use of low molecular weight heparin in pediatric patients: a prospective cohort study. J Pediatr. 2000;136(4):439–45.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Pediatric Trauma Surgery.
Rights and permissions
About this article
Cite this article
Bachier, M., Eubanks, J.W. Blunt Cerebrovascular Injuries in Children: When is Aggressive Management Necessary?. Curr Surg Rep 2, 64 (2014). https://doi.org/10.1007/s40137-014-0064-z
Published:
DOI: https://doi.org/10.1007/s40137-014-0064-z