Abstract
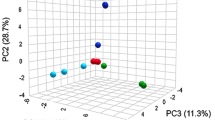
Kohlrabi (Brassica oleracea var. gongylodes) is a dietary Brassica vegetable with noted health-beneficial properties associated with its numerous metabolites. The aim of this study was to elucidate phenotypic variation between the two cultivars through comprehensive analysis of the relationship of their primary and secondary metabolites. High-performance liquid chromatography (HPLC) and gas chromatography time-of-flight mass spectrometry (GC-TOFMS) are considered useful tools for profiling primary and secondary metabolites. A total of 45 metabolites, including organic acids, amino acids, sugars, and an amine, were identified in pale green and purple kohlrabies using GC-TOFMS-based metabolic profiling. The resulting data sets were analyzed by principal component analysis to determine the overall variation, and the purple and pale green vegetables were separated by the score plots generated. Additionally, HPLC analysis of anthocyanins in both cultivars revealed that green kohlrabies did not contain any anthocyanidins, while 11 anthocyanins were quantified in the purple ones. Cyanidin was the dominant anthocyanin found in the purple cultivar, with cyanidin-3-(feruloyl)-diglucoside-5-glucoside being the major one. This study suggests that GC-TOFMS and HPLC are suitable tools to determine metabolic connection among various metabolites and describe phenotypic variation between green and purple kohlrabies.



Similar content being viewed by others
Change history
21 May 2018
Unfortunately, in the online published article, the second author’s given name was wrongly published. The correct given name should be “Hyeon Ji”.
Similarly the fourth author’s given name and family name was swapped. The given name should be “Ye Eun” and family name should be “Park”.
References
Ambrosone CB, Tang L (2009) Cruciferous vegetable intake and cancer prevention: role of nutrigenetics. Cancer Prev Res 2:298–300
Asadujjaman M, Hossain M, Khan M, Anisuzzaman A, Ahmed M, Islam A (2011) Antihyperglycemic and glycogenesis effects of different fractions of Brassica oleracea in Alloxan induced diabetic rats. Int J Pharm Sci Rev Res 2:1436
Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC (2003) A prospective study of cruciferous vegetables and prostate cancer. Cancer Epidemiol Biomarkers Prev 12:1403–1409
Melchini A, Traka MH (2010) Biological profile of erucin: a new promising anticancer agent from cruciferous vegetables. Toxins 2:593–612
Sankhari JM, Thounaojam MC, Jadeja RN, Devkar RV, Ramachandran A (2012) Anthocyanin-rich red cabbage (Brassica oleracea L.) extract attenuates cardiac and hepatic oxidative stress in rats fed an atherogenic diet. J Agric Food Chem 92:1688–1693
Thirumalai T, Therasa SV, Elumalai E, David E (2011) Hypoglycemic effect of Brassica juncea (seeds) on streptozotocin induced diabetic male albino rat. Asian Pac J Trop Biomed 1:323–325
Abdel-Farid IB, Kim HK, Choi YH, Verpoorte R (2007) Metabolic characterization of Brassica rapa leaves by NMR spectroscopy. J Agric Food Chem 55:7936–7943
Ayaz FA, Hayırlıoglu-Ayaz S, Alpay-Karaoglu S, Grúz J, Valentová K, Ulrichová J, Strnad M (2008) Phenolic acid contents of kale (Brassica oleraceae L. var. acephala DC.) extracts and their antioxidant and antibacterial activities. Food Chem 107:19–25
Cartea ME, Francisco M, Soengas P, Velasco P (2010) Phenolic compounds in Brassica vegetables. Molecules 16:251–280
Ferreres F, Fernandes F, Oliveira JM, Valentão P, Pereira JA, Andrade PB (2009) Metabolic profiling and biological capacity of Pieris brassicae fed with kale (Brassica oleracea L. var. acephala). Food Chem Toxicol 47:1209–1220
Jung HA, Karki S, Ehom N-Y, Yoon M-H, Kim EJ, Choi JS (2014) Anti-diabetic and anti-inflammatory effects of green and red kohlrabi cultivars (Brassica oleracea var. gongylodes). Prev Nutr Food Sci 19:281
Lin J-Y, Li C-Y, Hwang I-F (2008) Characterisation of the pigment components in red cabbage (Brassica oleracea L. var.) juice and their anti-inflammatory effects on LPS-stimulated murine splenocytes. Food Chem 109:771–781
Lippmann D, Lehmann C, Florian S, Barknowitz G, Haack M, Mewis I, Wiesner M, Schreiner M, Glatt H, Brigelius-Flohé R (2014) Glucosinolates from pak choi and broccoli induce enzymes and inhibit inflammation and colon cancer differently. Food Funct 5:1073–1081
Park WT, Kim JK, Park S, Lee S-W, Li X, Kim YB, Uddin MR, Park NI, Kim S-J, Park SU (2012) Metabolic profiling of glucosinolates, anthocyanins, carotenoids, and other secondary metabolites in kohlrabi (Brassica oleracea var. gongylodes). J Agric Food Chem 60:8111–8116
Podsędek A (2007) Natural antioxidants and antioxidant capacity of Brassica vegetables: a review. LWT-Food Sci Technol 40:1–11
Singh J, Upadhyay A, Bahadur A, Singh B, Singh K, Rai M (2006) Antioxidant phytochemicals in cabbage (Brassica oleracea L. var. capitata). Sci Hortic 108:233–237
Park S-Y, Lim S-H, Ha S-H, Yeo Y, Park WT, Kwon DY, Park SU, Kim JK (2013) Metabolite profiling approach reveals the interface of primary and secondary metabolism in colored cauliflowers (Brassica oleracea L. ssp. botrytis). J Agric Food Chem 61:6999–7007
Savithramma N, Rao ML, Suhrulatha D (2011) Screening of medicinal plants for secondary metabolites. Middle East J Sci Res 8:579–584
Stamp N (2003) Out of the quagmire of plant defense hypotheses. Q Rev Biol 78:23–55
Bagchi D, Sen C, Bagchi M, Atalay M (2004) Anti-angiogenic, antioxidant, and anti-carcinogenic properties of a novel anthocyanin-rich berry extract formula. Biochemistry (Moscow) 69:75–80
Bowen-Forbes CS, Zhang Y, Nair MG (2010) Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J Food Comp Anal 23:554–560
Hayashi K, Mori M, Matsutani-Knox Y, Suzutan T, Ogasawara M, Yoshida I, Hosokawa K, Tsukui A, Azuma M (2003) Anti influenza virus activity of a red-fleshed potato anthocyanin. Food Sci Technol Res 9:242–244
Matsui T, Ebuchi S, Kobayashi M, Fukui K, Sugita K, Terahara N, Matsumoto K (2002) Anti-hyperglycemic effect of diacylated anthocyanin derived from Ipomoea batatas cultivar Ayamurasaki can be achieved through the α-glucosidase inhibitory action. J Agric Food Chem 50:7244–7248
Kong J-M, Chia L-S, Goh N-K, Chia T-F, Brouillard R (2003) Analysis and biological activities of anthocyanins. Phytochemistry 64:923–933
Mazza G, Miniati E (1993) Anthocyanins in fruits, vegetables, and grains. CRC Press, London, pp 1–362
Pojer E, Mattivi F, Johnson D, Stockley CS (2013) The case for anthocyanin consumption to promote human health: a review. Compr Rev Food Sci Food Saf 12:483–508
Prior RL, Wu X (2009) Anthocyanins: structural characteristics that result in unique metabolic patterns and biological activities. Free Radic Res 40:1014–1028
Sun J, **ao Z, L-z Lin, Lester GE, Wang Q, Harnly JM, Chen P (2013) Profiling polyphenols in five Brassica species microgreens by UHPLC-PDA-ESI/HRMSn. J Agric Food Chem 61:10960–10970
Tatsuzawa F, Saito N, Shinoda K, Shigihara A, Honda T (2006) Acylated cyanidin 3-sambubioside-5-glucosides in three garden plants of the Cruciferae. Phytochemistry 67:1287–1295
Alkema J, Seager SL (1982) The chemical pigments of plants. J Chem Educ 59:183
Dyrby M, Westergaard N, Stapelfeldt H (2001) Light and heat sensitivity of red cabbage extract in soft drink model systems. Food Chem 72:431–437
Stintzing FC, Stintzing AS, Carle R, Frei B, Wrolstad RE (2002) Color and antioxidant properties of cyanidin-based anthocyanin pigments. J Agric Food Chem 50:6172–6181
Clarke CJ, Haselden JN (2008) Metabolic profiling as a tool for understanding mechanisms of toxicity. Toxicol Pathol 36:140–147
Harrigan GG, Goodacre R (2003) Metabolic profiling: its role in biomarker discovery and gene function analysis. Springer, New York, pp 1–335
Lisec J, Schauer N, Kopka J, Willmitzer L, Fernie AR (2006) Gas chromatography mass spectrometry–based metabolite profiling in plants. Nat Protoc 1:387–396
Kim JK, Bamba T, Harada K, Fukusaki E, Kobayashi A (2007) Time-course metabolic profiling in Arabidopsis thaliana cell cultures after salt stress treatment. J Exp Bot 58:415–424
Kim JK, Park S-Y, Lee SM, Lim S-H, Kim HJ, Oh S-D, Yeo Y, Cho HS, Ha S-H (2013) Unintended polar metabolite profiling of carotenoid-biofortified transgenic rice reveals substantial equivalence to its non-transgenic counterpart. Plant Biotechnol Rep 7:121–128
Kim YB, Kim JK, Uddin MR, Xu H, Park WT, Tuan PA, Li X, Chung E, Lee J-H, Park SU (2013) Metabolomics analysis and biosynthesis of rosmarinic acid in Agastache rugosa Kuntze treated with methyl jasmonate. PLoS ONE 8:e64199
Thwe AA, Kim JK, Li X, Kim YB, Uddin MR, Kim SJ, Suzuki T, Park NI, Park SU (2013) Metabolomic analysis and phenylpropanoid biosynthesis in hairy root culture of tartary buckwheat cultivars. PLoS ONE 8:e65349
Ding M-Z, Cheng J-S, **ao W-H, Qiao B, Yuan Y-J (2009) Comparative metabolomic analysis on industrial continuous and batch ethanol fermentation processes by GC-TOF-MS. Metabolomics 5:229–238
Jiang W, Qiu Y, Ni Y, Su M, Jia W, Du X (2010) An automated data analysis pipeline for GC–TOF–MS metabonomics studies. J Proteome Res 9:5974–5981
Fischer J (1992) Sulphur-and nitrogen-containing volatile components of kohlrabi (Brassica oleracea var. gongylodes L.). Eur Food Res Technol 194:259–262
Kim D-b, Oh J-W, Shin G-H, Kim Y-H, Lee JS, Park I-J, Cho JH, Lee O-H (2014) Inhibitory effect of kohlrabi juices with antioxidant activity on oxidative stress in human dermal fibroblasts (LB394). FASEB J 28:394
Kim JK, Kim YS, Kim Y, Uddin MR, Kim YB, Kim HH, Park SY, Lee MY, Chung SO, Park SU (2014) Comparative analysis of flavonoids and polar metabolites from hairy roots of Scutellaria baicalensis and Scutellaria lateriflora. World J Microbiol Biotechnol 30:887–892
Lee J-W, Lee D-Y, Baek D-R, Jeong R-H, Lee D-S, Kim Y-C, Kim G-S, Baek N-I, Lee Y-H (2014) Phenylpropanoids from red kohlrabi sprouts inhibits nitric oxide production in RAW 264.7 macrophage cells. Food Sci Biotechnol 23:965–969
Zhang Y, Hu Z, Zhu M, Zhu Z, Wang Z, Tian S, Chen G (2015) Anthocyanin accumulation and molecular analysis of correlated genes in purple Kohlrabi (Brassica oleracea var. gongylodes L.). J Agric Food Chem 63:4160–4169
Kim YB, Park S-Y, Park CH, Park WT, Kim S-J, Ha S-H, Arasu MV, Al-Dhabi NA, Kim JK, Park SU (2016) Metabolomics of differently colored Gladiolus cultivars. Appl Biol Chem 59:597–607
Fouad WMM (2004) Metabolic engineering for beta-alanine overproduction and stress tolerance in plants: Expression of Escherichia coli L-aspartate-alpha-decarboxylase in transgenic tobacco. University of Florida, pp 106
Betsche T, Eising R (1986) Refixation of photorespiratory ammonia and the role of alanine in photorespiration: studies with 15 N. Plant Soil 91:367–371
McCue KF, Conn EE (1990) Induction of shikimic acid pathway enzymes by light in suspension cultured cells of parsley (Petroselinum crispum). Plant Physiol 94:507–510
Kwon Y, S-i Yu, Lee H, Yim JH, Zhu J-K, B-h Lee (2012) Arabidopsis serine decarboxylase mutants implicate the roles of ethanolamine in plant growth and development. Int J Mol Sci 13:3176–3188
Hahlbrock K, Scheel D (1989) Physiology and molecular biology of phenylpropanoid metabolism. Annu Rev Plant Biol 40:347–369
Yonekura-Sakakibara K, Nakayama T, Yamazaki M, Saito K (2008) Modification and stabilization of anthocyanins. Anthocyanins. Springer, New York, pp 169–190
Bridle P, Timberlake C (1997) Anthocyanins as natural food colours—selected aspects. Food Chem 58:103–109
Hernández-Herrero JA, Frutos MJ (2011) Degradation kinetics of pigment, colour and stability of the antioxidant capacity in juice model systems from six anthocyanin sources. Int J Food Sci Technol 46:2550–2557
Malien-Aubert C, Dangles O, Amiot MJ (2001) Color stability of commercial anthocyanin-based extracts in relation to the phenolic composition. Protective effects by intra-and intermolecular copigmentation. J Agric Food Chem 49:170–176
Saito N, Tatsuzawa F, Yoda K, Yokoi M, Kasahara K, Iida S, Shigihara A, Honda T (1995) Acylated cyanidin glycosides in the violet-blue flowers of Ipomoea purpurea. Phytochemistry 40:1283–1289
Dixon RA (2001) Natural products and plant disease resistance. Nature 411:843–847
Harborne JB (2001) Twenty-five years of chemical ecology. Nat Prod Rep 18:361–379
Nugroho LH, Verpoorte R (2002) Secondary metabolism in tobacco. Plant Cell, Tissue Organ Cult 68:105–125
Yeoman M, Yeoman C (1996) Manipulating secondary metabolism in cultured plant cells. New Phytol 134:553–569
Zheng Z-L (2009) Carbon and nitrogen nutrient balance signaling in plants. Plant Signal Behav 4:584–591
Zulak KG, Weljie AM, Vogel HJ, Facchini PJ (2008) Quantitative 1 H NMR metabolomics reveals extensive metabolic reprogramming of primary and secondary metabolism in elicitor-treated opium poppy cell cultures. BMC Plant Biol 8:1
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, Massachusetts, pp 1–889
Scheible W-R, Morcuende R, Czechowski T, Fritz C, Osuna D, Palacios-Rojas N, Schindelasch D, Thimm O, Udvardi MK, Stitt M (2004) Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol 136:2483–2499
Causin HF (1996) The central role of amino acids on nitrogen utilization and plant growth. J Plant Physiol 149:358–362
Lee J, Finn CE (2012) Lingonberry (Vaccinium vitis-idaea L.) grown in the Pacific Northwest of North America: anthocyanin and free amino acid composition. J Funct Foods 4:213–218
Kim YB, Park S-Y, Thwe AA, Seo JM, Suzuki T, Kim S-J, Kim JK, Park SU (2013) Metabolomic analysis and differential expression of anthocyanin biosynthetic genes in white-and red-flowered buckwheat cultivars (Fagopyrum esculentum). J Agric Food Chem 61:10525–10533
Jain A, Srivastava H (1984) Effect of phenolic acids on anthocyanin content in maize roots. Biol Plant 26:241–245
Acknowledgments
This research was supported by Agriculture, Food and Rural Affairs Research Center Support Program, Ministry of Agriculture, Food and Rural Affairs and Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Agri-Bio Industry Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (316006-5).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Chang Ha Park and Hyun Ji Yeo have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Park, C.H., Yeo, H.J., Kim, N.S. et al. Metabolic profiling of pale green and purple kohlrabi (Brassica oleracea var. gongylodes). Appl Biol Chem 60, 249–257 (2017). https://doi.org/10.1007/s13765-017-0274-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13765-017-0274-z